Abstract
Chronic granulomatous disease (CGD) is a clinical syndrome of recurrent bacterial and fungal infections caused by a rare disorder of phagocytic cells. In CGD, the phagocytes are unable to generate oxygen radicals after stimulation of these cells, due to a defect in the NADPH oxidase system. This NADPH oxidase is a multicomponent enzyme of at least four subunits, of which the β-subunit of cytochrome b558, gp91-phox, is encoded by an X-linked gene (called CYBB). We report here five patients from two families; in each family we found a different mutation in the promoter region of CYBB. Both mutations prevented the expression of gp91-phox in the patients' neutrophils and thus caused inability of these cells to generate oxygen radicals. However, the mutations left the gp91-phox expression and the function of the NADPH oxidase in the patients' eosinophils intact. The relatively mild course of the CGD in these patients can probably be attributed to the fact that the eosinophils have retained their oxidative capacity. Furthermore, our results indicate that neutrophils and eosinophils differ in their regulation of gp91-phox expression.
Keywords: chronic granulomatous disease, CYBB, promoter region
INTRODUCTION
Chronic granulomatous disease (CGD) is a rare congenital syndrome (prevalence 1:250 000) characterized by recurrent, sometimes life-threatening bacterial and fungal infections, with granuloma formation in the urogenital and gastro-intestinal tract as well as in the airways (reviewed in [1]). These symptoms are caused by the inability of the phagocytic leucocytes (neutrophils, eosinophils, monocytes, macrophages) to kill ingested microorganisms, due to a defective NADPH oxidase enzyme [1–3]. This enzyme system reduces oxygen to superoxide, which is subsequently converted to other reactive oxygen species used for the killing of microbes [4]. NADPH oxidase is a multicomponent enzyme system, consisting of the plasma membrane-bound cytochrome b558 as well as the cytosolic components p47-phox, p67-phox and p40-phox. Cytochrome b558 is composed of a large β-subunit (gp91-phox) and a smaller α-subunit (p22-phox) [5,6]. Gp91-phox is the actual enzymatic unit of the NADPH oxidase enzyme. It accepts electrons from NADPH at the cytosolic side of the plasma membrane and transmits these via a FAD and two haem groups to molecular oxygen at the extracellular (or intraphagosomal) side of the membrane [7].
Upon stimulation of the neutrophils (e.g. through binding of opsonized microorganisms to cell surface receptors) the cytosolic components translocate to cytochrome b558, together with a GTP-binding rac protein (rac-1 in macrophages, rac-2 in neutrophils), thus forming an activated NADPH oxidase that binds NADPH and generates superoxide [8–10].
Defects in either gp91-phox, p22-phox, p47-phox or p67-phox have been described to lead to CGD [1,11]. The disease is inherited either in an X-linked way (with absent or non-functional gp91-phox) or autosomal recessive way (with absent or non-functional p22-phox, p47-phox or p67-phox). Although it has been described that the autosomal recessive form of CGD runs a somewhat milder course than the X-linked form [12], probably because of a very small rest activity of the NADPH oxidase [13], it has proved impossible to correlate the genotype of the CGD patients with the phenotype of their phagocytes or the clinical expression of their disease. We describe here for the first time the clinical course of five patients from two families with mutations in the promoter region of the CYBB gene, clearly linked to a mild form of CGD.
CASE REPORTS
Family A
Patient 1 (III-4, Fig. 1) was born in 1994 after an uneventful pregnancy. He was admitted to the Hospital in 1995, for a pharyngitis. After oral treatment with erythromycin (40 mg/kg body weight per 24 h) he developed a running left ear with spiking fever. After 5 days he had a parotitis left, which developed into an abscess. This abscess was drained, and the antibiotic treatment was changed to i.v. augmentin (100/25 mg amoxi/clavulamic acid/kg body weight per 24 h). Auscultation of heart and lungs revealed no abnormalities. Liver and spleen were not palpable. C-reactive protein (CRP) was elevated to 132 mg/l (normal <5 mg/l) and on the x-ray picture of his lungs multiple inflammatory lesions were seen on both sides. The computed tomography (CT) scan of his ear and neck region showed abscesses left cervical and parapharyngeal with pressure on the oropharynx. The parapharyngeal and cervical abscesses were drained. Nocardia farcinica was cultured from the pus. He was discharged 1·5 months after admission, with oral augmentin (25/6·25 amoxi/clavulamic acid/kg body weight per 24 h). After the diagnosis of chronic granulomatous disease had been confirmed by laboratory investigations, treatment with interferon-gamma (IFN-γ; 50 μg/kg body weight, three times per week, s.c.) was started and the prophylactic antibiotics were changed to trimethoprim (5 mg/kg body weight per 24 h). Since then he has been relatively well.
