Abstract
Immune parameters were compared in four groups of Ugandan subjects: HIV− and HIV+ adult patients with active pulmonary TB (HIV− PTB n = 38; HIV+ PTB n = 28), patients with HIV infection only (n = 26) and PPD+ healthy controls (n = 25). Compared with healthy controls, CD4 and CD8 T cells from patients with HIV and/or PTB expressed more activation markers (HLA-DR, CD38); their CD8 T cells expressed more CD95 (pre-apoptosis) and less CD28 (co-stimulatory receptor). Peripheral blood mononuclear cells (PBMC) of patients with either HIV or PTB were impaired in interferon-gamma (IFN-γ) production upon antigenic stimulation. PTB (with or without HIV) was characterized by monocytosis, granulocytosis, increased transforming growth factor-beta 1 production and PPD-induced apoptosis. In vivo CD4 T cell depletion, in vitro increased spontaneous CD4 T cell apoptosis and defects in IFN-γ responses upon mitogenic stimulation were restricted to HIV+ subjects (with or without PTB). Overlapping and distinctive immune alterations, associated with PTB and HIV, might explain mutual unfavourable influences of both diseases.
Keywords: HIV, tuberculosis, apoptosis, interferon-gamma, transforming growth factor-beta
INTRODUCTION
Protective immunity against Mycobacterium tuberculosis (MTB) essentially depends on ‘cell-mediated immunity’ (CMI), involving interactions between MTB-specific CD4 T cells and cells of the monocyte-macrophage (MO/MA) lineage [1–3]. A key factor in MO/MA activation is interferon-gamma (IFN-γ), produced by the CD4+ effector T cells [3–5]. The in vitro correlates of decreased CMI in active pulmonary TB (PTB) include lowered IFN-γ production after stimulation with PPD or MTB antigens [1,4,5], increased production of the immune suppressive cytokines transforming growth factor-beta 1 (TGF-β1) and IL-10 [1,5] as well as increased MTB-stimulated apoptosis [6]. Infection with HIV, on the other hand, is characterized by a more generalized defect in CMI, including decreased proliferative responses to both antigens and mitogens [7,8], increased spontaneous and activation-induced apoptosis [9,10] and deficient IFN-γ production in more advanced stages of HIV infection [11,12]. Both HIV infection and PTB are also characterized by overproduction of MO/MA-derived inflammatory factors, including tumour necrosis factor-alpha (TNF-α), neopterin and β2-microglobulin [13]. During HIV infection particular phenotypic changes are present in both CD4 and CD8 T cell subsets, including increased expression of HLA-DR and CD38 (related to immaturity and activation) [14], increased CD95 (related to apoptosis) [15,16] and decreased expression of CD28, which is a receptor for the important co-stimulatory molecules of the B7 family [17–22]. T cell phenotyping has not extensively been studied in PTB, but we described an increased HLA-DR expression on both CD4 and CD8 T cells [20].
MTB and HIV are well known to act synergistically both clinically and pathogenically [21,22]. Most previous pathogenic studies concentrated on each disease separately. In order to establish the point at which immune parameters in PTB and HIV interact, HIV− and HIV+ controls were compared with HIV− and HIV+ PTB patients. The following in vivo parameters were selected: major leucocyte subsets, T cell activation markers and viral load (the latter in HIV+ subjects only). Immune function was evaluated by measuring T cell proliferation, apoptosis and production of important cytokines (IFN-γ, TGF-β1, TNF-α) in vitro. The relationship between immune parameters and clinical staging of HIV and PTB infection was also investigated.
SUBJECTS and METHODS
Subjects
Immune parameters were studied in four groups of subjects at the CWRU Research Collaboration Tuberculosis Project at Mulago Hospital and at the JCRC facilities in Kampala, Uganda.
Thirty-eight HIV− and 28 HIV+ adults with sputum smear-positive and culture-confirmed PTB were investigated before the start of a 6-month short course chemotherapy. The PTB patients were compared with 25 healthy HIV− PPD+ and 26 HIV+ controls, with no signs or symptoms of acute AIDS-defining event. Adult subjects (18–60 years) were enrolled in the study after HIV pretest counselling and informed consent. Pregnant women were excluded.
