Abstract
Eosinophils have a variety of functions. Although increasing evidence links the presence of eosinophils to airway damage, studies have not examined in detail if, and how, eosinophils affect skin inflammation. The purpose of this study was to determine whether eosinophil infiltration augments the contact sensitivity reaction in vivo. Guinea-pigs were sensitized with 2, 4-dinitrochlorobenzene and challenged on the dorsal skin or on the right ear lobe. The number of eosinophils and macroscopic changes of the skin lesion in the presence or absence of human recombinant IL-5 (rIL-5) administered at the remote site was assessed. The reaction on the dorsal skin was acutely eczematous with considerable basophil infiltration. In contrast, eosinophils had extensively infiltrated the right ear lobe and major basic protein was deposited in the dermis. A subcutaneous injection of rIL-5 (10 pmol/kg) at the remote site (left ear lobe) 12 h after challenge induced transient blood eosinophilia and enhanced eosinophil accumulation in the challenged ear lobe. These changes were accompanied by increased ear swelling and severe erythema. In contrast, eosinophil infiltration was significantly inhibited by rIL-5 administered at the time of challenge. Ear thickness, as well as the erythema and oedema, were also reduced. These data suggest that marked eosinophil infiltration enhances skin inflammation in allergic contact dermatitis. Moreover, locally administered IL-5 functions remotely by controlling eosinophil recruitment into the skin. The guinea-pig model of contact sensitivity may be useful for evaluating therapies and pharmaceuticals targeted at eosinophil infiltration.
Keywords: basophils, contact sensitivity, eosinophils, IL-5
INTRODUCTION
Eosinophils perform several functions. They contain histaminase, arylsulphatase and phospholipase D, all of which inactivate chemical mediators [1–3]. During the mid-1970s, considerable effort was directed towards investigating eosinophil function based on the notion that eosinophils removed debris, detoxified components of tissue reactions and down-regulated inflammation in allergic diseases [4]. However, the current view of eosinophil functions is that of defence mechanisms against large harmful organisms, such as parasites. Eosinophils secrete toxic granule components, namely major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil cationic protein and eosinophil-derived neurotoxin, as well as newly formed lipid mediators and products of oxygen metabolism. Eosinophils are responsible for endomyocardial fibrosis in patients with the hypereosinophilic syndrome [5]. Eosinophil granule proteins are detected mostly within necrotic lesions of the endocardium. They are also thought to contribute significantly to the inflammation associated with asthma. Eosinophil accumulation in the lungs of animal models causes airway hyperresponsiveness and striking pathological changes, including fibrotic reactions [6–8]. Neutralization of MBP with either anti-MBP antibody or acidic polyamino acids prevents both airway hyperresponsiveness and the destruction of ciliated epithelial cells in the trachea [9,10].
Tissue eosinophila is a frequent component of allergic skin diseases, such as allergic contact dermatitis, atopic dermatitis, drug-induced skin eruption, auto-immune bullous diseases. Eosinophils are considered to be important effector cells in atopic dermatitis, since MBP is extracellularly deposited in lesional skin [11], and eosinophils can secrete cytokines including interleukin-4 and interleukin-5 (IL-5) [12,13]. Major basic protein potently stimulates histamine release from basophils [14] and an intradermal injection of MBP induces an immediate erythematous wheal [15].
In contrast, Beck et al. [16] have reported a lack of clinical reactivity in human skin despite extensive eosinophil infiltration and MBP deposition induced by cutaneous injections of RANTES (regulated upon activation: normal T cell expressed/secreted). Consistent with their findings, we also found that eliminating eosinophils from inflamed skin tissue did not affect the ear swelling response in murine contact sensitivity [17]. Although considerable in vivo evidence supports the notion that eosinophils play important roles in the pathophysiology of bronchial asthma, no reports have directly demonstrated that eosinophils augment skin inflammation. Hence, this study questions whether or not eosinophil infiltration is involved in the inflammation associated with allergic skin diseases such as contact dermatitis. To address this question, we used a guinea-pig model of contact dermatitis, since the histological changes associated with contact sensitivity in mice differ from those of human contact dermatitis [18]. Moreover, oedema, erythema and vesiculobullous changes are not present in mice, but can be identified in the guinea-pig model. We examined the roles of eosinophils by regulating eosinophil recruitment into an inflamed area by administering recombinant IL-5 at a remote site. This study also raises an issue about the mode of action of locally secreted IL-5 in the regulation of blood and tissue eosinophilia.
