Abstract
Profiles of ICAM-1 expression on cultured murine peritoneal macrophages infected with Mycobacterium avium complex (MAC) were examined, with special reference to modulating roles of TNF-α, TGF-β, and IL-10. When macrophages were infected with MAC, ICAM-1 expression, measured by microscopic counting of ICAM-1+ macrophages stained with anti-ICAM-1 antibody, ELISA, and flow cytometric analysis, was rapidly increased, peaking at day 3 (early-phase up-regulation) due to endogenous TNF-α, and thereafter gradually declined to the normal level within 1 week or more (late-phase down-regulation). The late-phase ICAM-1 down-regulation was also seen in macrophages phagocytosing heat-killed MAC and those stimulated with lipopolysaccharide but not in macrophages phagocytosing latex beads. ICAM-1 mRNA expression was augmented markedly at day 1 after MAC infection and thereafter decreased. While TNF-α and IL-10 production by MAC-infected macrophages was observed during the first 3 days, TGF-β production was initiated from day 3 and continued until day 14. Exogenously added TGF-β strongly inhibited the early-phase increase in ICAM-1 expression by infected macrophages, and the blockade of endogenous TGF-β with anti-TGF-β antibody markedly inhibited late-phase ICAM-1 down-regulation. Moderate blocking effect was also observed for anti-IL-10 antibody. On the other hand, late-phase ICAM-1 down-regulation was not prevented by the addition of exogenous TNF-α. Therefore, TGF-β and IL-10, especially the former, appear to play active roles in the late-phase down-regulation of ICAM-1 in MAC-infected macrophages during long-term cultivation.
Keywords: intercellular adhesion molecule-1, macrophage, tumour necrosis factor-alpha, transforming growth factor-beta, IL-10, Mycobacterium avium complex
INTRODUCTION
Disseminated and fatal Mycobacterium avium complex (MAC) infections develop frequently in immunocompromised hosts such as in AIDS patients [1]. MAC organisms persist at sites of infection for long periods without producing the severe foci in target organs which are observed in the case of tuberculosis [2]. We previously found that the persistence of MAC at sites of infection is due in part to high resistance of MAC organisms to microbicidal mechanisms of host macrophages [3–5]. Immunosuppressive cytokines, TGF-β and IL-10, which are endogenously produced by macrophages infected with MAC, play roles in persistence of the organisms in host macrophages [6–9]. These cytokines reduce T cell functions [10,11] and down-regulate macrophage anti-mycobacterial activity [6–9]. Thus, MAC infection frequently causes impairment of host cellular immunity including DTH reaction and antigen response of T cells in hosts [12], due in part to immunosuppressive macrophages which produce these cytokines [13].
Adhesion molecules expressed on immunocompetent cells are involved in cellular interactions, playing roles in the development of immunological responses [14]. The interaction of leucocyte function-associated antigen-1 (LFA-1) with ICAM-1 is required for conjugate formation of T cells with antigen-presenting cells (APC), leading to the activation of resting T cells [14–16]. ICAM-1 plays an important role in the antigen response of T cells to purified protein derivative of M. tuberculosis (MTB) [17,18]. It was reported that ICAM-1 expression by the THP-1 human macrophage-like cell line was strongly increased due to MTB infection during 3-day cultivation and that this increase was mediated by TNF-α [18]. However, profiles of ICAM-1 expression during macrophage cultivation longer than 3 days have not yet been examined. In this study we therefore studied the profiles of ICAM-1 expression during long-term cultivation of macrophages after mycobacterial infection. Moreover, we also determined the roles of TNF-α, TGF-β, and IL-10 in the modulation of macrophage ICAM-1 expression.
MATERIALS AND METHODS
Organisms
MAC N-260 SmT variant was isolated from a clinical specimen of the patient with MAC infection and identified as M. intracellulare by a DNA probe test. It belonged to serovar 16 in Schaefer's seroagglutination test.
Special agents
Recombinant mouse TNF-α, recombinant mouse IL-10, ultrapure natural human TGF-β1, mouse anti-human TGF-β MoAb (also specific to mouse TGF-β), and rat anti-mouse IL-10 MoAb were purchased from Genzyme (Cambridge, MA). These agents were essentially free from lipopolysaccharide (LPS) contamination by Limulus testing. Rat anti-mouse ICAM-1 MoAb purified from ascites by affinity column chromatography was obtained from Seikagaku Co. (Tokyo, Japan). FITC-conjugated hamster anti-mouse ICAM-1 MoAb purified from tissue culture supernatant by affinity column chromatography was purchased from PharMingen (San Diego, CA). These MoAbs recognize the mouse ICAM-1 molecule in a specific manner (the manuals of these MoAbs written by Seikagaku Co. and PharMingen Co.).
Peritoneal macrophages
Three types of peritoneal macrophage cultures were prepared using 7–10-week-old female BALB/c mice (Japan Clea Co., Osaka, Japan), as follows.
