Abstract
Autoantibodies to EEA1 have been described in patients with neurological diseases, subacute cutaneous lupus and a variety of other conditions, including a patient with Wegener's granulomatosis (WG). EEA1 is a hydrophilic peripheral membrane protein transiently associated with the cytoplasmic face of early endosomes. Antibodies to EEA1 produce a staining pattern that resembles the C-ANCA pattern produced by anti-proteinase 3 (PR3) antibodies in WG sera. Co-localization studies show incomplete overlap of the staining produced by anti-EEA1 with anti-PR3. We showed that 0/40 unselected sera, from a cohort of WG patients and antibodies to PR3, reacted with EEA1. In addition, 1/15 sera that have a C-ANCA staining pattern but do not react with PR3 in an ELISA, immunoprecipitated the recombinant EEA1 protein. We conclude that although antibodies to EEA1 produce a staining pattern that resembles anti-PR3 and C-ANCA, antibodies to EEA1 in WG are rare. However, some C-ANCA+ sera that do not react with PR3 may contain EEA1 autoantibodies.
Keywords: autoantibodies, endosome, anti-neutrophil cytoplasmic antibodies, Wegener's granulomatosis
INTRODUCTION
Autoantibodies are useful serologic markers of many autoimmune diseases and have been successfully used as reagents to isolate and characterize their respective autoantigens. For example, human autoantibodies have been used to identify unique nuclear [1,2], Golgi complex [3], mitochondrial [4] and ribosomal [5] antigens. Over the past 3 years, our attention turned to a number of sera referred for autoantibody analyses that produced a unique vesicular staining pattern in the cytoplasm of HEp-2 cells. We screened an expression cDNA library with serum from a patient with rapidly progressive lower motor neurone disease and high-titre antibodies to this cytoplasmic antigen and isolated a cDNA that encoded a recombinant protein identical to a previously described antigen, named EEA1 [6,7].
Early endosomes are major sorting compartments of materials taken up by receptor-mediated endocytosis [8]. The endocytosed material may be transported to lysosomes or to the opposite side of polarized cells, or recycled to the plasma membrane [9]. Human EEA1 is a hydrophilic 180-kD peripheral membrane protein of early endosomes that contains a calmodulin-binding motif, and its cysteine-rich, metal-binding fingers have a role in endocytic vesicular transport [6]. EEA1 is recruited onto early endosome membranes and then promotes vesicle fusion by tethering the early endosomes and endocytic vesicles [10,11].
Autoantibodies directed against EEA1 were initially reported in the sera of individual patients with subacute cutaneous systemic lupus erythematosus (SLE), polyarthritis, and rheumatoid arthritis (RA) [6,7]. Our previous study of sera that had a cytoplasmic vesicular staining pattern showed that 8/36 (22%) had EEA-1 antibodies [12]. The observation that one of the patients in this study had Wegener's granulomatosis (WG) led us to the present study of the relationship of anti-EEA1 with WG and ANCA.
ANCA represent a group of antibodies directed to granular constituents of neutrophils and monocytes [13–15]. Based on the fluorescence appearance on ethanol-fixed neutrophil preparations, two major ANCA patterns have been established: C-ANCA is characterized by a coarse granular cytoplasmic immunofluorescence pattern resulting from reactivity with anionic or neutral proteins, whereas P-ANCA is identified by a perinuclear staining pattern resulting from reactivity with cationic proteins that migrate and rearrange around the negatively charged nuclear membrane [14].
In more than 80% of cases, the classic C-ANCA pattern is caused by antibodies against proteinase 3 (PR3), a neutral serine protease present in the azurophil granules of neutrophils [14,16–18]. Anti-PR3 (C-ANCA) is strongly associated with WG, and over the last few years it has provided a reliable tool for the diagnosis of the patients with systemic vasculitis [16,19]. Perinuclear ANCA staining pattern (P-ANCA) can be caused by antibodies directed against several antigens, including myeloperoxidase (MPO), elastase, cathepsin G, lactoferrin, lysozyme, azurocidin and bactericidal/permeability-increasing protein (BPI) [13,20–22]. Even though a number of P-ANCA target antigens have been characterized, the antigens responsible for the C-ANCA fluorescence pattern are less thoroughly catalogued. Besides anti-PR3, in small number of cases the C-ANCA pattern may arise due to antibodies directed to BPI, and rarely MPO [23]. We report that anti-EEA1 antibodies produce a C-ANCA staining pattern but only rare sera from WG patients react with recombinant EEA1. In addition, sera that produce a C-ANCA staining pattern but do not react with PR3 by ELISA may contain anti-EEA1 antibodies.