MATERIALS AND METHODS
Animals
Female Hartley guinea-pigs weighing 350–400 g purchased from Japan SLC (Hamamatsu, Japan) were given free access to food and water and were studied in accordance with the guidelines of our institution for animal care.
Chemicals
The following chemicals were used without further purification: 2, 4-dinitrochlorobenzene (DNCB) (Nakalai Tesque Inc., Kyoto, Japan); polymyxin B sulphate (Sigma-Aldrich Japan KK, Tokyo, Japan); O-phenylendiamine dihydrochloride (Sigma-Aldrich Japan KK); 3-amino-1, 2, 4-triazole (AMT) (Sigma-Aldrich Japan KK); hexadecyltrimethylammonium bromide (HTAB) (Wako Pure Chemical Industries, Ltd, Osaka, Japan).
Sensitization and challenge
On days 0 and 1, guinea-pigs were sensitized by applying 100 µl of 10% DNCB in acetone to the clipped abdomen. On day 14, 10 µl of 0·15% DNCB in acetone was painted on each ear lobe. Ear thickness was measured using a dial thickness gauge (Peacock, Tokyo, Japan) before and after challenge and is expressed as the mean increment in thickness above the basal line control value. The shaved dorsal skin was challenged by painting with 10 µl of 0·15%, 0·08% and 0·04% DNCB in acetone.
Histological assessment
Excised specimens of skin tissue were fixed in 10% formalin and embedded in paraffin. Each specimen was sectioned into 2 µm slices, deparaffinized, and stained with 10% Giemsa in 2% sodium tetraborate. Basophils were differentiated from eosinophils and mast cells by subsequent staining with 10% Giemsa in 0·05 m acetate buffer (pH 4·2) [19,20].
Immunohistochemistry
One ear lobe from each guinea-pig was embedded in OCT compound (Miles Laboratories, Naperville, IL, USA) in liquid nitrogen. Acetone-fixed cryostat sections were incubated with mouse antihuman eosinophil MBP monoclonal antibody (BMK-13, YLEM srl, Rome, Italy). Sections were then immersed in rabbit antiserum to mouse immunoglobulin labelled with alkaline phosphatase-mouse antialkaline phosphatase complex (APAAP method). The reaction products were identified by reacting with alkaline phosphatase substrate containing naphthol AS-MX phosphate, FastRed, and levamizole (DAKO, Carpinteria, CA, USA).
Subcutaneous injection of human recombinant IL-5
The dorsal surface of the left ear lobes of sensitized or challenged guinea-pigs were administered subcutaneously (s.c.) with several doses of human recombinant IL-5 (rIL-5) (PeproTech EC Ltd, London, UK) in 0·1 ml PBS.
Blood eosinophil counts
Guinea-pigs were bled from the retro-orbital plexus. Absolute numbers of eosinophils were determined by adding 10 µl of blood to 90 µl Discombe's solution, then counting cells containing stained granules 10 min later in a haemocytometer.
Bone marrow eosinophil counts
Femurs were removed from sacrificed guinea-pigs. Bone marrow was gently expressed by flushing the femurs with RPMI-1640. A single cell suspension was generated by forcing the marrow through a 23-gauge needle, then washing and resuspending the cells in a final volume of 1 ml f RPMI-1640. Ten microliters of bone marrow suspension was added to 90 µl of Discombe's solution to estimate the absolute numbers of eosinophils.
Tissue eosinophil counts
The dorsal skin (15 mm in diameter) including epidermis and entire dermis or whole thickness of each ear lobe (18 × 10 mm) was excised and frozen at −70°C. The number of accumulated eosinophils in the skin was estimated by measuring eosinophil peroxidase (EPO) activity in skin homogenates. Eosinophil peroxidase was measured as described by Collins et al. [21] with some modification. Briefly, frozen skin sites were chopped, homogenized in 4 ml PBS containing 0·5% HTAB, sonicated for 20 s, and stored at −70°C. On the day of measurement, thawed samples were centrifuged twice (2800 g, 10 min, 20°C and 15 000 g, 20 min, 20°C), and the supernatants were incubated at 60°C for 2 h before a final centrifugation (15 000 g, 10 min, 20°C). Samples were placed in duplicate wells (50 µl per well) in 96-well flat-bottomed microtiter plates, then 100 µl of substrate (8·6 mm O-phenylendiamine dihydrochloride and 2·9 mm hydrogen peroxide in 100 mm Tris/HCl, pH 8·0) were added. After 30 min at room temperature, the reaction was stopped by adding 50 µl 4 m sulphuric acid and the absorbance was read at 492 nm in the presence or absence of 4 mm AMT. The standard sample for EPO measurement was prepared using eosinophils collected from the peritoneal cavities of donor guinea-pigs given 5 × 103 units of polymyxin B sulphate i.p. every week for 8–12 weeks, and purified over Percoll gradients. Eosinophils (1 × 106 cells/ml PBS containing 0·5% HTAB) were sonicated, incubated at 60°C for 2 h, then centrifuged (15 000 g, 20 min, 20°C) and the supernatant was collected. The equivalent number of eosinophils per dorsal skin site or ear lobe was calculated from an EPO calibration curve constructed from the absorbance of serially diluted EPO standard samples. The degree of tissue eosinophilia estimated by EPO activity almost exactly correlated with the number of eosinophils/mm basement membrane determined by light microscopy (data not shown).