Method A
Ten millilitres each of peptone-starch-elicited peritoneal exudate cell (PEC) suspension in RPMI 1640 medium supplemented with 25 mm HEPES, 2 mm glutamine, and 10% (v/v) heat-inactivated fetal bovine serum (FBS; BioWhittaker Co., Walkersville, MD) at a cell density of 5 × 106/ml were poured onto a 90-mm cell culture plate which was overlaid with 14-mm plastic culture sheets (about 20 sheets/plate). After 2 h incubation at 37°C in a CO2 incubator (5% CO2−95% humidified air), the resultant plastic sheets were removed and rinsed with Hanks' balanced salt solution (HBSS) containing 2% FBS.
Method B
The PEC (3 × 107 cells) suspended in 10 ml of 10% FBS–RPMI 1640 medium were seeded into FBS-coated 90-mm cell culture plate and incubated at 37°C for 2 h. After washing with 2% FBS–HBSS, adherent cells were scraped off using a rubber policemen and collected by subsequent centrifugation.
Method C
The PEC (1 × 106 cells) suspended in 1·0 ml of 10% FBS–RPMI 1640 medium were seeded into 16-mm culture wells and incubated at 37°C for 2 h. After washing, culture medium was overlaid onto the resultant macrophage monolayer culture.
These three methods gave essentially pure macrophage cultures without granulocyte contamination, with potent pinocytic ability for neutral red and phagocytic activity against latex beads.
ICAM-1 expression
The following methods were used for measurement of macrophage ICAM-1 expression.
Microscopic assay
Macrophage monolayer cultures (method A) were immersed into 16-mm culture wells containing 1·0 ml of 10% FBS–RPMI 1640 medium. The macrophages infected with 1 × 106 colony-forming units (CFU)/ml of MAC were then cultured in the medium (1·0 ml) with or without the addition of test agents at 37°C for up to 7 days, unless otherwise specified. At intervals, the macrophage monolayer culture was removed, washed with PBS containing 0·2% bovine serum albumin (BSA), fixed with 1% paraformaldehyde, incubated in 1% BSA–PBS at room temperature for 30 min, and then reacted with rat anti-mouse ICAM-1 MoAb (Seikagaku) at a concentration of 1:200 for 1 h. After rinsing with 0·1% BSA–PBS, the macrophages were reacted with alkaline phosphatase (AP)-conjugated anti-rat immunoglobulin MoAb (Seikagaku) for 1 h, and then washed with 0·1% BSA–PBS. Colour development was achieved using nitroblue tetrazolium/5′-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) substrate and the ratio of blue-stained macrophages containing ≥20 formasan granules (ICAM-1+ cells) was enumerated by microscopic counting.
ELISA
Macrophages (1 × 105 cells; Method B) suspended in 0·2 ml of 10% FBS–RPMI 1640 medium were infected with 1 × 106 CFU/ml of MAC or allowed to phagocytose heat (121°C, 15 min)-killed MAC or latex beads (3 μm diameter) at 1 × 106/ml, and then cultivated in 96-well flat-bottomed microculture plates in the medium (0·2 ml) with or without the addition of test agents at 37°C for up to 7 days. In some experiments, macrophages were stimulated with LPS (Escherichia coli O111:B4; Sigma Chemical Co., St Louis, MO) at 1 ng/ml or 10 μg/ml. At intervals, macrophage monolayers were fixed with 1% paraformaldehyde and subjected to blocking with 2% BSA–PBS for 1 h. The resulting macrophages were reacted with rat anti-mouse ICAM-1 MoAb (Seikagaku) at a concentration of 1:200 for 1 h, washed with 0·2% BSA–PBS, and thereafter reacted with AP-conjugated anti-rat immunoglobulin MoAb. After washing with 0·2% BSA–PBS, colour development was achieved using p-nitrophenyl phosphate as substrate. The plates were read on an ELISA reader at 405 nm.
Flow cytometric analysis
Macrophages (1 × 105 cells; Method B) suspended in 1·0 ml of 10% FBS–RPMI 1640 medium were infected with 1 × 106 CFU/ml of MAC and then cultivated in polypropylene tubes (12 × 75 mm) in the medium (1·0 ml) at 37°C for up to 7 days. At intervals, a monodispersed cell suspension was obtained by treatment with PBS containing 0·02% EDTA. After blocking with 10% FBS–PBS, the resultant cells were reacted with FITC-conjugated hamster anti-mouse ICAM-1 MoAb (PharMingen) at a concentration of 1:625 for 1 h, washed twice with 0·2% BSA–PBS, and fixed with 1% paraformaldehyde in PBS pH 7·2 for 10 min. After washing with PBS, the resulting macrophage cells were subjected to flow cytometry using FACStar (Becton Dickinson, Mountain View, CA).