MATERIALS AND METHODS
Human sera and patients
WG and other sera for this study were obtained from serum banks at the University of Calgary (Calgary, Alberta); Innova (San Diego, CA); and Immuno Concepts Inc. (Sacramento, CA). Fifteen sera from patients being investigated for the possibility of WG that had a positive C-ANCA and a negative ELISA for PR3 were included in the study. These patients did not meet the American College of Rheumatology (ACR) 1990 criteria [24]. The serum samples were stored at −20°C or −70°C. Control sera were randomly selected from a bank of 2000 female normal blood donors [25] or sera pooled from healthy volunteers. Clinical data were obtained by retrospective chart review.
Indirect immunofluorescence and ELISA
The reactivity of antibodies was identified by indirect immunofluorescence (IIF) microscopy [26] using commercially prepared HEp-2 cell substrates (Immuno Concepts Inc.) and a fluorescein-conjugated goat anti-human IgG (light and heavy chain), and a rhodamine-conjugated goat anti-rabbit IgG, as previously described [27]. ANCA was detected on commercially prepared human polymorphonuclear leucocyte substrate kits complete with control positive and negative sera (Innova). Slides were viewed with a Zeiss Universal microscope (Carl Zeiss Inc., Thornwood, NY). Co-localization of EEA1 and PR3 was studied using a Leica digital confocal microscope. ELISA detection of PR3 and MPO antibodies was performed with a commercial kit (Innova).
SDS-PAGE and immunoblotting
Primary and secondary granules were isolated from human polymorphonuclear leucocytes using published protocols [28]. Proteins or cellular preparations were solubilized in SDS sample buffer, separated by discontinuous SDS–PAGE [29], and transferred to nitrocellulose [30]. The nitrocellulose strips were then blocked with 5% non-fat milk in PBS containing 0·05% Tween-20 (PBS–T), and overlaid with the primary antibody and washed with PBS–T; bound antibody was traced with polyvalent goat anti-human immunoglobulin, conjugated with horseradish peroxidase (HRP; Calbiochem-Behring Corp., La Jolla, CA). Reactions were visualized by incubating the washed nitrocellulose strips in the enhanced chemiluminescence (ECL) substrate solution (Amersham Life Science Ltd, Aylesbury, UK), and by exposing the wet nitrocellulose strips to X-OMAT AR film (Eastman Kodak Co., Rochester, NY).
Immunoprecipitation
Immunoprecipitation of 35S-labelled in vitro translation product of the EEA1 cDNA was performed as previously described [12]. Briefly, 10 μl of human serum and 2–5 μl of in vitro translation product were incubated with protein A-Sepharose beads 4 h or overnight at 4°C. After incubation, the beads were washed five times with buffer, resuspended in SDS sample buffer, and then analysed by SDS–PAGE and autoradiography.
Rabbit immunization
Control polyvalent antibodies to EEA1 were prepared by immunizing New Zealand white rabbits with 2·5 mg of purified recombinant protein in an equal volume of Freund's complete adjuvant. The rabbits were boosted 2 weeks later with subcutaneous injections of 2·5 mg of the protein in Freund's incomplete adjuvant. The appearance and titre of EEA1 antibodies were monitored by IIF using goat anti-rabbit IgG (H + L chain) antibody (Calbiochem-Behring Corp.).