RESULTS
Histological features of guinea-pig contact sensitivity to DNCB
Guinea-pigs were sensitized on the clipped abdomen with 10% DNCB on days 0 and 1. They were challenged with various concentrations of DNCB on the dorsal skin or with 0·15% of DNCB on each ear lobe on day 14. The dorsal skin or each ear lobe was excised 24 h after challenge.
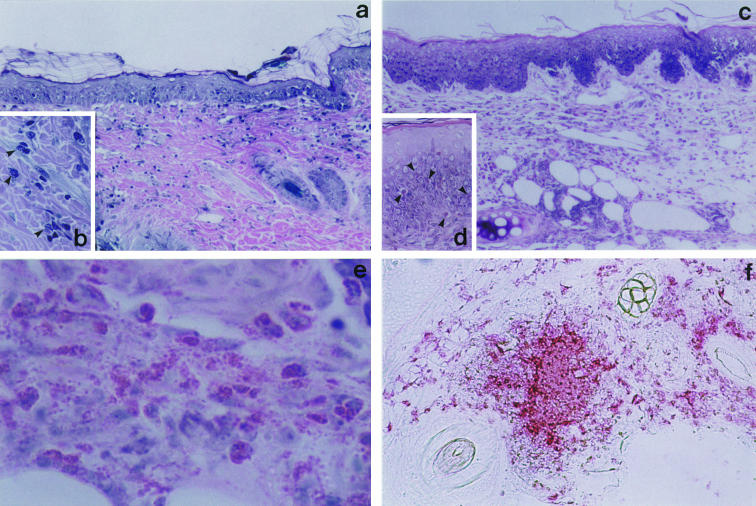
Histologically, the dorsal skin showed weak intercellular oedema with lymphocytic exocytosis in the epidermis (Fig. 1a). Cellular infiltrate including numerous basophils (Fig. 1b) in the upper dermis was observed, which was consistent with the previous reports [20,22]. On the other hand, the histological changes in the ear lobe were characterized by mild spongiosis in the epidermis and marked oedema with prominent eosinophil infiltration, but few basophils in the entire dermis (Fig. 1c,e). Eosinophils were also present within the epidermis (Fig. 1d). Figure 1(f) shows an immunohistochemical staining with antihuman eosinophil MBP monoclonal antibody (BMK13, IgG1). A number of MBP+ cells and prominent extracellular deposition of MBP were observed. Since data (not shown here) demonstrated that BMK13 cross-reacted with guinea-pig eosinophils, but not guinea-pig basophils, MBP deposited in the dermis was derived from eosinophils.
Fig. 1.
Microscopic features of skin reaction in guinea-pig contact sensitivity.Guinea-pigs were sensitized with DNCB and challenged on the dorsal skin or ear lobe on day 14. Excised skin specimens were stained with 10% Giemsa as described in Materials and methods. (a) Dorsal skin. The epidermis shows focal intercellular oedema with mononuclear cell infiltrate (spongiosis). Cellular infiltrate in the upper dermis consists of mononuclear cells, numerous basophils, and a few eosinophils. (b) High magnification view of basophils in the dermis (arrow head). (c) Ear lobe. The epidermis shows spongiosis. The entire dermis contains a remarkable number of eosinophils and mononuclear cells (d) Eosinophils have also infiltrated the epidermis (arrow head). (e) Prominent degranulation of eosinophilic granules. (f) Immunohistochemical staining for MBP in the challenged ear lobe. Numerous cells are positive for MBP and the extracellular deposition of MBP in the dermis is extensive.