Cytokine production
The macrophage monolayer cultures in 16-mm culture wells (Method C) were infected with 1 × 107 CFU/ml of MAC and then cultivated in 1·0 ml of 10% FBS–RPMI 1640 medium at 37°C for up to 14 days. The 10 times higher multiplicity of infection than those for the ICAM-1 expression assay was employed in order to achieve significant levels of TNF-α and TGF-β production by MAC-infected macrophages. At intervals, culture fluid was withdrawn and concentrations of TNF-α, IL-10, and TGF-β were measured by ELISA as previously described [19], using rat anti-mouse TNF-α MoAb (PharMingen), mouse anti-human TGF-β MoAb (specific to mouse TGF-β) (Genzyme), and rat anti-mouse IL-10 MoAb (Genzyme) as capture antibodies.
ICAM-1 mRNA expression
ICAM-1 mRNA in MAC-infected macrophages was measured by the reverse transcription-polymerase chain reaction (RT-PCR) method as previously described [19]. Macrophages (Method B) were infected with 1 × 106 CFU/ml of MAC and cultured in 10 ml of 10% FBS–RPMI 1640 medium in 25-cm2 tissue culture flasks at a density of 1·6 × 105 cells/flask, at 37°C for up to 14 days. At intervals total RNA was isolated from macrophages, using the ISOGEN kit (Nippon Gene Co., Toyama, Japan) followed by reverse transcription reaction using random hexamer primers (Gibco BRL, Rockville, MD) and Superscript II reverse transcriptase (Gibco). The resulting cDNAs were amplified by PCR (25 cycles: denaturing at 94°C for 1 min; annealing at 58°C for 2 min; extension at 72°C for 2 min) in the standard reaction mixture using Taq DNA polymerase (Takara Biomedicals Co., Tokyo, Japan) and sense and antisense primers for ICAM-1 (CAGGAGAGCACAAACAGCAGTG/AGAGCGGCAGAGCAA AAGAAGC). PCR products were analysed by electrophoresis on ethidium bromide-stained 2% agarose gels.
RESULTS
ICAM-1 expression by MAC-infected macrophages during cultivation
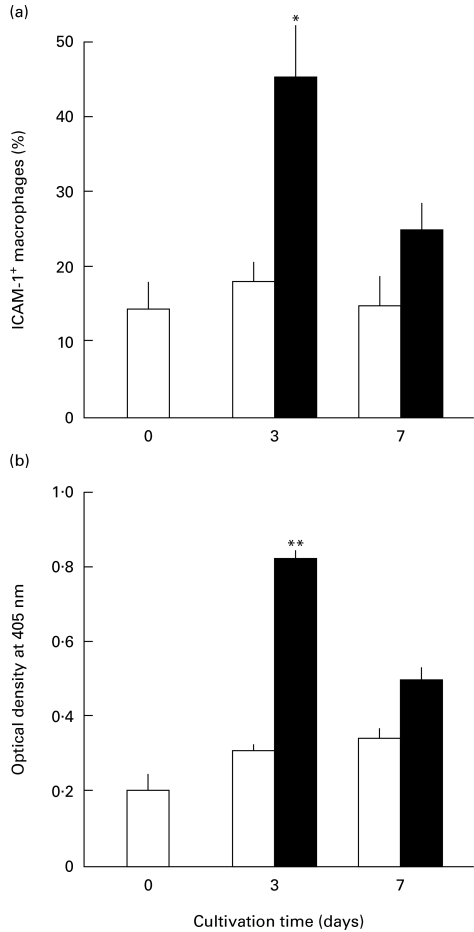
In the first series of experiments, we examined the mode of macrophage ICAM-1 expression in response to MAC infection. As shown in Fig. 1a, when ICAM-1 expression was measured by the microscopic method, a temporary increase in the ratio of ICAM-1+ macrophages was observed in the early periods of macrophage cultivation after MAC infection, reaching a peak at day 3 (the early phase ICAM-1 up-regulation) and thereafter declining to nearly the normal level by day 7 (the late phase ICAM-1 down-regulation). In contrast, uninfected control macrophages did not show such changes in ICAM-1 expression. The same profiles of ICAM-1 expression were observed when MAC-infected macrophages were cultured in serum-free GPI medium (data not shown). In a separate experiment performed under the same conditions, the number of bacterial CFU associated with macrophage monolayer on a plastic sheet was enumerated as follows (106 CFU/sheet; n = 3): time 0, 1·00; day 1, 4·27 ± 0·59; day 3, 8·32 ± 0·39; day 7, 17·0 ± 2·0 (the number of CFU recovered from macrophages by SDS (0·07%) treatment was counted on Middlebrook 7H11 agar plates). This increase in CFU values principally reflects the intramacrophagial growth of infected MAC, since similar profiles of intracellular growth of MAC were observed by microscopic counting of MAC residing in macrophages on plastic sheets after Ziehl–Neelsen staining (data not shown). Moreover, no significant growth of the MAC organisms was noted in the medium for macrophage cultivation used in this study.
Fig. 1.