RESULTS
IIF
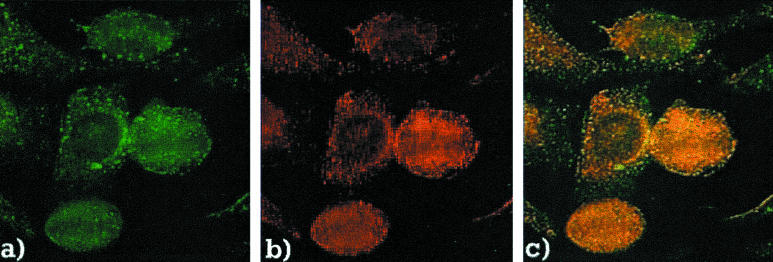
The prototype anti-EEA1 human serum chosen for this study was from a patient with a motor neurone disorder and muscular dystrophy [12]. The IIF pattern produced by this serum was characterized by distinct cytoplasmic dots that were evenly dispersed throughout the cytoplasm (Fig. 1a). The antibodies of the rabbit immunized with the recombinant EEA1 protein produced an identical vesicular staining pattern (Fig. 1b) that co-localized with the patient's antibodies on HEp-2 cell substrates (Fig. 1c).
Fig. 1.
Co-localization of human and rabbit anti-EEA1 sera on HEp-2 substrate. The prototype human serum containing anti-EEA1 produced a vesicular pattern of staining (a) that appears identical to the pattern produced by a rabbit serum with antibodies to recombinant EEA1 (b). Co-localization showed overlap of the vesicles attained by the two sera (c).
Identification of EEA1 as a component of polymorphonuclear leucocytes
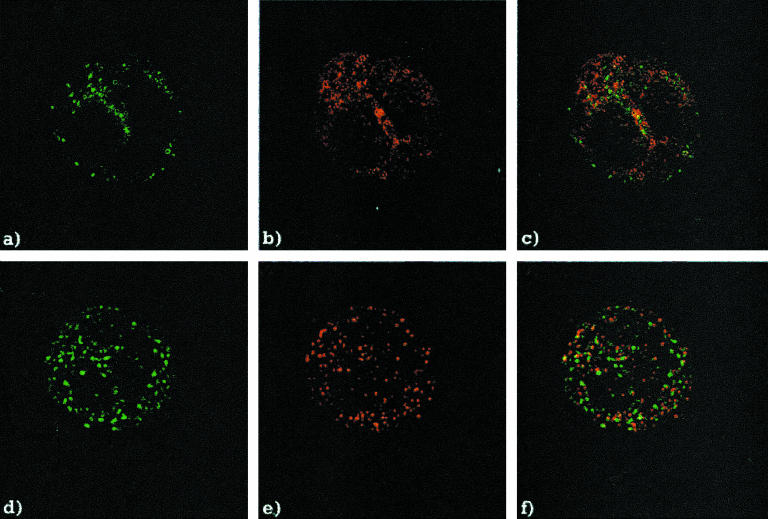
Rabbit sera containing antibodies to recombinant EEA1 produced a C-ANCA staining pattern on the ethanol-fixed human neutrophil substrate that was indistinguishable from the C-ANCA pattern produced by sera with anti-PR3 antibodies (Fig. 2a,b). Although these patterns appeared to be identical, co-localization studies showed that the staining produced by the human anti-PR3 (Fig. 2a) and the rabbit anti-EEA1 sera (Fig. 2b) had no obvious overlap (Fig. 2c). Similar results were obtained using formalin-fixed neutrophil slides (Fig. 2d,e,f).
Fig. 2.
Co-localization of the human anti-proteinase 3 (PR3) serum and rabbit anti-EEA1 serum on human neutrophil substrate. (a) Indirect immunofluorescence (IIF) of the human anti-PR3 serum, and (b) the rabbit anti-EEA1 serum, on the ethanol-fixed neutrophil substrate. (c) Overlay of the two images. (d) IIF of the human anti-PR3 serum, and (e) the rabbit anti-EEA1 serum on the formalin-fixed neutrophil substrate. (f) Overlay of the two images.
The sera from the 15 patients with a positive C-ANCA who did not fulfil the ACR criteria for the classification of WG were studied. Since none of these sera reacted with PR3 in an ELISA, we pursued the possibility that they might react with EEA1. Only one serum from these 15 patients immunoprecipitated recombinant EEA1.
We then determined if any of the 40 WG C-ANCA+ sera that reacted with PR3 produced a vesicular staining pattern on HEp-2 cells resembling anti-EEA1. This study showed that they displayed diffuse cytoplasmic staining on HEp-2 cells, although none produced a vesicular staining pattern resembling EEA1 (data not shown). When eight sera with EEA1 antibodies that displayed a C-ANCA-like pattern on neutrophil substrates were tested by ELISA, only one reacted with PR3 and none reacted with MPO (Table 1).
