Abstract
We investigated, by Northern blotting, ELISA, and a chemotaxis assay, the expression of IL-8 mRNA, the production of IL-8 protein, and the biological activity of mononuclear cells (MNC), polymorphonuclear neutrophils (PMN) and plasma, respectively, from patients with Kawasaki disease (KD) who received intravenous immunoglobulin (IVIG). IL-8 mRNA expression by MNC and PMN, the level of IL-8 protein, and the neutrophil chemoattractant activity within plasma were all increased in the acute phase of KD, and were significantly elevated following IVIG therapy. The level of chemotactic activity of neutrophils, but not that of monocytes, in response to F-met-leu-phe was decreased in patients with KD after IVIG. The increased expression of IL-8 in PMN and MNC, the increased plasma level of IL-8 and the decreased level of neutrophil chemotactic activity of the patients who received IVIG therapy might inhibit the accumulation of neutrophils at the sites of inflammation, and may thus reduce the risk of aneurysm formation.
Keywords: Kawasaki disease, IL-8, gamma globulin therapy
INTRODUCTION
Kawasaki disease (KD) is an acute multisystem vasculitis that mainly occurs in children under the age of 5 years [1]. The treatment used to prevent the development of coronary artery aneurysms involves a combination of intravenous immunoglobulin (IVIG) therapy and orally administered aspirin [2]. In Japan, IVIG is now the first line of therapy for KD, but it is not known how this reduces the risk of coronary aneurysms [3].
IL-8 is a novel C-X-C chemokine that attracts and activates neutrophils [4]. It also exhibits a wide range of other proinflammatory activities, such as the degranulation of neutrophil-specific granules and azurophilic granules, and the enhancement of adhesion molecule expression on the cell surface of neutrophils [5, 6]. IL-8 can be induced by a variety of agents, including lipopolysaccharide and liposomal encapsulated muramyl tripeptide [7].
Elevated serum levels of IL-8 have been reported in patients with a variety of conditions, including inflammatory bowel disease [8], idiopathic pulmonary fibrosis [9], β-thalassaemia, and graft-versus-host disease [10]; however, there are few reports on IL-8 in KD (see for example [11]). In addition to the paucity of information about the kinetics of chemokine action in this disease, the effects of IVIG therapy on chemokine production and function in KD patients have not been elucidated. Because IL-8 is involved in inflammation [12], we investigated the expression of IL-8 mRNA and protein by polymorphonuclear neutrophils (PMN) and mononuclear cells (MNC), the chemotactic response of these cells and the chemoattractant activity of plasma from patients with KD, as well as the effects of IVIG on these parameters.
PATIENTS AND METHODS
Patients
Peripheral blood was obtained from 15 Japanese patients with KD who were admitted to Nippon Medical School Hospital. All cases satisfied the diagnostic criteria for KD established by the Japanese Kawasaki Disease Research Committee [13]. This study was approved by our Institutional review board and was performed with the informed consent of each subject. Ten age-matched Japanese children were selected as healthy controls. The mean age (21 ± 16 months versus 25 ± 21 months) and the male/female ratio (5/10 versus 4/6) were comparable between the patients and healthy controls. Blood was sampled during the acute phase (i.e. upon admission) and 3–6 days after IVIG therapy (200–500 mg/kg × 5 days). The duration of illness prior to blood collection was 1–5 days. None of the patients studied had coronary artery aneurysms 1 year after follow up.
Reagents and drugs
Hanks' balanced salt solution (HBSS) was purchased from Gibco (Grand Island, NY). Lymphocyte separation medium (LSM) was purchased from Organon Teknika (Durham, NC). Bovine serum albumin (BSA) and F-met-leu-phe (FMLP) were obtained from Sigma (St Louis, MO).
Molecular probes
The cDNA probes for IL-8 (300-bp, Eco RI-Eco RI fragment) were a generous gift from Dr K. Matsushima (Department of Molecular Preventive Medicine, School of Medicine, University of Tokyo, Tokyo, Japan) and the chicken β-actin cDNA, 1800-bp Pst I-Pst I fragment, was a generous gift from Dr E. S. Kleinerman (Department of Cell Biology, The University of Texas M.D. Anderson Cancer Center, Houston, TX). Both have been described previously [7].