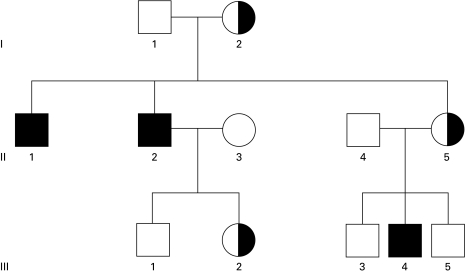
Fig. 1.
Pedigree of family A. Open symbols, no mutation at position −52 in CYBB; half-filled symbols, heterozygous C-52T mutation in CYBB; filled symbols, males with C-52T in CYBB. Patient II-2 was not investigated, because he had died before the start of our study; his mutation was deduced from the fact that his daughter but not his wife was heterozygous for the mutation.
Patient 2 (II-2, Fig. 1) is a maternal uncle of patient 1. He was born in 1961 and died in 1995, 5 weeks before the admission of patient 1, from a Nocardia infection. During his life he suffered from lupus erythematosus-like lesions, but he had no previous serious infections. He could not be tested, but was diagnosed posthumously by the fact that his daughter but not his wife proved to be a carrier for CGD (see Results).
Patient 3 (II-1, Fig. 1) is another uncle of patient 1, born in 1959. He has no complaints and has been healthy until now. He was diagnosed during the family investigations.
Family B
Patient 4 (III-4, Fig. 2) is a male born in 1980 after an uneventful pregnancy. In 1989 he had a lymph node abscess right and left in the neck region, which fistulated after incision by the general physician and recurred several times. The diagnosis of CGD was made in 1998. At that time, no bacteria or fungi could be cultured from the pus. Infection with Epstein–Barr virus (EBV), cytomegalovirus (CMV) or Brucella, and cat-scratch disease were ruled out serologically. The Ziehl–Nielsen ‘acid-fast’ assay was negative, as was the PPD skin reaction. Skin tests to non-tuberculous mycobacteria showed negative responses to Mycobacterium kansasi and M. scrofulaceum, but a positive reaction to M. avium (10 mm). This indicates infection with atypical mycobacteria.
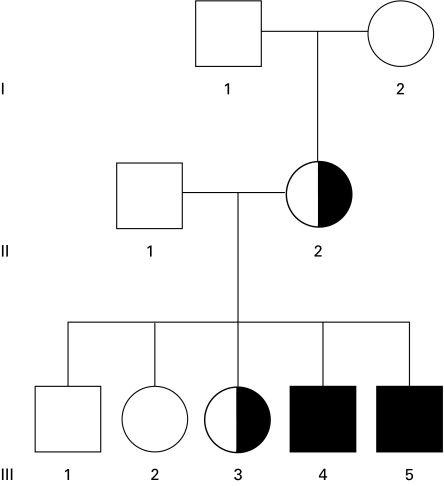
Fig. 2.
Pedigree of family B. Open symbols, no mutation at position −53 in CYBB; half-filled symbols, heterozygous C-53T mutation in CYBB; filled symbols, males with C-53T in CYBB.
Patient 5 (III-5, Fig. 2) is the brother of patient 4. He was born after an uneventful pregnancy in 1981. He suffered from multiple aphtous lesions in the mouth and constipation e.c.i. from early childhood. His clinical picture resembled that of his brother because he, too, had persistent lymph node abscesses with fistula. The pus was negative for the Ziehl–Nielsen acid-fast assay and for bacterial cultures, except for Corynebacterium minutissimum. He was diagnosed as a CGD patient at the same time as his brother, late in 1998.
The mother of these two patients suffers from the discoid form of lupus erythematodes.
METHODS
Granulocytes were isolated as described [14]. The cell preparations from healthy donors contained about 95% neutrophils and 2–5% eosinophils. Cell viability was always >95%.
Double-colour FACS analysis
Granulocytes (1 × 106) were resuspended in 150 μl of PBS with 1% (v/v) bovine serum albumin (BSA) and 5 mm EDTA. After addition of 7D5 MoAb against cytochrome b558 to a final concentration of 10 μg/ml, the cells were incubated for 30 min on ice, washed with PBS–BSA–EDTA and were subsequently incubated with PE-conjugated goat anti-mouse immunoglobulin (CLB) for 30 min on ice. The cells were washed again with PBS–BSA–EDTA and incubated with FITC-conjugated CD9 MoAb (4E1; CLB) for 30 min on ice. Finally, the cells were washed, resuspended in PBS–BSA–EDTA and kept on ice until analysis in a flow cytometer (FACScan; Becton Dickinson, San Jose, CA).