Time course changes in the number of tissue eosinophils
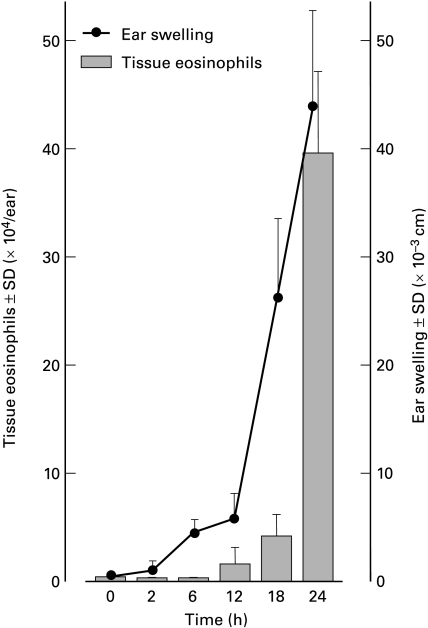
The number of eosinophils accumulating in the dorsal skin challenged with 0·15% DNCB at 24 h after challenge was 0·13 ± 0·05 × 104 cells/site. The dorsal skin samples challenged with 0·08% or 0·04% DNCB did not have detectable levels of EPO activity (less than 0·04 × 104 cells/site), although well-demarcated erythema was observed. The eosinophil number in the ear lobe challenged with 0·15% DNCB was approximately 300 times greater compared with the dorsal skin samples (Fig. 2). There was a dramatic increase in eosinophil accumulation between 12 and 24 h after challenge. This was in contrast to the guinea-pig airway disease model where the tissue eosinophil count was significantly elevated at 6 h after challenge [23].
Fig. 2.
Time course changes of ear thickness and tissue eosinophil count in dermis of the ear lobe. Flank skin of guinea-pigs was sensitized with 10% DNCB, then animals were challenged on each ear lobe with 0·15% DNCB. Ear swelling responses were measured and the absolute number of eosinophils in the lesional skin was determined. Both ear thickness and tissue eosinophil count dramatically increased after 12 h. Each group consisted of at least four animals.
Subcutaneous injection of IL-5 induces blood eosinophilia
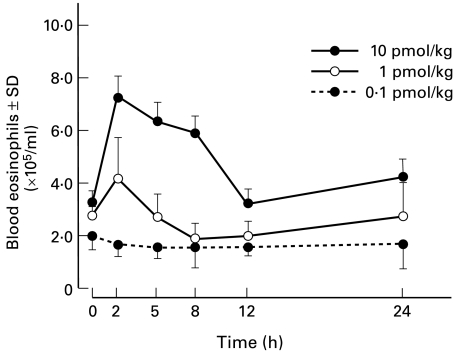
IL-5 has been described as a selective eosinophil chemotactic factor in vitro and in vivo [6,8,24–26]. Sensitized guinea-pigs were injected s.c. with several doses of human recombinant IL-5 (rIL-5) (0·1–10 pmol/kg) into the dorsal surface of the left ear lobe. After 2, 6, 12 and 24 h, the eosinophils accumulating in the area of rIL-5-injection were enumerated. However, EPO activity could not be detected, indicating that the count of eosinophils was less than 0·04 × 104 cells per ear. On the other hand, it was interesting to note that the peripheral blood of these animals showed eosinophilia that started 2 h after the injection and lasted 12 h (Fig. 3). There was a significant decrease in the numbers of bone marrow eosinophils when rIL-5-treated groups (2·0 ± 0·55 × 107 cells/femur) were compared with PBS-treated control groups (5·5 ± 1·22 × 107 cells/femur, P < 0·01). Since intravenous administration of IL-5 induces a rapid increase in circulating eosinophil numbers [21], blood eosinophilia in guinea-pigs treated with s.c. injection of rIL-5 was probably achieved by the circulating IL-5 leaking out from the injected site.
Fig. 3.
Subcutaneous injection of rIL-5 induces transient peripheral blood eosinophilia.Recombinant IL-5 in 0·1 ml PBS was injected s.c. into the dorsal surface of the left ear lobe. Peripheral blood obtained from the retro-orbital plexus was serially sampled to determine the absolute eosinophil count. Each point is the mean of counts in four animals.