Preparation of peripheral blood-derived leucocytes for RNA extraction
Peripheral blood PMN and MNC were isolated from heparinized whole blood. MNC were isolated by density gradient centrifugation over LSM for 10 min (1100 g) at 20°C and the interphase layer containing MNC was collected. After removing this layer, the erythrocytes containing PMN, present at the bottom of the tube, were incubated with lysis buffer containing 0·11 m sucrose, 3·3 mm Tris–HCl pH 7·6, 1·3 mm MgCl2, 0·33% Triton X-100, 0·01% sodium azide, for 2 min at 4°C. The PMN were then centrifuged at 500 g for 15 min and the pelleted PMN were collected and utilized for RNA preparation. The purity of the PMN and MNC was >95% as determined by Diff Quik staining (Baxter Healthcare Corp., Scientific Division, McGraw Park, IL).
Northern blot analysis
Total RNA was prepared by the acid guanidine isothiocyanate-phenol-chloroform extraction method and the RNA was size-fractionated, blotted and hybridized according to the previously described standard procedure [7]. The specific activity of the probe was found to be between 1 × 109 and 2 × 109 ct/min per mg DNA. The hybridized membranes were exposed to Kodak XAR-5 film (Eastman Kodak, Rochester, NY) at −70°C as described previously [7].
ELISA assay for IL-8
Plasma IL-8 concentrations were determined by ELISA kits (human IL-8 Quantikine™; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Assay of neutrophil chemotaxis
Neutrophil chemoattractant activity in plasma from the patients with KD was assayed using the blind well-chamber method described previously [12]. PMN were isolated from whole heparinized blood obtained from healthy volunteers. After incubation with 3% dextran for 30 min, PMN and MNC were isolated by density gradient centrifugation. After removal of the MNC, the erythrocytes and PMN at the bottom of the tube were incubated in lysis buffer containing 0·2% NaCl for 45 s and then an equal volume of 1·6% NaCl was added. A 200-μl aliquot of plasma was placed in each well of a 24-well plate (Iwaki, Chiba, Japan). The upper wells, created using Chemotaxicell (Kurabo, Tokyo, Japan) that separated the upper and lower wells by a 5-μm polycarbonate filter, were filled with 0·3 ml of Gey's balanced salt solution (Gibco) containing 2% BSA and 8 × 104 neutrophils. After incubation for 90 min at 37°C in humidified air with 5% CO2, the filters were removed and stained with Diff Quik stain (Baxter Health Corp.), then 10 oil immersion fields (high power fields (HPF)) were examined and the migrated cells in the fields counted.
Chemotaxis by patients' neutrophils or monocytes was investigated as follows: MNC and PMN from the patients with KD were obtained as described above for the healthy volunteers. FMLP (250 nm) in HBSS was used as the chemoattractant agent in this assay. Two hundred microlitres of this solution, or the control solution (HBSS), were placed in each well of a 24-well plate, then 8 × 104 neutrophils or monocytes from a patient were added to the upper wells. After incubation for 90 min at 37°C in humidified air with 5% CO2, the filters were stained and migrating neutrophils or monocytes were counted as described above.
The inhibition assays for IL-8-induced chemotaxis were performed by preincubation of the plasma with rabbit anti-human IL-8 (Monosan, Uden, The Netherlands) antibodies at 5 μg/ml for 30 min at 37°C. Rabbit IgG was employed as the negative control.
Statistical analysis
Data were analysed by the Mann–Whitney test. P < 0·05 was accepted as statistically significant.
RESULTS
Expression of IL-8 mRNA by peripheral blood PMN and MNC after gamma globulin therapy
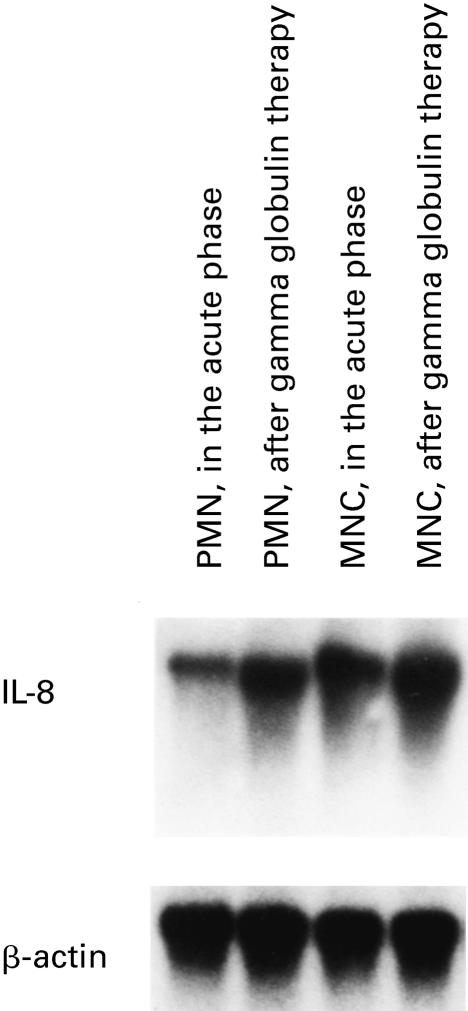
We first evaluated the expression of IL-8 mRNA by peripheral blood PMN and MNC in patients with KD. Unstimulated PMN and MNC from 10 healthy controls showed either no or very weak expression of IL-8 mRNA (data not shown), whereas PMN and MNC both showed high IL-8 mRNA expression in the acute phase of KD as determined by Northern blotting (Fig. 1). IL-8 mRNA expression was further enhanced in PMN and MNC after IVIG therapy (Fig. 1). There were no differences in the results relating to the variability in the sampling times in either the acute phase or after IVIG (data not shown).