Routine lab determinations
In all subjects, total blood count, differential leucocyte count and serum HIV ELISA testing were performed. In patients with PTB a chest x-ray was performed and graded using a numeric scale [23].
Viral load
Viral load was determined in HIV+ controls and in the HIV+ PTB co-infected patients by polymerase chain reaction (PCR) technique, using Cobas Amplicor HIV-1 Monitor™ version 1.5 (Roche Diagnostics, Branchburg, NJ).
Immune phenotypic studies
EDTA-whole blood (50 μl) were incubated with seven combinations of MoAbs at 5 μl each (Becton Dickinson, Erembodegem, Belgium). The major T cell subsets as well as monocytes were counted using (i) anti-CD45–FITC + anti-CD4–PE + anti-CD3–PerCP and (ii) anti-CD4–FITC + anti-CD3–PE + anti-CD8–PercP. Activation and differentiation markers on CD4 and CD8 T cells were measured with combinations of anti-CD4–FITC + anti-CD8–PerCP and one of the following: (iii) isotypic IgG1–PE (background control); (iv) anti-HLA-DR–PE; (v) anti-CD38–PE; (vi) anti-CD28–PE and (vii) anti-CD95–PE. After 30 min incubation, erythrocytes were lysed (Becton Dickinson lysing solution), the leucocytes were washed once with PBS–bovine serum albumin (BSA) 1% pH 7·2 and fixed with 250 μl 1% paraformaldehyde in PBS; samples were acquired in a FACScan with the LYSYS II software and analysed with WinMDI software version 2.4. The percentage cells within the CD4 or CD8 T population, expressing HLA-DR, CD38, CD28 and CD95 (above the threshold of channel 325) were calculated.
T cell functional parameters
Peripheral blood mononuclear cells (PBMC) were isolated from lithium-heparinized whole blood by differential centrifugation over Ficoll–Hypaque, washed three times with RPMI and resuspended in medium RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 1% l-glutamine and 5% normal human serum, without antibiotics. One millilitre of PBMC (final concentration 106/ml) was dispensed in 5-ml polystyrene culture tubes, using the following culture conditions: (i) medium alone; (ii) Candida albicans antigen (Hollister, Greer Laboratories, Lenore, NC) at 20 μg/ml; (iii) PPD (provided by R. D. Wallis, CWRU, Cleveland, OH) at 10 μg/ml; (iv) 5 μl MTB (live avirulent H37Ra, 9·8 × 108 colony-forming units (CFU)/ml, provided by R. Silver, CWRU); and (v) pokeweed mitogen (PWM; Sigma, Bornem, Belgium) at 1 ng/ml. All cultures were set up in triplicate and incubated at 37°C with 5% CO2.
Measurement of TNF-α, IFN-γ and TGF-β1
Supernatants (100 μl) were harvested from each triplicate after 16 h (= day + 1) for TNF-α determination and after 90 h (= day + 4) for IFN-γ and TGF-β1. These supernatants were stored at −70°C. Cytokines were measured by ELISA: TNF-α and TGF-β1 kits were from Quantikine HS (R&D Systems, Minneapolis, MN) and the kit for IFN-γ was from Endogen Inc. (Woburn, MA). Results were expressed in pg/ml.
T cell proliferative responses
After harvesting the supernatants on day 4, triplicate PBMC suspensions were mixed together, and from this mixture four replicates of 150 μl were dispensed in 96-well plates. 3H-methyl-thymidine (20 μl; 0·4 μCi) was added to each well and the plates were incubated for 20 h at 37°C. Cells were harvested on filter paper and radioactivity measured using a Topcount scintillation counter (Packard, Meriden, CT). Thymidine incorporation was expressed in ct/min.
CD4 T cell apoptosis
Triplicate cultures of 106 PBMC from each subject were set up in 1 ml medium with 10% fetal bovine serum and incubated for 4 days in a CO2 incubator at 37°C, using the same conditions as listed above. Apoptosis was measured using the TUNEL reagent, combined with anti-CD4–PE surface labelling, as described [6].