Effect of subcutaneous injection of IL-5 on tissue eosinophil accumulation and eczematous reaction at a remote site
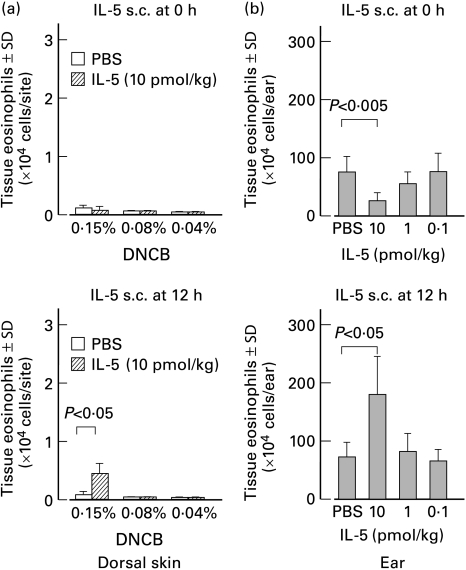
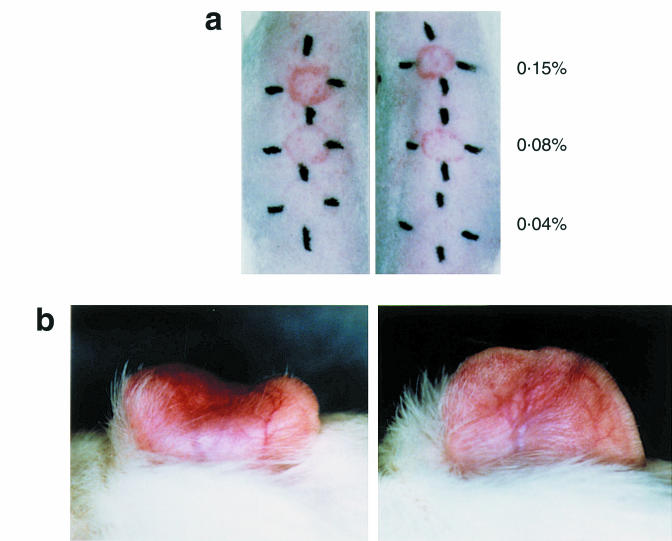
It has been reported that the number of eosinophils accumulating in response to intradermal eotaxin is correlated with an increase in circulating eosinophil numbers [21]. These findings raised our interest in studying the effect of s.c. injection of rIL-5 on tissue eosinophil accumulation at a remote site. Guinea-pigs were challenged on the dorsal skin or on the right ear lobe. At 0 or 12 h after challenge, rIL-5 was injected s.c. into the left ear lobe. As seen in Figs. 4, 10 pmol/kg rIL-5 administered at 12 h after challenge significantly enhanced the eosinophil accumulation into both the dorsal skin and ear lobe. Although there was no difference in the severity of erythema for the dorsal skin (Fig. 5a), erythema and oedema of right ear lobe were enhanced by the increased numbers of infiltrative eosinophils. On the other hand, 10 pmol/kg rIL-5 injected into the left ear lobe at the time of challenge (0 h) inhibited eosinophil recruitment in right ear lobe (Fig. 4). These guinea-pigs showed decreased erythematous and oedematous reactions in the right ear lobe (Fig. 5b). Table 1 shows the ear swelling responses of these animals. It seemed likely that the ear swelling responses correlated with the number of eosinophils accumulating in the dermis.
Fig. 4.
Effect of subcutaneous rIL-5 administration on eosinophil recruitment into challenged areas of skin. (a) Guinea-pigs were challenged with several doses of DNCB on the dorsal skin. Thereafter, 10 pmol/kg rIL-5 was injected s.c. into the dorsal surface of the left ear lobe 0 or 12 h after challenge. At 24 h after challenge, lesional skin was excised and the number of eosinophils was enumerated. Recombinant IL-5 administered 12 h after challenge induced a small but significant increase in the number of accumulating eosinophils in the dorsal skin challenged with 0·15% DNCB. (b) Guinea-pigs challenged on the right ear lobe with 0·15% DNCB were administered s.c. with several doses of rIL-5 into the left ear lobe 0 or 12 h after challenge. At 24 h after challenge, the right ear lobe was removed and the number of eosinophils was enumerated. Whereas rIL-5 (10 pmol/kg) injected 12 h after challenge remarkably enhanced eosinophil infiltration into the right ear lobe, tissue eosinophila was suppressed when rIL-5 was administered at the time of challenge (0 h). Each group consisted of at least four animals.
Fig. 5.