Fig. 1.
Northern blot analysis of IL-8 from polymorphonuclear neutrophils (PMN) and mononuclear cells (MNC) in patients with Kawasaki disease. Increased expression of IL-8 mRNA was observed in neutrophils and monocytes in the acute phase of disease, and a further increase in IL-8 mRNA expression was observed after intravenous immunoglobulin therapy. Representative data from one of 15 patients are shown.
Plasma level of IL-8
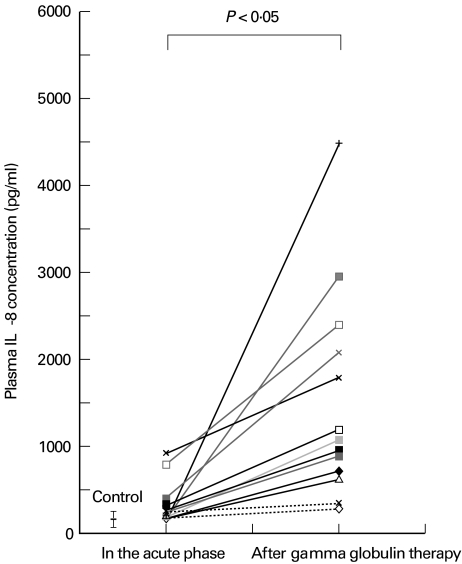
The plasma levels of IL-8 were elevated in the acute phase of KD (210 ± 200 pg/ml) compared with healthy controls (65 ± 22 pg/ml, n = 10), but the difference was not statistically significant (P = 0·15). Plasma IL-8 was significantly increased after IVIG compared with the controls or untreated patients with KD (589 ± 490 pg/ml, P < 0·05 versus healthy controls; P < 0·05 versus the acute phase) (Fig. 2). There were no differences in the results relating to the time of sampling in either the acute phase or after IVIG (data not shown). IL-8 was undetectable by ELISA in the IVIG used to treat our patients (data not shown).
Fig. 2.
Plasma concentration of IL-8 in Kawasaki disease. The average plasma level of IL-8 increased during the acute phase of disease (210 ± 200 pg/ml, n = 15) compared with the level in healthy controls (65 ± 22 pg/ml, n = 10), and was significantly (P < 0·05) increased after intravenous immunoglobulin therapy (589 ± 490 pg/ml, n = 15).