Statistical analysis
All statistical analysis was performed using the SPSS program for Windows (version 5.0.1). The results in each group of patients were expressed as mean ± s.e.m. To compare subject groups the t-test for independent samples was used, and differences were considered significant at P < 0·05. A simple factorial anova test was performed to investigate the influence of covariates (CD4 count, age, sex) and correlations between various parameters within each patient group were calculated with the two-tailed Spearman's rank test.
RESULTS
Characteristics of the subject groups (Table 1)
Table 1.
Clinical and routine laboratory data of the four subject groups
| Subject groups n = | HIV− PPD+ 25 | HIV− PTB+ 36 | HIV+ PTB− 26 | HIV+ PTB+ 28 |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | ||||
| (mean, s.e.m.) | 29·8 (1·9) | 25·4 (0·8)* | 32·2 (1·4)†† | 29·5 (1·6)† |
| Sex ratio, M/F | 19/6 | 26/10 | 11/15*† | 11/17**†† |
| Body mass index (%) | 31·0 (0·5) | 30·6 (0·8) | ||
| Sputum FAB smear | ||||
| scarce/1+/2+/3 + | ||||
| (% of subjects) | 3 17 19 61 | 7 14 32 47 | ||
| Chest x-ray | ||||
| grade 1/2/3 | ||||
| (% of subjects) | 2·5 30·5 67 | 14 25 61 | ||
| Laboratory data | ||||
| Leucocyte count/μl | 5104 (259) | 9333 (711)** | 4969 (346)†† | 8014 (795)**‡‡ |
| Granulocytes | 2057 (154) | 4326 (356)** | 2040 (229)†† | 4057 (532)**‡‡ |
| Lymphocytes | 2244 (140) | 2253 (146) | 2100 (134) | 1911 (248) |
| Monocytes | 803 (68) | 2754 (323)** | 829 (168)†† | 2046 (214)**‡‡ |
| CD4 T cell count | 939 (56) | 888 (54) | 401 (60)** | 303 (49)**†† |
| CDC classification (% of subjects) 1, 2, 3 | 92 8 0 | 94 6 0 | 38·5 23 38·5** | 25 32 43**†† |
| CD8 T cell count | 524 (45) | 485 (43) | 1087 (85)**†† | 926 (162)*† |
| Viral load (log) | 4·9 (0·2) | 5·2 (0·1) | ||
Mean values ±s.e.m. in each subject group are represented. Differences between the subject groups were calculated using Student's t-test for independent samples:
P < 0·05
P < 0·01 versus HIV− PPD+ controls.
P < 0·05
P < 0·01 versus HIV− PTB+ patients.
P < 0·05
P < 0·01 versus HIV+ PTB− controls.
CD4 classes are according the CDC classification [40]: Class 1, CD4 ≥ 500/μl; Class 2, 200 ≥ CD4 < 500/μl; Class 3, CD4 < 200/μl.
The HIV− and HIV+ controls, as well as the dually infected subjects, were similar in age, but the HIV− PTB+ patients were significantly younger. Men predominated in the HIV− groups (controls and PTB patients), whereas both HIV+ groups contained more women than men. HIV− and the HIV+ PTB did not differ in body mass index (BMI), degree of smear positivity or chest x-ray grading.
Leucocyte, granulocyte and monocyte counts were higher in the PTB patients (HIV− or HIV+), compared with the respective controls, but the absolute lymphocyte count was similar in the four groups. As expected, CD4 T cell numbers as well as CD4/CD8 ratio (not shown) were significantly lower in the HIV+ than in the HIV− groups, but these parameters did not differ between the HIV− controls and HIV− PTB patients. Moreover, the distribution of CD4 counts, according to CDC classification, was similar in the dually infected patients and in the HIV+ controls, but viral load tended to be higher in the dually infected patients (P = 0·09).