ICAM-1 expression by Mycobacterium avium complex (MAC)-infected macrophages during cultivation. Macrophage ICAM-1 expression was measured by the microscopic method (a) or ELISA method (b). MAC-infected macrophages (▪) or uninfected macrophages (□) were cultivated for up to 7 days. Each bar indicates the mean ± s.e.m. (n = 3). Experiments were repeated three times or more and the representative results are indicated. Significantly larger than the value of uninfected macrophages: *P < 0·05; **P < 0·01.
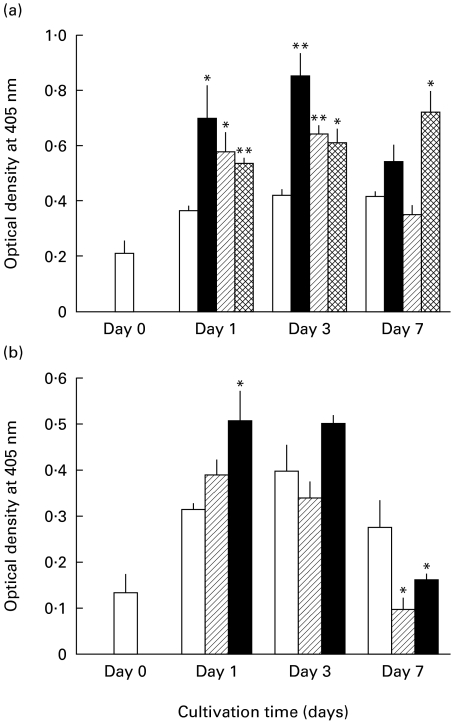
We next examined macrophage ICAM-1 expression by ELISA testing. As shown in Fig. 1b, a similar mode of the ICAM-1 expression was observed for MAC-infected macrophages during 7-day cultivation. Macrophage ICAM-1 expression markedly increased during the first 3 days, followed by a subsequent decline at day 7. In separate experiments, the ICAM-1 expression returned to the base line by day 9. In contrast, uninfected macrophages did not show such marked changes in their ICAM-1 expression. As shown in Fig. 2a, macrophages phagocytosing heat-killed MAC also displayed significantly increased expression of ICAM-1 at days 1 and 3, followed by subsequent reduction at day 7. This change was not so marked as observed in the case of macrophages infected with live MAC organisms. On the other hand, macrophages phagocytosing latex beads (3·0 μm diameter) displayed a progressive increase of ICAM-1 expression until day 7. As shown in Fig. 2b, macrophages stimulated with high-dose (10 μg/ml) LPS also displayed significantly increased ICAM-1 expression during day 1 to day 3, as previously reported by other investigators [20,21], followed by a reduction to normal levels at day 7. Macrophages stimulated with low-dose (1 ng/ml) LPS showed small increases in ICAM-1 expression in the early phase but displayed marked down-regulation of ICAM-1 in the late phase. Notably, in LPS-stimulated macrophages, the late-phase ICAM-1 down-regulation was much more marked than in the case of MAC-infected macrophages (Fig. 2a,b).
Fig. 2.
ICAM-1 expression by macrophages in response to stimulation with various irritants during long-term cultivation. Macrophage ICAM-1 expression was measured by ELISA method. (a) Macrophages infected with Mycobacterium avium complex (MAC) (▪), or those phagocytosing heat-killed MAC (hatched bars) or latex beads (cross-hatched bars) were measured for their ICAM-1 expression. (b) Macrophages stimulated with lipopolysaccharide (LPS) at 1 ng/ml (hatched bars) or 10 μg/ml (▪) were measured for their ICAM-1 expression. □, ICAM-1 expression on unstimulated control macrophages. Each bar indicates the mean ± s.e.m. (n = 3). Significantly different from the value of unstimulated control macrophages: *P < 0·05; **P < 0·01.
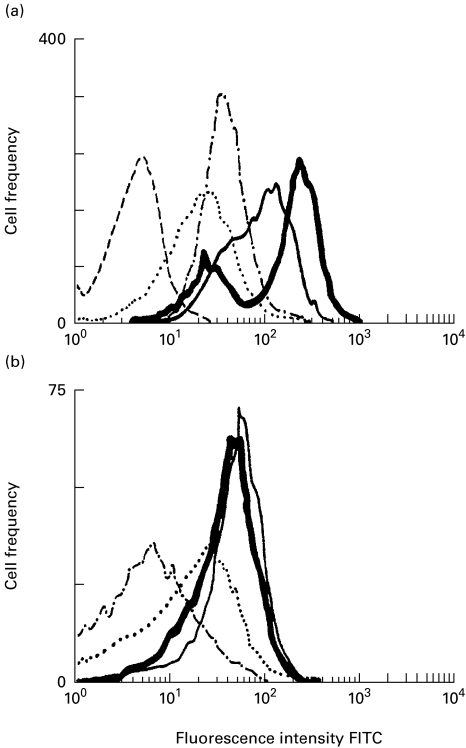
As indicated in Fig. 3, flow cytometric analysis also revealed a transient increase in the number of ICAM-1-positive macrophages in the early phase (day 3) of cultivation after MAC infection, followed by subsequent decline to the normal level at day 7 (Fig. 3a). In contrast, there was only a slight increase in the fluorescence intensity in the case of uninfected macrophages (Fig. 3b).