Neutrophil chemoattractant activity in plasma is related to the plasma IL-8 level, and is partially blocked by anti-IL-8 antibody
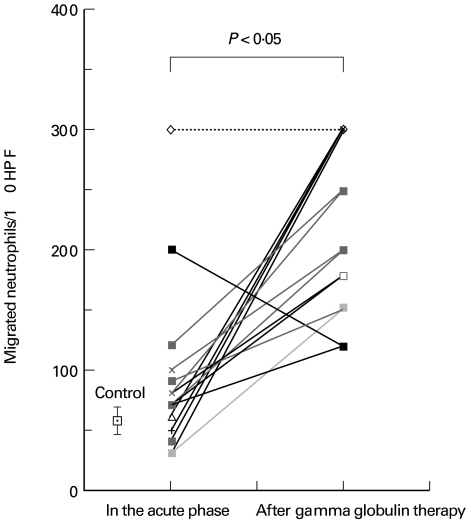
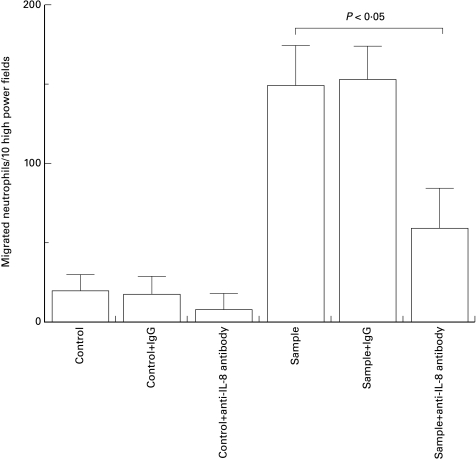
We next determined the chemoattractant activity for neutrophils in patient plasma. As shown in Fig. 3, this activity was elevated in the acute phase of KD (93 ± 71 neutrophils per 10 HPF) compared with the healthy controls (66 ± 12 neutrophils per 10 HPF, n = 10), but the elevation was not statistically significant (P = 0·2). Chemoattractant activity was significantly increased after IVIG therapy (220 ± 69 neutrophils per 10 HPF, P < 0·01 versus control, P < 0·01 versus the acute phase). There were no differences in the results relating to the time of sampling in either the acute phase or after gamma globulin therapy (data not shown). The chemoattractant activity was partially blocked by preincubation of the plasma with an anti-IL-8 antibody (Fig. 4).
Fig. 3.
Neutrophil chemotactic activity in the plasma of patients with Kawasaki disease. The average neutrophil chemotactic activity in the acute phase (93 ± 71 neutrophils per 10 high power fields (HPF), n = 15) was increased compared with healthy controls (66 ± 12 neutrophils per 10 HPF, n = 10). This activity was further increased (220 ± 69 neutrophils per 10 HPF, n = 15) after intravenous immunoglobulin therapy (P < 0·05).
Fig. 4.
Increased neutrophil chemotactic activity in the plasma of patients with Kawasaki disease is partially blocked by preincubation with IL-8 antibody. Hanks' balanced salt solution was used as control. Data were measured as the average ± s.d. in triplicate. Representative data from one of 15 experiments are shown.
Decrease of neutrophil chemotaxis in patients with KD after IVIG therapy
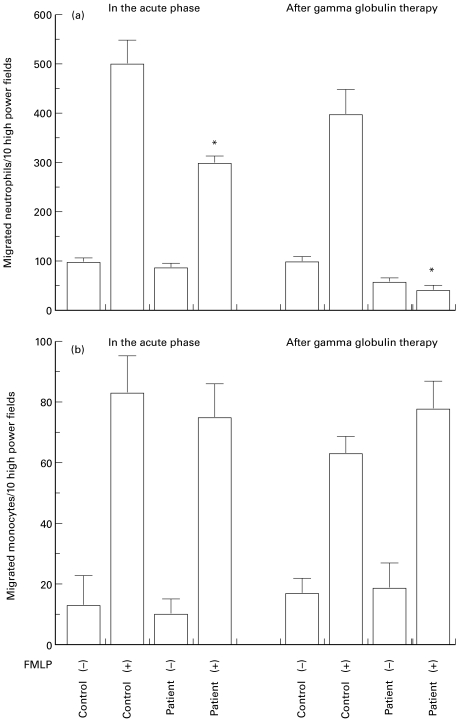
We then determined the level of neutrophil and monocyte chemotaxis using cells from patients with KD, and observed a significant decrease in neutrophil chemotaxis in response to FMLP after IVIG therapy (P < 0·05, Fig. 5a). The level of monocyte chemotaxis was unchanged in patients with KD after IVIG therapy compared with that in either healthy controls or the acute phase (Fig. 5b). There were no differences in the results relating to the time of sampling in either the acute phase or after IVIG therapy (data not shown).
Fig. 5.
(a) Decrease in the chemotactic activity of neutrophils obtained from patients with Kawasaki disease (KD) following intravenous immunoglobulin (IVIG) therapy. *A significant decrease in chemotactic activity was noted in the neutrophils from patients with KD following IVIG therapy. Neutrophils from healthy volunteers were used as controls. Hanks' balanced salt solution (HBSS) was used as the FMLP (–) solution. FMLP (250 nm) in HBSS was used as the chemotactic solution, FMLP (+). Data are the average ± s.d. of triplicates. Representative data from one of 15 experiments are shown. (b). Chemotactic activity of monocytes from patients with KD. Monocytes from healthy volunteers were used as controls. HBSS was used as the FMLP (–) solution. FMLP (250 nm) in HBSS was used as the chemotactic solution, FMLP (+). Data are the average ± s.d. of triplicates. Representative data from one of 15 experiments are shown.