Table 1.
ELISA of the human anti-EEA1 sera performed using proteinase 3 (PR3) and myeloperoxidase (MPO) as target antigens
| Patient* | IP EEA1 | IIF titre HEp-2† | IIF‡ neutrophil | Pattern neutrophil | PR3 ELISA† | MPO ELISA† |
|---|---|---|---|---|---|---|
| CH | + | 1/5120 | + + | C-ANCA | – | – |
| MS | + | 1/5120 | + + + | C-ANCA | – | – |
| DM | + | 1/2560 | + + | C-ANCA | + + | – |
| KR | + | 1/1280 | + + + | C-ANCA | – | – |
| RA | + | 1/640 | + | C-ANCA | – | – |
| BI | + | 1/640 | + + + | C-ANCA | – | – |
| ED | + | 1/640 | + + | C-ANCA | – | – |
| SJ | + | 1/640 | + + | C-ANCA | – | – |
| SS-A | – | 1/640 | – | – | – | – |
| NHS | – | – | – | – | – | – |
| MPO+ | – | – | + + | P-ANCA | – | + + + |
| PR3+ | – | – | + + | C-ANCA | + + + | – |
Clinical details published in [ 12].
The titre of the sera was determined by end-point serial dilutions on HEp-2 cells.
The intensity of C-ANCA staining on the ethanol-fixed neutrophil slides was determined based on the comparison with the PR3 and MPO controls: +, weak; ++, moderate; and ++ +, very strong staining. A similar semiquantitative scoring was applied to ELISA results. IIF, Indirect immunofluorescence; IP, immunoprecipitation of recombinant EEA1.
Immunoprecipitation
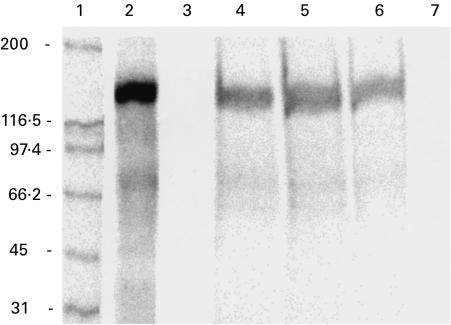
The 35S-labelled in vitro transcription and translation (TnT) product of the EEA1 cDNA migrated in SDS–PAGE at approx. 160 kD (Fig. 3, lane 2). The TnT product was immunoprecipitated by the serum of the rabbit immunized with EEA1 (Fig. 3, lane 4) and by the prototype human serum (Fig. 3, lane 5). The serum of a patient with WG displaying the C-ANCA staining pattern on polymorphonuclear neutrophil (PMN) substrate, but not reacting with PR3, also immunoprecipitated the EEA1 TnT product (Fig. 3, lane 6). Control normal human serum (Fig. 3, lane 7) and preimmune rabbit serum (Fig. 3, lane 3) did not immunoprecipitate recombinant EEA1. None of the 40 WG sera that demonstrated a C-ANCA pattern and reacted with PR3, immunoprecipitated the recombinant EEA1 protein (data not shown).
Fig. 3.
Immunoprecipitation of the in vitro translation product of the EEA1 cDNA with sera displaying C-ANCA pattern on ethanol-fixed neutrophil substrates. The translation product of EEA1 (EEA1-TnT) migrated at approx. 160 kD (lane 2). EEA1-TnT was immunoprecipitated by the rabbit anti-EEA1 serum (lane 4), prototype human anti-EEA1 serum (lane 5), and the serum of a patient with Wegener's granulomatosis displaying C-ANCA pattern but not reacting with proteinase 3 (PR3) (lane 6). Preimmune rabbit serum (lane 3), and control normal human serum (lane 7) did not immunoprecipitate EEA1-TnT.