Increased expression of activation markers on CD4 T cells and CD8 T cells of HIV− PTB+ and HIV+ PTB+ patients
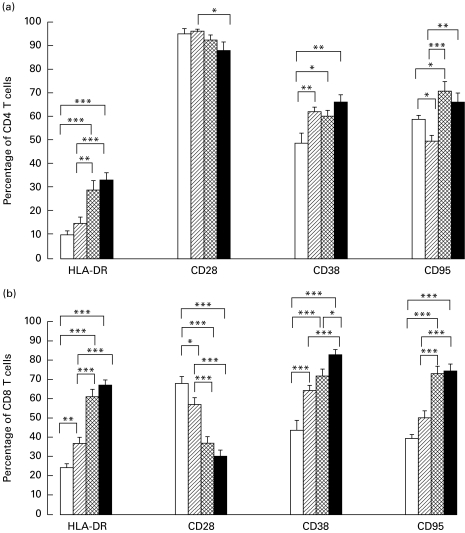
Compared with the PPD+ healthy controls, a larger proportion of CD8 T cells from the PTB patients (without HIV) expressed HLA-DR, CD38, and CD95 (Fas), but a lower proportion was CD28+. Their CD4 T cells expressed more CD38, but less CD95. Amongst the four patient groups, the HIV and PTB co-infected patients showed the highest proportions of HLA-DR, CD38 and CD95, and the lowest proportion of CD28 within both CD4 and CD8 T cells (more pronounced in the latter subset). However, only the CD38 marker on CD8 T cells was significantly different between dually and HIV-only infected subjects (Fig. 1a,b). A simple factorial anova test indicated that the number of CD4 T cells was the main determinant of CD38 expression in co-infected subjects with CD4 count <200/μl (P < 0·05). In subjects with CD4 counts >200/μl however, both the CD4 T count and the presence of PTB significantly contributed to enhanced CD38 expression.
Fig. 1.
Differentiation markers on CD4 T cells (a) and on CD8 T cells (b). Whole blood from 23 HIV− PPD+ controls (□), 36 HIV− pulmonary TB (PTB)+ patients (hatched), 25 HIV+ PTB− subjects (cross-hatched) and 25 HIV+ PTB+ patients (▪) was incubated with anti-CD4 FITC, anti-CD8 PerCP and one of the following PE-labelled monoclonals: isotypic control IgG1, anti-HLA-DR, anti-CD28, anti-CD38 or anti-CD95. Using the isotypic control, the threshold of positivity in PE was fixed on channel 325 and the activation marker expression was calculated as a percentage within the gated CD4, respectively, CD8 T cells. Results are given as mean ±s.e.m. within each patient group. The significance of differences between the groups was calculated using Student's t-test for independent samples, with the following levels of significance: *P < 0·05; **P < 0·01; ***P < 0·001.
Proliferation and apoptosis induced by antigenic and mitogenic stimulation
Compared with healthy controls, PBMC from HIV− PTB patients showed similar proliferative responses to PPD, decreased proliferation to Candida and an increased response to PWM (data not shown). PBMC from HIV-infected subjects, with or without PTB, proliferated less to PPD, Candida and PWM compared with the HIV− controls (data not shown).
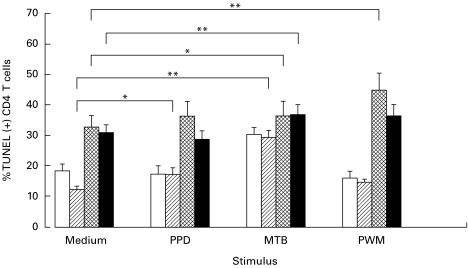
In accordance with previous studies [15,16], we confirmed that CD4 T cell apoptosis was systematically higher in the HIV+ groups (with or without PTB) compared with the respective HIV− groups. This was particularly true in non-stimulated cultures and in those stimulated with PPD or PWM (P < 0·01), but not in those stimulated with MTB.
Comparing unstimulated and stimulated cultures within each group indicated that PPD slightly, but significantly, increased the CD4 T cell apoptosis in the HIV− PTB patients, whereas activation with PWM selectively enhanced CD4 T cell death in the HIV+ controls. Stimulation with MTB increased apoptosis in all patient groups (Fig. 2: only the significance of differences between spontaneous and stimulated apoptosis within each group is indicated).
Fig. 2.