Eosinophil depletion
Granulocytes were resuspended in HEPES medium containing CD9 MoAb (4E1, 7·5 μg/ml) and incubated for 30 min on ice. After this incubation the cells were washed once and incubated in a head-over-head rotator for 45 min at 4°C with goat anti-mouse immunoglobulin-coated magnetic beads (ratio beads:cells 3:1; Dynal A.S., Oslo, Norway). Beads with adhering eosinophils were removed by a magnet. The eosinophil content of the cell suspensions was determined before and after the procedure by microscopic examination of cytocentrifuge preparations stained with May–Grünwald–Giemsa. Chemotaxis and random migration of purified granulocytes were measured in a modified Boyden chamber [15].
Measurement of NADPH oxidase activity
Oxygen consumption of granulocyte suspensions incubated with phorbol myristate acetate (PMA; 100 ng/ml) or serum-treated zymosan (STZ; 1 mg/ml) was measured with an oxygen electrode essentially as described [16]. Incubation of PMA-activated granulocytes with nitroblue tetrazolium (NBT) and microscopic scoring of the formazan reduction product in individual cells were performed as described by Meerhof & Roos [17]. Dihydrorhodamine-1,2,3 (DHR) oxidation was measured essentially as described [18]. In short, neutrophils (2·5 × 106/ml in HEPES medium (132 mm NaCl, 6 mm KCl, 1 mm MgSO4, 1·2 mm potassium phosphate, 20 mm HEPES, 5·5 mm glucose and 0·5% w/v BSA, pH 7·4) + 1 mm CaCl2) were incubated for 5 min at 37°C in a shaking water bath before the addition of DHR (1 μm) (Molecular Probes, Eugene, OR). Catalase (Boehringer, Mannheim, Germany) at a concentration of 0·2 mg/ml was added to avoid carry-over of hydrogen peroxide from NADPH oxidase-active cells to inactive cells. After another 5 min, the cells were stimulated by the addition of 100 ng/ml PMA. The reaction was stopped by the addition of 2 ml of ice-cold PBS containing 1% (v/v) BSA. Samples were kept on ice until analysis in a flow cytometer (FACScan; Becton Dickinson). Data were collected from 5000 cells. Killing of Escherichia coli ML-35 (Dr A. Kepes, Institut Pasteur, Paris, France) was measured by following the kinetics of phagocytosis, perforation of the bacterial cell envelope and inactivation of bacterial proteins by human granulocytes [19].
Mutation analysis
Genomic DNA was isolated from circulating blood leucocytes with the Puregene kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. The 13 exons of CYBB with their adjacent intronic sequences (exon 1 + promoter region) were amplified with the appropriate primer combinations. For exon 1 +the adjacent 800 bp of the promoter region, the sense primer was 5′-GCT GGT TAG TTA AAA AGT TAT TTC ACT GTG-3′ and the antisense primer was 5′-GAT AAC CCC AGA AGT CAG AG-3′. Genomic DNA (50–500 ng) was amplified by means of the Rapid Cycler (Idaho Technology, Idaho Falls, ID) with 50 cycles of 95°C for 5 s, 60°C for 30 s and 72°C for 15 s and slope S9. The reaction mixture contained 15 μl 2 U of Taq DNA polymerase (Promega, Madison, WI), 2 U of TaqStart antibody (Clontech Labs, Palo Alto, CA), 50 ng of each primer, 200 μm of each of the dNTPs (Promega) and reaction buffer (50 mm KCl, 1·5 mm MgCl2, 0·1% Triton X-100, 10 mm Tris and 8% DMSO, pH 9 at 25°C). The reaction took place in 10-μl glass capillaries (Idaho Technology). The polymerase chain reaction (PCR) products were purified with the GFX PCR DNA Gel Band Purification kit (Amersham Pharmacia Biotech, Uppsala, Sweden) and cycle-sequenced by means of the Big Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin Elmer Applied Biosystems, Warrington, UK) run on an ABI 377 XL Automated DNA Sequencer (Perkin Elmer). Sequence analysis was performed by means of the Sequence Analysis, Sequence Navigator and Auto Assembler software (Perkin Elmer).