SDS–PAGE and immunoblotting
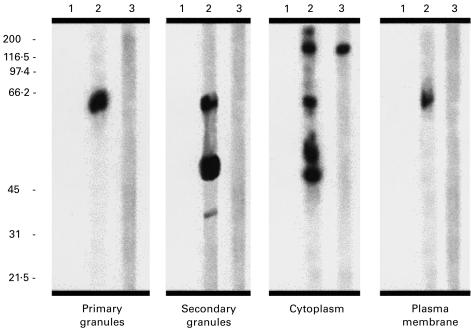
The neutrophil subcellular fractions separated by SDS–PAGE and immunoblotted with anti-EEA1 sera are shown in Fig. 4. The prototype human (lane 3) and rabbit EEA1 sera (lane 2) immunoblotted an approx. 180-kD protein in the cytosol preparation. The immunoblot of neutrophil primary granules indicated no reactivity with the expected molecular weight of PR3 or EEA1, although a few proteins of a lower molecular weight reacted with the rabbit anti-EEA1 serum.
Fig. 4.
Immunoblot of the neutrophil subcellular fractions with anti-EEA1 sera. Rabbit anti-EEA1 serum (lane 2) and the prototype human serum (lane 3) immunoblotted a protein of approx. 180 kD present in the cytosolic neutrophil fraction. No similar reactivity was observed with other subcellular fractions, although rabbit anti-EEA1 reacted with a few proteins of a lower molecular weight. Lane 1 indicates the reactivity of the preimmune rabbit serum.
DISCUSSION
This is the first study showing that anti-EEA1 antibodies produce a staining pattern that resembles C-ANCA. This is an important observation because C-ANCA antibodies are used as a diagnostic screen to identify patients with WG and other conditions [31]. In our earlier study, one WG patient was shown to have anti-EEA1 [12], although in the present study we show that this patient did not have antibodies to PR3. Since none of the 40 WG patients had EEA1 antibodies, we conclude that anti-EEA1 antibodies are rare in WG. However, antibodies to EEA1 should be considered in sera that produce a C-ANCA pattern but do not react with PR3.
Previous studies have shown that anti-EEA1 antibodies are relatively common (> 20%) in sera that show a cytoplasmic vesicular staining pattern on HEp-2 cells [12]. In this study, 22% of patients that had an IIF staining pattern resembling antibodies to EEA1, immunoprecipitated recombinant EEA1. These patients had a number of diagnoses, but neurological disease was a common feature [12]. The presence of neurological diseases in a cohort of patients with EEA1 autoantibodies is of interest, because early endosomes are key functional components of both pre- and post-synaptic neurones [32]. In other studies, EEA1 antibodies were reported in patients with subacute cutaneous SLE [6], polyarthritis and RA [7].
PMN are short-lived, non-mitotic cells generated in large numbers in the bone marrow through a highly controlled process of myelopoiesis [33]. Cytoplasmic granules start to form during neutrophil maturation, so the differences in protein content that define different subsets of granules are determined by the maturation stage at which particular granule proteins are being synthesized [34]. Based on their size, morphology and protein content several subsets of granules have been identified: azurophil (primary) granules, specific (secondary) granules, secretory vesicles and gelatinase granules. The common C-ANCA and P-ANCA target antigens are constituents of the azurophil granules. The azurophil granules have a spherical shape resembling lysosomes, whereas secondary granules have more irregular, elongated, endosome-like shape. Secretory vesicles arise from endosomes and their membrane is rich in receptors important for immune function [34]. Although the size and distribution of EEA1 demonstrated by IIF in our study resembled primary granules bound by anti-PR3, we were unable to show the presence of EEA-1 in primary granules by immunoblotting. PR3 can be detected by immunoblotting and immunoprecipitation [35,36], but because reactivity may depend on conformational epitopes, great care must be taken to prevent excessive denaturation of the antigen during preparation [15,36,37]. It appears that epitopes on EEA1 are not as sensitive as PR3 to denaturation, since a variety of techniques, including immunoblotting of denatured proteins, is able to identify the cognate approx. 180-kD protein [12].
EEA1 is a 180-kD hydrophilic peripheral membrane protein lacking a hydrophobic transmembrane domain [6]. It is present in the cytosol and recruited to the cytoplasmic face of early endosomes with the help of Rab5, a small GTP-binding protein, and phosphatidylinositol 3-phosphate (PI3P), a membrane phospholipid [7], on the endosomal membrane EEA1 is a key factor controlling vesicle fusion [6,38]. It is interesting that Rab5, an EEA1 vesicle fusion partner in other cell types, also plays an important role in neutrophil granule exocytosis. Rab5 is present in neutrophil cytosol in large amounts, but upon neutrophil stimulation the amount of cytosolic Rab5 decreases with a concomitant increase in the amount of membrane-associated Rab5 [39]. Considering the evidence that Rab5 is necessary for recruitment of EEA1 to the early endosome membrane [40,41] and our observation that EEA1 is also present in the neutrophil cytosol, it is plausible that EEA1 may play a role in neutrophil granule vesicle fusion. However, the significance of EEA1 and anti-EEA1 antibodies as they relate to the normal function of polymorphonuclear cells remains to be determined.