Apoptosis of CD4+ T cells after antigenic and mitogenic stimulation. Three replicate cultures of peripheral blood mononuclear cells were incubated with medium, PPD, Mycobacterium tuberculosis (MTB) and pokeweed mitogen (PWM) from 24 HIV− PPD+ controls (□), 30 HIV− PTB+ patients (hatched), 14 HIV+ PTB− subjects (cross-hatched) and 16 HIV+ PTB+ (▪) patients. Apoptosis was assessed using the TUNEL test in combination with anti-CD4–PE. Results are expressed as percentage of TUNEL (+) CD4 T cells. Indicated in the figure are the statistical significant differences (paired t-test), between the non-stimulated and the stimulated cultures within each subject group.
Decreased IFN-γ and increased TGF-β1 production in HIV− and HIV+ PTB
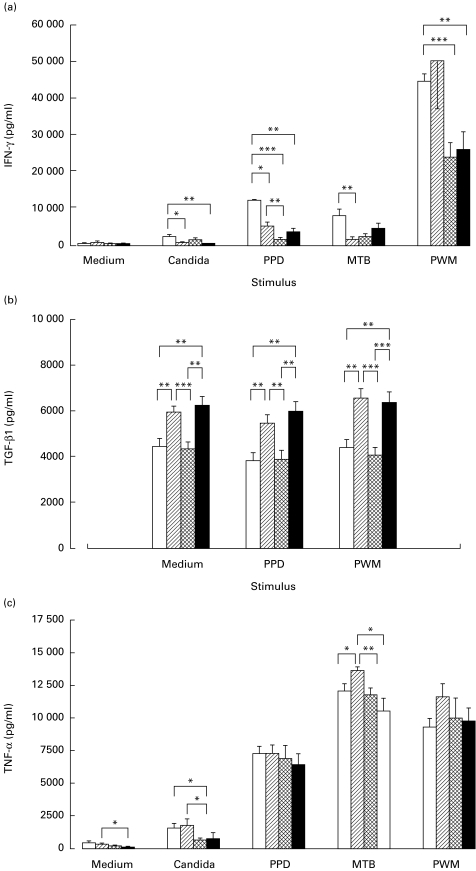
IFN-γ was strongly induced by PWM > PPD > MTB > Candida in PBMC from HIV− control subjects. Subjects with active PTB only, showed a selectively decreased IFN-γ production to antigenic stimulation with Candida, PPD or MTB and a normal response to PWM. In HIV-infected subjects, with or without PTB, IFN-γ production was lowered, after either antigenic or mitogenic stimulation (Fig. 3a).
Fig. 3.
Secretion of cytokines in cultured peripheral blood mononuclear cells (PBMC). PBMC from 23 HIV− PPD+ controls (□), 31 HIV− PTB+ patients (hatched), 24 HIV+ PTB− subjects (cross-hatched) and 21 HIV+ PTB+ (▪) patients, were cultured in vitro. The respective stimuli are indicated on the abscissa. (a) IFN-γ, (b) transforming growth factor-beta 1, (c) tumour necrosis factor-alpha were measured in the supernatants by ELISA and results were expressed in pg/ml. The differences in cytokine secretion between subject groups were calculated and presented as in Fig. 1.
The spontaneous production of TGF-β was significantly higher in cell cultures from PTB patients (with or without HIV) compared with respective controls. Remarkably, TGF-β1 production was not increased by any stimulation (Fig. 3b).
TNF-α was strongly induced by MTB > PWM > PPD > Candida and did not differ much between the subject groups, except for MTB-induced TNF-α, which was highest in patients with PTB only (Fig. 3c).
Correlation between clinical and laboratory parameters
Influence of age and sex
In view of the younger age of HIV− PTB patients and the sex differences between the HIV− and HIV+ groups (Table 1), we wondered whether immunological alterations were related to the HIV− PTB infection per se or to sex/age differences. Statistical correction (anova) for age between the HIV− PTB patients and the HIV− controls did not abrogate the changes of immune phenotype and function, with the exception of the lowered CD95 on CD4 T cells from PTB patients, which disappeared after age correction. Similarly, all significant immune differences between the HIV− and the HIV+ groups persisted after correction for sex.