RESULTS
The CGD diagnosis of patient 1, born in 1994 (III-4 in Fig. 1) was first established by means of the NBT test with PMA (30 min), in which only 20% formazan-positive (NADPH oxidase active) cells were counted (Table 1). In his granulocyte preparation were 54% neutrophils, 20% eosinophils and 20% lymphocytes. Formazan-positive cells were no longer identifiable as neutrophils or eosinophils. A granulocyte preparation from the mother of this patient showed a mosaic of 62% formazan-positive cells and 38% formazan-negative cells. Control cell preparations with >95% neutrophils showed >97% formazan-positive cells.
Table 1.
Nitroblue tetrazolium (NBT) tests of granulocyte preparations
| Percent formazan-positive cells | ||||
|---|---|---|---|---|
| Percent eosinophils in cell preparation | NBT test resting cells | NBT test + PMA | DNA analysis | |
| Normal controls | ≤ 5 | < 1 | > 97 | |
| Family A (Fig. 1) | ||||
| Patient III-4 | 20 | 0 | 20 | C-52T hemizygote |
| 32 | 0 | 32 | ||
| Patient II-1 | 13 | 0 | 13 | C-52T hemizygote |
| Patient II-2 | NT | NT | NT | NT |
| I-2 | NT | NT | NT | C-52T heterozygote |
| II-5 | 4 | 0 | 62 (mosaic) | C-52T heterozygote |
| III-2 | 4 | 0 | 36 (mosaic) | C-52T heterozygote |
| Family B (Fig. 2) | ||||
| Patient III-4 | 6 | 0 | 6 | C-53T hemizygote |
| Patient III-5 | 11 | 0 | 11 | C-53T hemizygote |
| II-2 | 8 | 0 | 26 (mosaic) | C-53T heterozygote |
| 2 | 0 | 30 (mosaic) | ||
| III-3 | 4 | 0 | 80 (mosaic) | C-53T heterozygote |
All other family members were normal.
NT, Not tested.
The oxygen consumption of the granulocytes from patient III-4 (Fig. 1) after stimulation with STZ was 2·5 nmol/106 cells per min, while control cells consumed 10·6 nmol of oxygen/106 cells per min (normal range 6·1–11·7 nmol/106 cells per min). Chemotaxis was normal. Perforation and breakdown of E. coli ML-35 (see Methods) was strongly diminished. The rate constants of perforation (k2) and degradation (k3) were 0·003 and 0·001, respectively, versus controls k2 = 0·25–0·93 and k3 = 0·040–0·160 (range, n = 25).
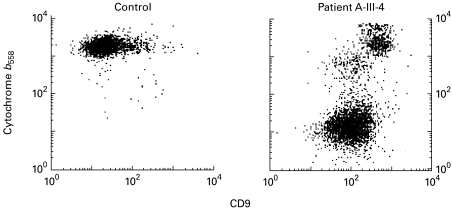
Surface expression of cytochrome b558, as indicated by 7D5 binding to granulocytes (Fig. 3), showed in the patient two cell populations: one with CD9 low (MFI 93) (neutrophils) and 7D5 low (MFI 12) and another with CD9 high (MFI 376) (eosinophils) and 7D5 high (MFI 1731). Thus, only his eosinophils (with high expression of CD9) expressed cytochrome b558.
Fig. 3.
Surface expression of cytochrome b558 on neutrophils and eosinophils. Granulocytes from a healthy control (left panel) and from patient III-4 in family A (right panel) were stained with anti-cytochrome b558 (MoAb 7D5) and PE-conjugated goat anti-mouse immunoglobulin, and with FITC-conjugated anti-CD9, and the cells were then analysed with a flow cytometer. In the patient's cells, the CD9-dull neutrophils lack cytochrome b558 expression, whereas the CD9-bright eosinophils show a normal cytochrome b558 expression (for exact values see text). The control cell preparation did not contain an appreciable number of eosinophils.
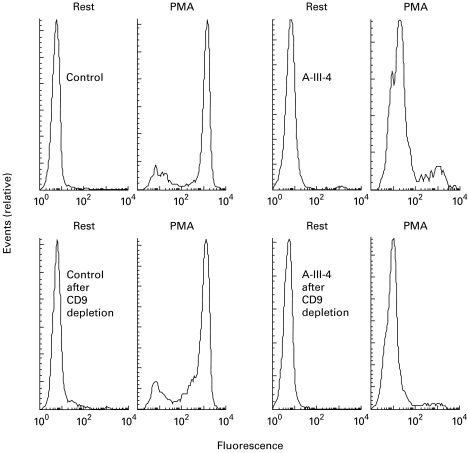
In the DHR oxidation assay (Fig. 4) this patient showed two activity peaks after stimulation with PMA: one large negative peak and one smaller positive peak. By treatment of the cell preparation with CD9-coated beads, the percentage of eosinophils was brought down from 20% to 2%. In the DHR assay only the negative peak remained (Fig. 4), indicating that only the eosinophils expressed NADPH oxidase activity.