Macroscopic features of skin reaction. (a) Dorsal skin. Sensitized guinea-pigs were administered s.c. with 10 pmol/kg rIL-5 into the ear lobe 12 h after challenge on the dorsal skin (right). Despite increased eosinophil infiltration into the dermis, macroscopic features were not altered compared with positive control animals (left). (b) Ear lobe. Sensitized guinea-pigs received 10 pmol/kg rIL-5 at the time of challenge (right). Inhibition of eosinophil recruitment reduced erythema and oedema, when compared with positive control animals (left).
Table 1.
Effect of subcutaneous injection of IL-5 on contact sensitivity reactions
| Experiment | IL-5 administration* | Group | Dose (pmol/kg) | Ear swelling ±SD† (× 10 cm) |
|---|---|---|---|---|
| 1 | 0 h | A (positive control) | – | 34·2 ± 4·63 |
| B | 10 | 21·6 ± 3·79‡ | ||
| C | 1 | 37·0 ± 4·48 | ||
| D | 0·1 | 30·5 ± 3·84 | ||
| E (negative control) | – | 3·5 ± 1·12 | ||
| 2 | 12 h | A (positive control) | – | 27·6 ± 3·37 |
| B | 10 | 39·0 ± 3·32§ | ||
| C | 1 | 29·2 ± 3·79 | ||
| D | 0·1 | 26·4 ± 2·42 | ||
| E (negative control) | – | 3·0 ± 2·12 |
Guinea-pigs were challenged on the right ear lobe with 0·15/ DNCB. 0 or 12 h after challenge, IL-5 was injected subcutaneously into the left ear lobe.
Ear swelling responses at 24 h.
P < 0·005
P < 0·025, compared with group A.
DISCUSSION
Basophils as well as eosinophils infiltrate skin lesions of human contact dermatitis and atopic dermatitis [19,27]. Dvorak [22] called basophil-rich skin reactions with a delayed time course, cutaneous basophil hypersensitivity. Contact sensitivity in guinea-pigs is also considered to represent a form of cutaneous basophil hypersensitivity. The present study found extensive eosinophil-predominant infiltration into the dermis when ear lobes were challenged with DNCB. This was in striking contrast to the considerable basophil infiltration in the dorsal skin. The mechanisms underlying the relatively selective eosinophil or basophil recruitment at distinct sites within the same animal remains unresolved. The balance of chemokines produced between dorsal skin and ear lobe might differ, although eosinophils and basophils share common chemoattractants, such as RANTES, eotaxin, and MCP-4 [28–31]. In human contact dermatitis, skin lesions of the face and ear tend to manifest as marked oedema, swelling and erythema, whereas papules and vesicles predominate in the trunk and extremities. Inflammatory reactions may depend on the anatomical site or tissue structure, although no studies have examined whether or not the composition of inflammatory cells differs. Regardless, this model allowed the roles of eosinophils in acute eczematous reaction to be clarified. Recombinant IL-5 (10 pmol/kg) administered into the left ear lobe at 12 h after challenge induced an increase in infiltrative eosinophil numbers (Fig. 4) and enhanced swelling (Table 1) of the right ear lobe. When rIL-5 was injected at the time of challenge (0 h), the number of tissue eosinophils dramatically decreased. This phenomenon was accompanied by inhibited ear swelling (Table 1) and macroscopically decreased oedema and erythema (Fig. 5b). Although we did not assess the degree of extracellular release of eosinophil granule proteins and other mediators, these data suggest that extensive eosinophil infiltration augmented skin inflammation in acute allergic contact dermatitis.
IL-5 is regarded as a key cytokine in eosinophilic inflammation. Despite the various activities of IL-5 on eosinophils [32–34], the chemoattractant activity of IL-5 remains controversial. Either intranasal administration of a large amount of IL-5 or adenovirus-mediated over expression of IL-5 in the lung induces local eosinophil accumulation [6,7]. The transfer of an ovalbumin-specific, IL-5-producing T cell clone induces eosinophil infiltration into the lung [35]. We have shown that locally secreted IL-5 is not crucial for inducing eosinophil recruitment into the skin of the murine model of contact sensitivity if peripheral blood eosinophilia is achieved [17]. Local injections of rIL-5 do not induce tissue eosinophilia in mice. Collins et al. [20] found that local eosinophil accumulation was not significantly induced in guinea-pigs given low intradermal doses of rIL-5 (0·32–2·90 pmol/site). They proposed that the action of IL-5 is hormonal and remotely acting, rather than as a chemoattractant. The present study further confirms and extends these observations. A subcutaneous injection of 10 pmol/kg rIL-5 (3·5–4·0 pmol/site) at 12 h after challenge did not induce tissue eosinophilia at the injected site, but induced peripheral blood eosinophilia and enhanced eosinophil accumulation at a remote site, namely the challenged right ear lobe. These data have potentially important implications for patients with various degrees of dermal eosinophilia, such as atopic dermatitis and erythroderma in Sezary syndrome, which is accompanied by the significant infiltration of cells expressing IL-5 mRNA [36,37]. Pivotal roles of locally secreted IL-5 may be the stimulation of eosinophil mobilization via leakage from inflamed skin tissue and the facilitation of eosinophil recruitment into lesional skin, rather than as a chemoattractant.