Effects of low CD4 counts
Amongst HIV+ subjects with or without PTB, the subgroups with CD4 counts <200 had a significantly higher viral load (+ 0·5 log/ml). T cells from HIV+ patients with low CD4 T counts expressed significantly more HLA-DR, CD38 and CD95 and less CD28 (P < 0·05). PBMC from patients with low CD4 counts displayed higher CD4 T apoptosis in all conditions as well as lower proliferation and IFN-γ response to PPD and PWM. Clearly, most differences between subgroups with higher and lower CD4 T counts were similar in HIV+ subjects with or without concomitant PTB. Nevertheless, only dually infected subjects with low CD4 counts also had a significantly lower BMI (−3·3%).
Correlates of sputum positivity and x-ray staging
In PTB patients without HIV, far-advanced disease on x-ray (grade 3) was associated with a higher degree of sputum positivity (P < 0·05). Both of these disease parameters were associated with higher granulocyte (P < 0·05) and lower lymphocyte counts (P = 0·065) in vivo as well as with higher spontaneous TNF-α (P < 0·05) and TGF-β1 production (P = 0·075) in vitro. In HIV+ PTB patients however, the radiographic and sputum gradings were not significantly correlated with each other. Dually infected patients with 3+ sputum tended to have lower CD4 counts in vivo (P = 0·07) and their T cells displayed more phenotypic evidence of activation: higher CD38, higher CD95 and lower CD28 expression (P < 0·05).
DISCUSSION
The present study prospectively compared immune parameters, in healthy subjects and patients with HIV and/or PTB. Some alterations were particular for each disease: PTB was characterized by granulo-monocytosis in vivo and increased TGF-β1 production in vitro; whereas HIV-infected subjects showed the expected in vivo CD4 T cell depletion, increased in vitro CD4 T cell apoptosis and generalized hyporesponsiveness to in vitro stimulation. These anomalies coexisted in dually infected subjects, without evidence of a synergistic effect of HIV and PTB. Other immune changes were present in both diseases, e.g. aberrant in vivo T cell activation (increased HLA-DR, CD38, decreased CD28) and decreased IFN-γ production upon in vitro antigenic stimulation. In general, these common alterations were less pronounced in patients with PTB only, compared with HIV only. Dual infection had an additive effect on CD38 expression in patients with CD4 T counts >200/μl. The effect of active PTB on HIV viral load was limited to a non-significant increase of +0·5 log.
The expression of differentiation markers on peripheral T cells is tightly regulated and relates to cell function. HLA-DR can present antigenic peptides to the CD4–T cell receptor complex. CD38 functions both as an adhesion molecule and as ecto-enzyme, mediating ADP-induced Ca2+ mobilization. Increased T cell expression of HLA-DR and CD38 is associated with activation and/or immaturity. Triggering of CD28 by co-stimulatory signals of the B7 family enhances and prolongs cytokine production. Lowered expression of CD28 reflects terminal differentiation, shortening of the telomeres and cellular senescence. Finally, CD95 is a receptor for pro-apoptotic signals [24–31].
During HIV infection changes in expression of these markers are linked to immune dysfunction and disease progression. Our present study confirmed HIV-associated up-regulation of HLA-DR, CD38 and CD95 and down-regulation of CD28 on both CD4 and CD8 T cells in a disease stage-related fashion. It was previously shown that these in vivo phenotypic changes correlate with hyporesponsiveness to in vitro re-stimulation and with a tendency to apoptosis. In addition, T cell (over)activation, especially CD38 increase on CD8 T cells, predicts disease progression in a CD4-independent way [14,17,18,32–38].
Increased HLA-DR expression on T cells of PTB patients in association with serum markers of immune activation was previously observed [28]. Our present data extend these findings by showing up-regulation of CD38 and down-regulation of CD28, especially on CD8 T cells from PTB patients. Since this pattern of T cell activation is qualitatively similar in HIV and PTB, we investigated whether similar immune dysfunction was associated too.