DISCUSSION
IL-8 mRNA was over-expressed in MNC and PMN in the acute phase of KD, compared with healthy controls. Following the administration of IVIG, the expression of IL-8 mRNA, production of IL-8 protein and plasma chemoattractant activity for neutrophils were further enhanced. Such enhancement has also been found following the administration of IVIG in patients with primary hypogammaglobulinaemia [14], although our results showed a longer duration of the effect of infused IVIG on IL-8 than the study by Aukrust et al. [14] (20 h versus 14 days, respectively). IVIG has also been shown to enhance IL-8 mRNA expression and protein production in cultured normal human monocytes [15]. In the patients with KD who did not receive IVIG therapy, the serum IL-8 level decreased during the second week of the disease [11]. Our data suggest that IVIG therapy enhanced the production of IL-8 by neutrophils, as well as by monocytes, in patients with KD.
In addition, we found a decrease in the chemotactic response of patients' neutrophils to FMLP after IVIG therapy. Neutrophils contain toxic granules and vacuoles during the acute phase of KD [16], and enhanced neutrophil activity is involved in the pathogenesis of coronary thrombosis and aneurysm formation by causing injury to the endothelial vascular smooth muscle cells [17]. Pathologically, 12–20 days after the onset of symptoms, KD is characterized by panvasculitis of the three major coronary arteries and by aneurysms with thrombus in the stem [18]. The samples obtained from the patients after IVIG therapy were collected approximately 14 days after the onset of symptoms and would, without therapy, coincide with the phase of panvasculitis.
IL-8 has been shown to suppress the accumulation of neutrophils at an inflammatory locus in vitro by inhibiting their attachment to the endothelium [19,20]. Increased plasma levels of IL-8 also suppress the accumulation of neutrophils at extravascular sites in patients with endotoxaemia [21], while the i.v. administration of IL-8 in rabbits prevents the local accumulation of neutrophils [22,23]. Infused IVIG is thought to bind with the Fcγ receptor on phagocytes, resulting in the down-regulation of the immune system [24]. We propose that IL-8 at concentrations present in the acute phase of disease promotes neutrophil adhesion and transmigration [22], whereas a further increase in intravascular IL-8 levels caused by immunoglobulin infusion could impair leucocyte adhesion and protect organs from neutrophil-mediated injury.
In conclusion, our results indicate that alteration of the IL-8 level by high-dose IVIG therapy may ameliorate the symptoms of Kawasaki disease, and decrease the risk of aneurysm formation.
Acknowledgments
The authors thank Drs Y. Katusbe, R. Fukazawa, T. Ohkubo, N. Takechi, Y. Kuramochi, M. Hashizume, T. Hatori, T. Matsudaira, T. Ohkawa, T. Yanagihara, J. Hayakawa, M. Hayashida, and H. Narazaki for the patients' blood sampling and for providing the relevant clinical information. This work was partially supported by The Uehara Memorial Research Foundation.