An injection of rIL-5 at the time of challenge (0 h) suppressed eosinophil recruitment into the challenged ear lobe (Fig. 4). Transient peripheral blood eosinophilia induced by rIL-5 peaked at 2 h, lasted until 12 h after the injection and was accompanied by a decrease in the number of bone marrow eosinophils. Whereas the eosinophil count in the blood returned to normal within 12 h, that in the bone marrow was not restored (data not shown). On the other hand, eosinophils infiltrated the dermis between 12 and 24 h after challenge (Fig. 2). Thus, we considered that the inhibitory effect of rIL-5 administered at the time of challenge on eosinophil accumulation was caused by the consumption of a mobilizable pool of bone marrow eosinophils. Alternative explanations are that immunoregulation such as either the release of soluble IL-5 receptor [38] or a decrease in the expression of IL-5 receptor α chain on eosinophils pre-exposed to IL-5 [39], reduced the numbers of infiltrative eosinophils. The mechanism(s) of synchronizing a stimulus for releasing an eosinophil pool, such as IL-5 and eotaxin [40], with the expression of adequate cell adhesion molecules on endothelial cells and the production of eosinophil specific chemokines including eotaxin and ecalectin [41], in inflamed tissue should be required to efficiently recruit eosinophils into the skin.
The present study shows that challenge to the ear lobes of a guinea-pig model of contact sensitivity stimulated remarkable eosinophil infiltration and degranulation in the skin. The number of eosinophils that accumulated in the dermis was controlled by remotely administered IL-5. The degree of eosinophil infiltration seemed to be correlated with contact sensitivity reactions.
Acknowledgments
We thank Ms Motoko Sekiya for excellent technical assistance.
REFERENCES
- 1.Herman JJ, Rosner KI, Davis AE, Zeiger RS, Arnaout MA, Colten HR. Complement-dependent histaminase release from human granulocytes. J Clin Invest. 1979;63:1195–202. doi: 10.1172/JCI109414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainton DF, Farquhar MG. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970;45:54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kater LA, Goetzl EJ, Austen KF. Isolation of human eosinophil phospholipase D. J Clin Invest. 1976;57:1173–80. doi: 10.1172/JCI108385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austen KF. Homeostasis of effector systems which can also be recruited for immunologic reactions. J Immunol. 1978;121:793–805. [PubMed] [Google Scholar]
- 5.Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987;1:643–7. doi: 10.1016/s0140-6736(87)90412-0. [DOI] [PubMed] [Google Scholar]
- 6.Van Oosterhout AJM, Fattah D, Van Ark I, Hofman G, Buckley TL, Nijkamp FP. Eosinophil infiltration precedes development of airway hyperreactivity and mucosal exudation after intranasal administration of interleukin-5 to mice. J Allergy Clin Immunol. 1995;96:104–12. doi: 10.1016/s0091-6749(95)70039-0. [DOI] [PubMed] [Google Scholar]
- 7.Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest. 1996;97:1102–10. doi: 10.1172/JCI118503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leifauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest. 1997;100:2254–62. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker RL, Gundel RH, Gleich GJ, Checkel JL, Loegering DA, Pease LR, Hamann KJ. Acidic polyamino acids inhibit human eosinophil granule major basic protein toxicity. J Clin Invest. 1991;88:798–805. doi: 10.1172/JCI115379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. New Engl J Med. 1985;313:282–5. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 12.Moqbel R, Ying S, Barkans J, Newman TM, Kimmit P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan CJ, Durham SR, Kay AB. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–47. [PubMed] [Google Scholar]
- 13.Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–8. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil major basic protein. J Exp Med. 1983;157:1981–91. doi: 10.1084/jem.157.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiferman KM, Davis MDP, George TJ, Checkel JL, Gleich GJ. Eosinophil granule proteins induce vasopermeability that is inhibitable by antihistamines in guinea pig skin. J Invest Dermatol. 1996;105:A792. [Google Scholar]
- 16.Beck LA, Dalke S, Leiferman KM, Bickel CA, Hamilton R, Rosen H, Bochner BS, Schleimer RP. Cutaneous injection of RANTES causes eosinophil recruitment: comparison of nonallergic and allergic human subjects. J Immunol. 1997;159:2962–72. [PubMed] [Google Scholar]