Compared with healthy controls, PBMC from PTB patients produced less IFN-γ upon stimulation with mycobacterial antigens, their spontaneous TGF-β1 production was increased, but their stimulated TNF-α was not different from controls, confirming previous data [39]. Remarkably, PBMC from PTB patients responded poorly to Candida (proliferation and IFN-γ), whereas their IFN-γ response to PWM was unaffected. In contrast, in HIV-infected subjects, with or without PTB, proliferation and IFN-γ responses to all stimuli were decreased. These findings indicate that immune deficiency during active PTB is not limited to MTB antigens alone, but it is still less generalized compared with HIV infection.
In a previous study, increased spontaneous as well as MTB-stimulated CD4 T cell apoptosis were selectively present in PTB patients [6]. The present data show no difference in spontaneous apoptosis between PTB patients and controls. Stimulation of T cells with MTB induced additional apoptosis in T cells from patients, but also in controls. CD4 T cells from HIV+ subjects displayed a more pronounced tendency to spontaneous and PWM-induced apoptosis, confirming previous data [15,16,37]. Co-infection with PTB had no additive effect on CD4 T cell death. The differential effect of MTB and PPD suggests that the former contains non-protein substances, directly or indirectly inducing cell death.
Taking all data together, it seems that similar T cell phenotype changes in PTB and HIV are reflected in T cell hyporesponsiveness to antigenic stimulation (e.g. decreased IFN-γ to PPD). Although apoptosis was much less pronounced in PTB, the PPD-induced IFN-γ production was inversely correlated with apoptosis in both HIV infection and PTB. Remarkably, decreased IFN-γ was also inversely correlated with in vivo HLA-DR expression in patients with PTB only, whereas in HIV+ patients (with or without PTB) it was directly correlated with in vivo CD4 T cell count, according to Spearman's rank test (data not shown).
Investigating the relation between clinical and biological parameters of PTB revealed different associations in HIV− versus HIV+ patients. In subjects with PTB only, a far-advanced radiographic stage and a strongly positive smear directly correlated with the PTB-specific biological alterations, including increased granulocytosis and TGF-β1 production, but there was no association with any T cell marker. In contrast, in dually infected subjects, far-advanced radiographic disease or grade 3 smears were associated with decreased CD4 T counts, aberrant T cell phenotypes and increased T cell apoptosis. Clearly, in dually infected subjects, PTB disease staging is correlated with typical immune parameters of HIV infection.
In conclusion, pulmonary tuberculosis and HIV display partly similar and partly distinct immune alterations. During active PTB, inappropriate in vivo T cell activation and spontaneous production of immune suppressive TGF-β may have a role in the observed hyporesponsiveness to both mycobacterial and other (Candida) antigens. In HIV-infected subjects, CD4 T cell depletion, together with even more pronounced T cell activation, is involved in a generalized hyporesponsiveness. In dually infected subjects, the HIV-related changes dominate the overall immunological picture, especially in patients with low CD4 T counts. Apparently, HIV and PTB have additive effects: each infection provides specific immune changes, which may contribute to the mutually unfavourable effects of co-infection.
Acknowledgments
Approval for this study was given by the Institute of Tropical Medicine, University Hospitals of Cleveland and Mulago Hospitals Investigational Review Committees and Uganda AIDS Commission. The study was supported by grants from the Damien Foundation, Brussels, Belgium with support from the Tuberculosis Research Unit of the National Institutes of Health, USA (grant no. AI-45244). The authors gratefully acknowledge Robert S. Wallis and Richard Silver (CWRU, Cleveland) for supplying PPD and MTB H37Ra; the medical, paramedical and counselling staff of the Uganda CWRU TB project Clinic and of the JCRC for dedicated and professional help; the HIV vaccine trial unit at JCRC for sharing some of their equipment.