REFERENCES
- 1.Yanagawa H, Nakamura Y, Yashiro M, Ojima T, Koyanagi H, Kawasaki T. Update of the epidemiology of Kawasaki disease in Japan. From the results of 1993–94 nation wide survey. J Epidemiol. 1996;6:148–57. doi: 10.2188/jea.6.148. [DOI] [PubMed] [Google Scholar]
- 2.Furusho K, Kamiya T, Nakano H, et al. High dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;ii:1055–8. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 3.Leung DYM. Kawasaki syndrome: immunomodulatory benefit and potential toxin neutralization by intravenous immune globulin. Clin Exp Immunol. 1996;104(Suppl.1):49–54. [PubMed] [Google Scholar]
- 4.Yoshimura T, Robinson EA, Appella E, Matsushima K, Showalter SD, Skeel A, Leonard EJ. Three forms of monocyte-derived neutrophil chemotactic factor (MDNCF) distinguished by different lengths of the amino-terminal sequence. Mol Immunol. 1989;26:87–93. doi: 10.1016/0161-5890(89)90024-2. [DOI] [PubMed] [Google Scholar]
- 5.Baum RK, Franchini M, Erard F, Rihs S, De Vries IJM, Blaser K, Hansel TT, Walker C. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. Eur J Immunol. 1993;23:956–60. doi: 10.1002/eji.1830230429. [DOI] [PubMed] [Google Scholar]
- 6.Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992;13:291–4. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 7.Asano T, McWatters A, An T, Matsushima K, Kleinerman ES. Liposomal muramyl tripeptide upregulates IL-1α, IL-1β, TNFα, IL-6, and IL-8 gene expression in human monocytes. J Pharmacol Exp Ther. 1994;268:1032–9. [PubMed] [Google Scholar]
- 8.Shuerer-Maly C-C, Eckmann L, Kagnoff MF, Falco MT, Maly FE. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Strieter RM, Koch AE, Antony VB, Fick Rb, Jr, Standiford TJ, Kunkel SL. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemo-attractant protein-1. J Lab Clin Med. 1994;123:183–97. [PubMed] [Google Scholar]
- 10.Uguccioni M, Meliconi R, Nesci S, Lucarelli G, Ceska M, Gasbarrini G, Facchini A. Elevated interleukin-8 serum concentrations in beta-thalassemia and graft-versus-host disease. Blood. 1993;81:2252–6. [PubMed] [Google Scholar]
- 11.Lin C-Y, Lin C-C, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor among patients with Kawasaki disease. J Pediatr. 1992;121:924–6. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 12.Asano T, An T, Jia S-F, Kleinerman ES. Altered monocyte chemotactic and activating factor gene expression in human glioblastoma cell lines increased their susceptibility to cytotoxicity. J Leuk Biol. 1996;59:916–24. doi: 10.1002/jlb.59.6.916. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Kawasaki Disease Research Committee. Diagnostic guidelines of Kawasaki disease. 4. Tokyo: Japan Kawasaki Disease Research Committee; 1984. [Google Scholar]
- 14.Aukrust P, Frøland SS, Liabakk N-B, Müller F, Nordøy I, Haug C, Espevik T. Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood. 1994;84:2136–43. [PubMed] [Google Scholar]
- 15.de Souza VR, Carreno M-P, Kaveri SV, Ledur A, Sadeghi H, Cavaillon J-M, Kazatchkine MD, Haeffner-Cavaillon N. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin) Eur J Immunol. 1995;25:1267–73. doi: 10.1002/eji.1830250521. [DOI] [PubMed] [Google Scholar]
- 16.Rowe PC, Quinian A, Luke BKH. Value of degenerative change in neutrophils as a diagnostic test for Kawasaki syndrome. J Pediatr. 1991;119:370–4. doi: 10.1016/s0022-3476(05)82047-5. [DOI] [PubMed] [Google Scholar]
- 17.Niwa Y, Sohmia K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984;104:56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Peditrarics. 1978;61:100–7. [PubMed] [Google Scholar]
- 19.Gimbrone Ma, Jr, Obin MS, Brock AF, et al. Endothelial interleukin-8: a novel inhibitor of leukocyte–endothelial interactions. Science. 1989;246:1601–3. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- 20.Luscinskas FW, Kiely J-M, Ding H, Obin MS, Hebert CA, Baker JB, Gimbrone Ma., Jr In vitro inhibitory effect of IL-8 and other chemo-attractants on neutrophil–endothelial adhesive interactions. J Immunol. 1992;149:2163–71. [PubMed] [Google Scholar]
- 21.Solomkin JS, Bass RC, Bjornson HS, Tindal CJ, Babcock GF. Alterations of neutrophil responses to tumor necrosis factor alpha and interleukin-8 following human endotoxemia. Infect Immun. 1994;62:943–7. doi: 10.1128/iai.62.3.943-947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hechtman DH, Cybulsky MI, Fuchs HJ, Baker JB, Gimbrone Ma., Jr Intravascular IL-8: inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991;147:883–92. [PubMed] [Google Scholar]
- 23.Ley K, Baker JB, Cybulsky MI, Gimbrone Ma, Jr, Luscinskas FW. Intravenous interleukin-8 inhibits granulocyte emigration from rabbit mesenteric venules without altering l-selectin expression or leukocyte rolling. J Immunol. 1993;151:6347–57. [PubMed] [Google Scholar]
- 24.Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Kawase H, Sekine I. Regulation of the expression of Fcγ receptor on circulating neutrophils and monocytes in Kawasaki disease. Clin Exp Immunol. 1999;117:418–22. doi: 10.1046/j.1365-2249.1999.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]