- 17.Satoh T, Chen QJ, Sasaki G, Yokozeki H, Katayama I, Nishioka K. Cyclophosphamide-induced blood and tissue eosinophilia in contact sensitivity: mechanism of hapten-induced eosinophil recruitment into the skin. Eur J Immunol. 1997;27:85–91. doi: 10.1002/eji.1830270113. [DOI] [PubMed] [Google Scholar]
- 18.Roupe G, Ridell B. The cellular infiltrate in contact sensitivity to picryl chloride in the mouse. Acta Dermatovener (Stockholm) 1979;59:191–5. [PubMed] [Google Scholar]
- 19.Dvorak HF, Mihm Mc., Jr Basophilic leukocytes in allergic contact dermatitis. J Exp Med. 1972;135:235–54. doi: 10.1084/jem.135.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz SI, Heather CJ, Parker D, Turk JL. Basophilic leukocytes in delayed hypersensitivity reactions. J Immunol. 1974;113:1073–8. [PubMed] [Google Scholar]
- 21.Collins PD, Marleau S, Griffiths-Johnson DA, Rose PJ, Willams TJ. Cooperation between interleukin-5 and chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–74. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvorak HF. Cutaneous basophil hypersensitivity. J Allergy Clin Immunol. 1976;58:229–40. doi: 10.1016/0091-6749(76)90159-7. [DOI] [PubMed] [Google Scholar]
- 23.Humbles AA, Conroy DM, Marleau S, Rankin SM, Palframan RT, Proudfoot AEI, Wells TNC, Li D, Jeffery PK, Griffiths-Johnson DA, Williams TJ, Jose PJ. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airway disease: analysis in a guinea pig model in vivo. J Exp Med. 1997;186:601–12. doi: 10.1084/jem.186.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K, Suda T. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival: IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–42. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sehmi R, Wardlaw AJ, Cromwell O, Kurihara K, Waltmann P, Kay AB. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992;79:2952–9. [PubMed] [Google Scholar]
- 26.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin 5 is a selective chemoattractant. Eur J Immunol. 1989;19:701–5. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell EB, Chapman MD, Pope FM, Crow J, Jouhal SS, Platts-Mills TAE. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet. 1982;1:127–30. doi: 10.1016/s0140-6736(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 28.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–95. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponath PD, Qin S, Ringler DJ, Clark- Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newmann W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin: expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, Murphy PM, Luster AD. Human monocyte chemoattractant protein (MCP-4) is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–26. [PubMed] [Google Scholar]
- 31.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA. High expression of the chemokine receptor CCR3 in human blood basophils: role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–43. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clutterbuck EJ, Sanderson CJ. Human eosinophil hematopoiesis studied in vitro by means of murine eosinophil differentiation factor (IL-5): production of functionally active eosinophils from normal human bone marrow. Blood. 1988;71:646–51. [PubMed] [Google Scholar]
- 33.Takafuji S, Bischoff SC, deWeck AL, Dahinden CA. IL-3 and IL-5 prime normal human eosinophils to produce leukotriene C4 in response to soluble agonists. J Immunol. 1991;147:3855–61. [PubMed] [Google Scholar]
- 34.Tai PC, Sun L, Spry CJF. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85:312–6. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminuma O, Mori A, Ogawa K, Nakata A, Kikkawa H, Naito K, Suko M, Okudaira H. Successful transfer of late phase eosinophil infiltration in the lung by infusion of helper T cell clones. Am J Respir Cell Mol Biol. 1997;16:448–54. doi: 10.1165/ajrcmb.16.4.9115756. [DOI] [PubMed] [Google Scholar]
- 36.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, Rook AH. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–73. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 38.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetnik G. A human high affinity interleukin-5 receptor (IL-5) is composed of an Il-5 specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–84. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 39.Wang P, Wu P, Cheewatrakoolpong B, Myers JG, Egan RW, Billah MM. Selective inhibition of IL-5 receptor α-chain gene transcription by Il-5, IL-3, and granulocyte-macrophage colony-stimulating factor in human blood eosinophils. J Immunol. 1998;160:4427–32. [PubMed] [Google Scholar]
- 40.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 41.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, Stevens RL, Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–84. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]