Acknowledgments
The authors appreciate the technical assistance of Mrs Joan Miller. This project was funded by Grant no. 17868 from the Medical Research Council of Canada.
REFERENCES
- 1.Tan EM. Autoantibodies and autoimmunity: a three-decade perspective—a tribute to Henry G. Kunkel. Ann NY Acad Sci. 1997;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- 2.Fritzler MJ. Autoantibodies: diagnostic fingerprints and etiologic perplexities. Clin Invest Med. 1997;20:50–66. [PubMed] [Google Scholar]
- 3.Fritzler MJ, Hamel JC, Ochs RL, Chan EKL. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J Exp Med. 1993;178:49–62. doi: 10.1084/jem.178.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell SH, Leung PS, Spivey JR, et al. Antimitochondrial antibodies in kindreds of patients with primary biliary cirrhosis: antimitochondrial antibodies are unique to clinical disease and are absent in asymptomatic family members. Hepatology. 1992;16:899–905. doi: 10.1002/hep.1840160408. [DOI] [PubMed] [Google Scholar]
- 5.Bonfa E, Weissbach H, Brot N, Elkon KB. Ribosomal P protein autoantibodies. In: Peter JB, Shoenfeld Y, editors. Autoantibodies. Elsevier Science BV; 1996. pp. 721–6. [Google Scholar]
- 6.Mu FT, Callaghan JM, Steele-Mortimer HS, et al. EEA1, an early endosomal protein. J Biol Chem. 1995;270:13503–11. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 7.Waite RL, Sentry JW, Stenmark H, Toh BH. Autoantibodies to a novel early endosome antigen 1. Clin Immunol Immunopathol. 1998;86:81–87. doi: 10.1006/clin.1997.4455. [DOI] [PubMed] [Google Scholar]
- 8.Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–20. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman E. Lysosomes. New York: Plenum Press; 1989. [Google Scholar]
- 10.Rubino M, Miaczynska M, Lippé R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem. 2000;275:3745–8. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–5. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- 12.Selak S, Scheonroth L, Senecal J-L, Fritzler MJ. Early endosome antigen 1: an autoantigen associated with neurological diseases. J Invest Med. 1999;47:311–8. [PubMed] [Google Scholar]
- 13.Hagen C, Ballieux BEPB, Van Es LA, Daha MR, van der Woude FJ. Antineutrophil cytoplasmic autoantibodies: a review of the antigens involved, the assays, and the clinical and possible pathogenetic consequences. Blood. 1993;81:1996–2002. [PubMed] [Google Scholar]
- 14.Hoffman GS, Wiegert E, Homburger HA. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41:1521–37. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Wiik A. Antineutrophil cytoplasmic antibodies. In: Rose NR, Conway de Macario E, Folds JD, Lane CL, Nakamura RM, editors. Manual of clinical laboratory immunology. Washington, DC: American Society for Microbiology; 1997. pp. 954–9. [Google Scholar]
- 16.Gross WL, Csernok E, Schmitt WH. Antineutrophil cytoplasmic autoantibodies: immunobiological aspects. Klin Wochenschr. 1991;69:558–66. doi: 10.1007/BF01649318. [DOI] [PubMed] [Google Scholar]
- 17.Ludemann J, Utecht B, Gross WL. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastolytic enzyme. J Exp Med. 1990;171:357–62. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldschmeding R, Tervaert JWC, Dolman KM, Kr von dem Borne AEG, Kallenberg CGM. ANCA: a class of vasculitis-associated autoantibodies against myeloid granule proteins: clinical and laboratory aspects and possible pathogenetic implications. Adv Exp Med Biol. 1991;297:129–39. doi: 10.1007/978-1-4899-3629-5_11. [DOI] [PubMed] [Google Scholar]
- 19.Specks U, Wiegert E, Homburger HA. Human mast cells expressing recombinant proteinase 3 (PR3) as substrate for clinical testing for anti-neutro cytoplasmic antibodies (ANCA) Clin Exp Immunol. 1997;109:286–95. doi: 10.1046/j.1365-2249.1997.4561353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauschildt S, Schmitt WH, Csernok E, Flescher E, Rautmann A, Grossbach U. ANCA in systemic vasculites, collagen vascular diseases, rheumatic disorders and inflammatory bowel diseases. Adv Exp Med Biol. 2000;336:245–51. doi: 10.1007/978-1-4757-9182-2_36. [DOI] [PubMed] [Google Scholar]