Fig. 3.
ICAM-1 expression by Mycobacterium avium complex (MAC)-infected macrophages (a) and uninfected macrophages (b) during cultivation for up to 7 days. Macrophage ICAM-1 expression was measured by flow cytometric analysis. Macrophages were harvested and subjected to flow cytometric analysis on day 0 (· · · · ·), day 1 (–––––), day 3 ( ), and day 7 (– · − · –). Broken line indicates the basal level of fluorescence on macrophages without anti-ICAM-1 antibody staining.
), and day 7 (– · − · –). Broken line indicates the basal level of fluorescence on macrophages without anti-ICAM-1 antibody staining.
These findings indicate that the early-phase ICAM-1 up-regulation on MAC-infected macrophages was the phenomenon specific to MAC infection. Moreover, in separate experiments using the microscopic method, late-phase ICAM-1 down-regulation was also noted, even when half medium changes were done every 3 days during cultivation (data not shown). This indicates that the late-phase ICAM-1 down-regulation was not due to nutrient defects during long-term cultivation of macrophages.
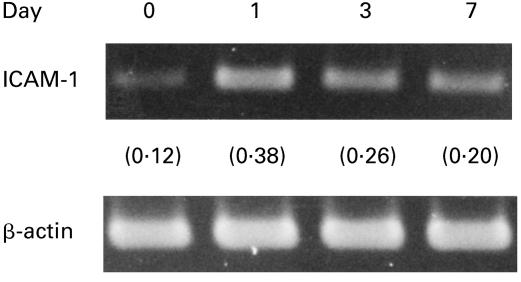
Figure 4 shows the expression of ICAM-1 mRNA in MAC-infected macrophages during 7-day cultivation after MAC infection. ICAM-1 mRNA expression rapidly increased during the first 24 h and thereafter decreased until day 7, but a significant level of ICAM-1 mRNA was detected even at this stage. Since the expression of ICAM-1 in protein level by MAC-infected macrophages was markedly decreased at day 7 (Figs 1b and 2a), it is thought that not only transcriptional but also post-transcriptional regulation of ICAM-1 expression is related to late-phase ICAM-1 down-regulation in MAC-infected macrophages.
Fig. 4.
ICAM-1 mRNA expression by Mycobacterium avium complex (MAC)-infected macrophages during cultivation. At intervals, total RNA was isolated from macrophages and subjected to reverse transcription-polymerase chain reaction analysis. In parentheses, the ratio of ‘ICAM-1 band/β-actin band’ is indicated.
The role of TNF-α in early-phase ICAM-1 up-regulation
In separate experiments using uninfected macrophages, exogenously added TNF-α increased the percentage of ICAM-1+ macrophages at day 3: +TNF-α, 62·0 ± 7%; −TNF-α, 21·0 ± 2%, indicating the roles of TNF-α in the early-phase ICAM-1 up-regulation in MAC-infected macrophages. Indeed, TNF-α production by MAC-infected macrophages increased in the early phase of macrophage cultivation, followed by a subsequent decline leading to diminishment at day 7: day 0, < 0·10 ng/ml; day 1, 0·27 ± 0·17 ng/ml; day 3, 0·66 ± 0·24 ng/ml; day 7, 0·14 ± 0·04 ng/ml (n = 3). This supports the concept that TNF-α plays central roles in early-phase ICAM-1 expression in MAC-infected macrophages. Therefore, it is possible that late-phase ICAM-1 down-regulation in MAC-infected macrophages is also due to a decrease in the amounts of macrophage-produced TNF-α in culture medium. However, exogenously added TNF-α (500 U/ml) failed to overcome the late-phase (day 7) down-regulation of ICAM-1 in MAC-infected macrophages (data not shown). This finding implies that alternative mediators are related to the late-phase ICAM-1 down-regulation.
Roles of TGF-β and IL-10 in late-phase ICAM-1 down-regulation
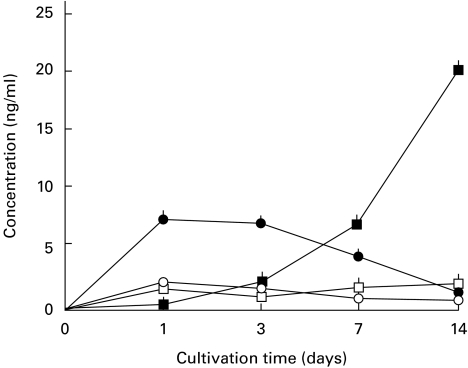
We determined the roles of immunosuppressive cytokines in the late-phase down-regulation of ICAM-1 expression by MAC-infected macrophages. Figure 5 shows the modes of IL-10 and TGF-β production by MAC-infected macrophages during cultivation. IL-10 production rapidly increased after MAC infection, peaked between day 1 and day 3, and thereafter decreased, returning to normal levels by day 7. On the other hand, TGF-β levels in macrophage culture fluids began to increase from day 3 and continuously increased until day 14.