REFERENCES
- 1.Kaplan G, Freedman VH. The role of cytokines in the immune response to tuberculosis. Res Immunol. 1996;147:565–72. doi: 10.1016/s0923-2494(97)85223-6. [DOI] [PubMed] [Google Scholar]
- 2.Rich EA. Pulmonary immune response to Mycobacterium tuberculosis and human immunodeficiency virus. Infect Agents Dis. 1996;5:108–18. [PubMed] [Google Scholar]
- 3.Vanham G, Toossi Z, Hirsch CS, Wallis RS, Schwander SK, Rich EA, Ellner JJ. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tuber Lung Dis. 1997;78:145–58. doi: 10.1016/s0962-8479(97)90021-6. [DOI] [PubMed] [Google Scholar]
- 4.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toossi Z. Cytokine circuits in tuberculosis. Infect Agents Dis. 1996;5:98–107. [PubMed] [Google Scholar]
- 6.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 7.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruters RA, Terpstra FG, De Jong R, Van Noesel CJ, Van Lier RA, Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990;20:1039–44. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 9.Oyaizu N, Pahwa S. Role of apoptosis in HIV disease pathogenesis. J Clin Immunol. 1995;15:217–31. doi: 10.1007/BF01540879. [DOI] [PubMed] [Google Scholar]
- 10.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–9. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 11.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Ullum H, Cozzi Lepri A, Bendtzen K, Victor J, Gotzsche PC, Phillips AN, Skinhoj P, Klarlund Pedersen B. Low production of interferon gamma is related to disease progression in HIV infection: evidence from a cohort of 347 HIV-infected individuals. AIDS Res Hum Retrovir. 1997;13:1039–46. doi: 10.1089/aid.1997.13.1039. [DOI] [PubMed] [Google Scholar]
- 13.Takashima T, Ueta C, Tsuyuguchi I, Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–92. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders RL, Gigase PL. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehri R, Hahn S, Rothen M, Steuerwald M, Nuesch R, Erb P. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS. 1996;10:9–16. [PubMed] [Google Scholar]
- 16.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–36. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vingerhoets J, Kestens L, Penne G, et al. CD8+ cells and not CD4+ T cells are hyporesponsive to CD28- and CD40L-mediated activation in HIV-infected subjects. Clin Exp Immunol. 1997;107:440–7. doi: 10.1046/j.1365-2249.1997.d01-964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinchmann JE, Dobloug JH, Heger BH, Haaheim LL, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 19.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 22.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 23.Falck A, O'connor JB, Pratt PC, Webb WR, Wier JA, Wolinski E. Diagnostic standards and classification of tuberculosis. 12. New York: National Tuberculosis and Respiratory Disease Association; 1969. Classification of pulmonary tuberculosis; pp. 68–78. [Google Scholar]
- 24.Horejsi V. Surface antigens of human leukocytes. Adv Immunol. 1991;49:75–147. [PubMed] [Google Scholar]
- 25.Mattern T, Scholz W, Feller AC, Flad HD, Ulmer AJ. Expression of CD26 (dipeptidyl peptidase IV) on resting and activated human T-lymphocytes. Scand J Immunol. 1991;33:737–48. doi: 10.1111/j.1365-3083.1991.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 26.Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38: a glycoprotein in search of a function. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 27.Boussiotis VA, Freeman GJ, Gribben JG, Nadler LM. The role of B7-1/B7-2: CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 28.Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 29.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 30.Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, Strauss K. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–8. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 31.Effros RB, Boucher N, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 32.Vanham G, Kestens L, Penne G, et al. Subset markers of CD8(+) cells and their relation to enhanced cytotoxic T-cell activity during human immunodeficiency virus infection. J Clin Immunol. 1991;11:345–56. doi: 10.1007/BF00918800. [DOI] [PubMed] [Google Scholar]
- 33.Kestens L, Vanham G, Gigase P, Young G, Hannet I, Vanlangendonck F, Hulstaert F, Bach BA. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–7. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleo G, De Maria A, Koenig S, Butini L, Moss B, Baseler M, Lane HC, Fauci AS. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci USA. 1990;87:4818–22. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo JJ, Bach BA. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–82. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho HN, Hultin LE, Mitsuyasu RT, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–9. [PubMed] [Google Scholar]
- 37.Silvestris F, Cafforio P, Frassanito MA, Tucci M, Romito A, Nagata S, Dammacco F. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS. 1996;10:131–41. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Mocroft A, Bofill M, Lipman M, et al. CD8+, CD38+ lymphocyte percent: a useful immunological marker for monitoring HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovir. 1997;14:158–62. doi: 10.1097/00042560-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]