- 21.Yang JJ, Falk RJ, Jennette JC. Frequency of anti-bactericidal/permeability-increasing protein (BPI) and anti-azurocidin in patients with renal disease. Clin Exp Immunol. 1996;105:125–31. doi: 10.1046/j.1365-2249.1996.d01-738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiik A. Anti-neutrophil cytoplasmic antibodies in Wegener's granulomatosis. Some clinical and pathogenetic aspects. Sem Clin Immunol. 1995;9:5–16. [Google Scholar]
- 23.Lesavre P. Antineutrophil cytoplasmic antibodies antigen specificity. Am J Kidney Dis. 1983;18:159–63. doi: 10.1016/s0272-6386(12)80873-0. [DOI] [PubMed] [Google Scholar]
- 24.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 25.Fritzler MJ, Pauls JD, Kinsella TD, Bowen TJ. Antinuclear, anticytoplasmic and anti-Sjögren's syndrome antigen-A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol. 1985;36:120–8. doi: 10.1016/0090-1229(85)90045-5. [DOI] [PubMed] [Google Scholar]
- 26.Fritzler MJ, Etherington J, Sokoluk C, Kinsella TD, Valencia DW. Antibodies from patients with autoimmune disease react with a cytoplasmic antigen in the Golgi apparatus. J Immunol. 1984;132:2904–8. [PubMed] [Google Scholar]
- 27.Fritzler MJ. Autoantibody testing: procedures and significance in systemic rheumatic diseases. Meth Achiev Exp Pathol. 1986;12:224–60. [PubMed] [Google Scholar]
- 28.Borregaard N, Heiple J, Simons E, Clark R. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Gordon J. Immunoblotting and dot immunobinding—current status and outlook. J Immunol Methods. 1984;72:313–40. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Mulder A, Broekroelofs J, Horst G, Limburg P, Nelis G, Kallenberg CGM. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease (IBD); relation to disease pattern and disease activity. Dig Dis Sci. 1994;39:545–9. doi: 10.1007/BF02088340. [DOI] [PubMed] [Google Scholar]
- 32.Parton RG, Dotti CG. Cell biology of neuronal endocytosis. J Neurosci Res. 1993;36:1–9. doi: 10.1002/jnr.490360102. [DOI] [PubMed] [Google Scholar]
- 33.Mollinedo F, Borregaard N, Boxer L. Novel trends in neutrophil structure, function and development. Immunol Today. 1999;20:535–7. doi: 10.1016/s0167-5699(99)01500-5. [DOI] [PubMed] [Google Scholar]
- 34.Borregaard N, Cowland J. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 35.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, Hack CE, van den Ende ME, Kallenberg CGM, von Dem Borne AEGK. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieslander J, Wiik A. ANCA antigens; proteinase 3. In: Van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. Dordecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–9. [Google Scholar]
- 37.Bini P, Gabay JE, Teitel A, Melchior M, Zhou JL, Elkon KB. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 38.Patki V, Virbasius J, Lane WS, Toh B-H, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–30. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vita F, Soranzo MR, Borelli V, Bertoncin P, Zabucchi G. Subcellular localization of the small GTPase Rab 5a in resting and stimulated neutrophils. Exp Cell Res. 1996;227:367–73. doi: 10.1006/excr.1996.0286. [DOI] [PubMed] [Google Scholar]
- 40.Simonsen A, Lippé R, Christoforidis S, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–8. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 41.Lawe DC, Patki V, Heller-Harrison R, Lambright D, Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding—critical role of this dual interaction for endosomal localization. J Biol Chem. 2000;275:3699–705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]