Fig. 5.
Production of IL-10 and TGF-β by Mycobacterium avium complex (MAC)-infected macrophages during cultivation for up to 14 days. At intervals, culture fluid was withdrawn and concentrations of IL-10 and TGF-β were measured by ELISA. Open symbols indicate IL-10 (○) and TGF-β (□) production by uninfected macrophages. Closed symbols indicate IL-10 (•) and TGF-β (▪) production by MAC-infected macrophages. Each symbol indicates the mean ± s.e.m. (n = 3).
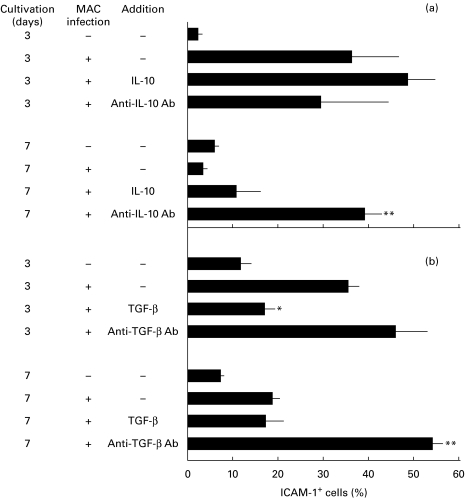
Next, we examined the effects of exogenous IL-10 and TGF-β, or anti-TGF-β and anti-IL-10 antibodies on ICAM-1 expression of MAC-infected macrophages measured by the microscopic method. In this experiment, ICAM-1 expression by MAC-infected macrophages temporarily increased at day 3, followed by subsequent decline, reaching normal levels by day 7. As shown in Fig. 6a, although exogenously added IL-10 did not affect ICAM-1 expression on days 3 and 7, anti-IL-10 antibody blocked the late-phase (day 7) down-regulation in MAC-infected macrophages. It thus appears that endogenous IL-10 produced in the early phase is partly responsible for the induction of late-phase ICAM-1 down-regulation. As shown in Fig. 6b, the exogenous addition of TGF-β significantly suppressed early-phase (day 3) ICAM-1 expression. In addition, anti-TGF-β antibody moderately potentiated ICAM-1 expression on day 3 and completely blocked subsequent diminishment of the ICAM-1 expression up to day 7. Therefore, it appears that TGF-β directly mediated late-phase ICAM-1 down-regulation in MAC-infected macrophages. In separate experiments, TGF-β-mediated ICAM-1 down-regulation was also demonstrated by ELISA testing (data not shown).
Fig. 6.
Effects of IL-10 (10 ng/ml), anti-IL-10 antibody (5 μg/ml), TGF-β (10 ng/ml), and anti-TGF-β antibody (30 μg/ml) on ICAM-1 expression by Mycobacterium avium complex (MAC)-infected macrophages. MAC-infected macrophages were cultivated in the presence or absence of indicated agents and macrophage ICAM-1 expression was measured by the microscopic method. Each bar indicates the mean ± s.e.m. (n = 3). Significantly different from the value of the control macrophages (untreated with cytokine or anti-cytokine antibody): *P < 0·01; **P < 0·005). Specificity of anti-IL-10 and anti-TGF-β antibodies was confirmed by separate experiments using control antibodies.
DISCUSSION
The present study indicates that MAC infection caused TNF-α-mediated up-regulation of ICAM-1 expression by murine peritoneal macrophages on day 3 of cultivation. A similar phenomenon has been reported for MTB-infected human macrophages [18]. These findings are consistent with the in vivo findings that granulomas in leprosy lesions and that keratinocytes in tuberculoid-type lesional skin of leprosy patients exhibited a pronounced expression of ICAM-1 [22,23]. Notably, it was previously reported that neither MAC infection nor TNF-α treatment of murine macrophages affected their LFA-1 expression [24]. It thus appears that the ICAM-1 and LFA-1 expression by MAC-infected macrophages are differentially modulated.
In the present study, we newly found that macrophage ICAM-1 expression peaked at day 3 after MAC infection and thereafter gradually decreased and returned to nearly normal levels within 1 week or more. Immunosuppressive cytokines, TGF-β and IL-10 [10,11], especially the former, appeared to play the roles in late-phase ICAM-1 down-regulation, as demonstrated by blocking experiments using anti-TGF-β antibody (Fig. 6). This concept is consistent with previous findings that TGF-β down-regulated ICAM-1 expression on interferon-gamma (IFN-γ)-stimulated rat microglial cells [25] and that IL-10 inhibited ICAM-1 expression on human monocytes [26].
Not only macrophages infected with live MAC but also macrophages phagocytosing heat-killed MAC displayed increased ICAM-1 expression during day 1 to day 3, followed by a marked decline in ICAM-1 expression at day 7 (Fig. 2a). On the other hand, macrophages phagocytosing latex beads displayed a progressive increase in ICAM-1 expression until day 7 (Fig. 2a). These findings suggest that the late-phase (day 3 to day 7) ICAM-1 down-regulation in MAC-infected macrophages was mediated by some cellular components specific to MAC organisms. It also appears that heat-stable components of MAC organisms are responsible for the late-phase ICAM-1 down-regulation, since not only macrophages infected with viable MAC but also macrophages phagocytosing heat-killed MAC displayed similar levels of reduction in ICAM-1 expression during day 3 to day 7. The most probable candidate for such bacterial components is lipoarabinomannan (mycobacterial LPS), since LPS-stimulated macrophages also displayed similar profiles of ICAM-1 expression during 7-day cultivation, as in the case of MAC-infected macrophages (Fig. 2b).
At the sites of mycobacterial infection, granuloma formation is important for CD4+ T cell- and macrophage-mediated blockade and elimination of parasites [27]. Since ICAM-1 plays important roles in the interaction between T cells and APC and transmission of antigen signals from APC to T cells [14–16], prolonged increase in ICAM-1 expression on APC, including macrophages, is required to maintain the structure of a granuloma or facilitate the activation of T cells leading to DTH reaction [28]. However, as indicated by the present study, the increase in ICAM-1 expression by MAC-infected macrophages did not last for long periods but was down-regulated by macrophage-derived endogenous TGF-β and IL-10 in the late phase of cultivation. This seems to cause the failure of continuous strong granuloma formation at sites of severe MAC infection in vivo. Indeed, Moncada et al. [23] reported lack of expression of ICAM-1 by keratinocytes in the epidermis of lepromatous leprosy patients, whose T cells are unable to react either in vivo or in vitro against M. leprae antigens, resulting in poor inflammatory response at the sites of infection. In this context, Pancholi et al. [29] reported the interesting finding that macrophages chronically infected with MTB failed to present mycobacterial antigens to CD4+ T cells. This inadequate antigen presentation appears to be due in part to TGF-β- or IL-10-mediated down-regulation of ICAM-1 expression in such macrophage populations.
We previously found that tissue levels of TNF-α, IL-10 and TGF-β in host spleens increased in a sequential fashion during MAC infection in mice [30]. That is, TNF-α levels rapidly increased after MAC infection, peaked at week 2, and thereafter gradually decreased, returning to normal levels by week 8. IL-10 levels gradually increased, reaching a peak at week 4 after infection, and thereafter rapidly decreased. On the other hand, TGF-β levels began to increase from week 2, peaked at week 4, and remained high during weeks 4–8. Thus, in the case of MAC infection in mice, TNF-α appears to cause up-regulation of ICAM-1 expression on host splenic macrophages in the early phase of infection. On the other hand, TGF-β and IL-10, particularly the former cytokine, appear to cause ICAM-1 down-regulation in host splenic macrophages in the advanced stage of MAC infection.
In any case, the present study revealed that TGF-β and IL-10, especially the former cytokine, play important roles in the late-phase down-regulation of ICAM-1 expression in MAC-infected macrophages. It is of interest to determine what kinds of macrophage factors other than TGF-β and IL-10 play additional roles in such a phenomenon. In our separate experiments, macrophage ICAM-1 expression was moderately down-regulated by prostaglandin E2 (unpublished observation). It is thus likely that this eicosanoid is also responsible for the late-phase down-regulation of ICAM-1 expression by MAC-infected macrophages. Further studies are currently underway to identify such macrophage factors and to elucidate the molecular basis of TGF-β-mediated ICAM-1 down-regulation.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (grant no. 07670310, no. 07307004, and no. 10670255).
REFERENCES
- 1.Benson CA, Ellner JJ. Mycobacterium avium complex infection in AIDS. Adv Theory Practice Clin Infect Dis. 1993;17:7–20. doi: 10.1093/clinids/17.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi N, David HL. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988;70:1101–20. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Tomioka H. The role of macrophages in host defence mechanisms against Mycobacterium avium complex infection induced in mice. Res Microbiol. 1990;141:206–12. doi: 10.1016/0923-2508(90)90032-l. [DOI] [PubMed] [Google Scholar]
- 4.Tomioka H, Sato K, Sano C, et al. Effector molecules of the host defence mechanism against Mycobacterium avium complex: the evidence showing that reactive oxygen intermediates, reactive nitrogen intermediates, and free fatty acids each alone are not decisive in expression of macrophage antimicrobial activity against the parasites. Clin Exp Immunol. 1997;109:248–54. doi: 10.1046/j.1365-2249.1997.4511349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaki T, Sato K, Tomioka H, et al. Effector molecules in expression of the antimicrobial activity of macrophages against Mycobacterium avium complex: the roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids. J Leuk Biol. 1997;62:795–804. doi: 10.1002/jlb.62.6.795. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez LE. Production of transforming growth factor-β by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-γ. J Immunol. 1993;150:1838–45. [PubMed] [Google Scholar]
- 7.Bermudez LE, Champsi J. Infection with Mycobacterium avium induced production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seyler I, Appel M, Devissagguet J-P, Legrand P, Barratt G. Modulation of nitric oxide production in RAW 264.7 cells by transforming growth factor-beta and interleukin-10: differential effects on free and encapsulated immunomodulator. J Leuk Biol. 1997;62:374–80. doi: 10.1002/jlb.62.3.374. [DOI] [PubMed] [Google Scholar]
- 9.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–21. [PubMed] [Google Scholar]
- 10.Wahl SM. Transforming growth factor beta (TGF-β) in inflammation: a cause and a cure. J Clin Immunol. 1992;12:61–73. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 11.De Waal Malefyt R, Yssel H, Roncarolo M-G, Spits H, Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 12.Edwards D, Kirkpatrick CH. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986;134:1062–71. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- 13.Tomioka H, Sato K, Maw WW, Saito H. The role of tumor necrosis factor, interferon-γ, transforming growth factor-β, and nitric oxide in the expression of immunosuppressive functions of splenic macrophages induced by Mycobacterium avium complex infection. J Leuk Biol. 1995;58:704–12. doi: 10.1002/jlb.58.6.704. [DOI] [PubMed] [Google Scholar]
- 14.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 15.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen-1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 16.Hagerty DT. Intercellular adhesion molecule-1 is necessary but not sufficient to activate CD4+ T cells. J Immunol. 1996;156:3652–9. [PubMed] [Google Scholar]
- 17.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–86. [PubMed] [Google Scholar]
- 18.Lopez Ramirez GM, Rom WN, Ciotoli C, et al. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–20. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T, Tomioka H, Sato K, et al. Effects of Chinese traditional medicine Mao-Bushi-Saishin-To on therapeutic efficacy of a new benzoxazinorifamycin, KRM-1648, against Mycobacterium avium infection in mice. Antimicrob Agents Chemother. 1999;43:514–9. doi: 10.1128/aac.43.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzelmann M, Mercer-Jones MA, Gardner SA, Wilson MA, Polk HC. Bacterial cell wall products increase monocyte HLA-DR and ICAM-1 without affecting lymphocyte CD18 expression. Cell Immunol. 1997;176:127–34. doi: 10.1006/cimm.1997.1089. [DOI] [PubMed] [Google Scholar]
- 21.Rutten H, Theimermann C, Perretti M. Upregulation of ICAM-1 expression on J774.2 macrophages by endotoxin involves activation of NF-κB but not protein tyrosine kinase: comparison to induction of iNOS. Med Inflammation. 1999;8:77–88. doi: 10.1080/09629359990568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan L, Sano S, Pirmez C, et al. Expression of adhesion molecules in leprosy lesions. Infect Immun. 1991;59:4154–60. doi: 10.1128/iai.59.11.4154-4160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncada B, Torres-Alvarez MB, Gonzalez-Amaro R, et al. Lack of expression of intercellular adhesion molecules ICAM-1 in lepromatous leprosy patients. Int J Lepr. 1993;61:581–5. [PubMed] [Google Scholar]
- 24.Pourshafie MR, Sonnenfeld G. Treatment of an infected murine macrophage cell line (J774A.1) with interferon-γ but not tumor necrosis factor-α or live Mycobacterium intracellulare alone modulates the expression of adhesion molecules. J Interferon Cytokine Res. 1997;17:69–75. doi: 10.1089/jir.1997.17.69. [DOI] [PubMed] [Google Scholar]
- 25.Xiao B-G, Zhang G-X, Ma C-G, Link H. Transforming growth factor-beta 1 (TGF-β1)-mediated inhibition of glial cell proliferation and down-regulation of intercellular adhesion molecule-1 (ICAM-1) are interrupted by interferon-gamma (IFN-γ) Clin Exp Immunol. 1996;103:475–81. doi: 10.1111/j.1365-2249.1996.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willems F, Marchant A, Delville J-P, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–9. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 27.Chan J, Kaufmann SHE. Immune mechanisms of protection. In: Bloom BR, editor. Tuberculosis, pathogenesis, protection, and control. Washington DC: ASM Press; 1995. pp. 389–415. [Google Scholar]
- 28.Johnson CM, Cooper AM, Frank AA, Orme IM. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intercellular adhesion molecule-1 gene. Infect Immun. 1998;66:1666–70. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pancholi P, Mirza A, Bhardwaji N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–6. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 30.Tomioka H, Sato K, Shimizu T, et al. Effects of benzoxazinorifamycin KRM-1648 on cytokine production at sites of Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1997;41:357–62. doi: 10.1128/aac.41.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]