Fig. 4.
Hydrogen peroxide production by the granulocytes from patient A III-4. Dihydrorhodamine (DHR)-loaded granulocytes from a healthy control (left) and from patient III-4 in family A (right) were incubated for 10 min with PBS (rest) or with phorbol myristate acetate (PMA), and the fluorescence of the H2O2-oxidized DHR was measured by flow cytometry. Each upper panel displays the NADPH oxidase activities (abscissa) before depletion of eosinophils with CD9-coated beads (control cells, 5% eosinophils; patient cells, 20% eosinophils). Each lower panel shows the activities after eosinophil depletion (control, 2% eosinophils; patient, 2% eosinophils). Events (cell numbers) are indicated on the ordinate, with the highest peak given maximal size.
The control granulocyte preparation, with about 5% eosinophils by morphological criteria, showed one cell population (Fig. 3) with low CD9 binding (MFI 23) and high 7D5 binding (MFI 1803). CD9+ eosinophils were not detected in this preparation. In the DHR assay (Fig. 4) this control granulocyte preparation showed two activity peaks after PMA stimulation: one small negative peak (presumably dead cells) and one large positive peak. Depletion of CD9+ cells induced a drop in the percentage of eosinophils from 5% to 2% (morphologically) but had hardly any effect on the oxidase activity peaks. This indicates normal cytochrome b558 expression and oxidase activity in the control neutrophils.
The granulocytes from the mother of patient 1 (93% neutrophils, 7% eosinophils) had two peaks in the 7D5 binding (not shown), one with high 7D5 binding (MFI: 1664) and one with low 7D5 binding (MFI: 14), with a low, broad CD9 binding distribution (MFI: 153 and 95, respectively). In the DHR assay her cells had two peaks, one without and the other with DHR oxidation, with about equal cell numbers (not shown). This cell preparation was not treated with CD9-coated beads.
Thus, the patient in this family appeared to suffer from an NADPH oxidase deficiency in his neutrophils but not in his eosinophils, correlating with lack of cytochrome b558 expression on the former but normal expression on the latter cells. His mother showed a normal oxidase activity in part of her granulocytes and lack of oxidase activity in the other part. This last pattern is reminiscent of an X-linked abnormality, and we therefore analysed the nucleotide sequence of the exons and flanking intron regions of the X-chromosome-localized CYBB gene, encoding the β-subunit of cytochrome b558. Analysis of genomic DNA from patient 1 (III-4, Fig. 1) revealed a mutation in the promoter region of the CYBB gene: at position −52, cytosine was replaced by thymine (C-52T). No other mutations were found. As indicated in Fig. 1 and Table 1, family investigations showed that the mother (II-5) of the patient was heterozygous for this mutation, as was the maternal grandmother (I-2) of the patient. The patient's father II-4 and the maternal grandfather I-1 showed no abnormalities. One maternal uncle (II-1) of the patient was found to be a hemizygote of the mutation; genetically, he must therefore be regarded as a CGD patient, although he never had any serious complaints about his health. The other maternal uncle (II-2) had died at age 34 from a Nocardia infection. His wife (II-3) did not carry the mutation, but his daughter (III-2) was a heterozygote, which proves that this uncle II-2 had also been a CGD patient.
Subsequent NBT testing of the leucocytes from the maternal uncle II-1 showed that his neutrophils were completely deficient in NBT reduction, whereas his eosinophils were normal in this respect. We now used a 5-min incubation period with PMA, which enabled us to identify the formazan-positive cells as eosinophils. The same test applied to the mononuclear leucocyte fraction showed that his monocytes had a weak NBT reducing capacity, in contrast to normal monocytes, which showed a much stronger formazan generation.
Figure 2 shows the pedigree of family B, a second family with CGD. Two brothers in this family suffer from recurrent infections. In the promoter region of CYBB we found a mutation next to the previous one: cytidine at position −53 was replaced by thymidine (C-53T). Both patients proved to be hemizygotes for this mutation, and no other mutations were found. Their mother and one of their sisters (III-3) were heterozygotes for this mutation; the other family members, including both parents of the mother, had a normal sequence.
Again, the NBT test performed with PMA-activated granulocytes showed that only the patients' eosinophils reduced the dye. The neutrophils in these preparations did not show any residual staining. In the mononuclear leucocyte fractions, their monocytes displayed very weak NBT reducing capacity. Their mother and their sister (III-3, Fig. 2) showed a mosaic of formazan-positive and -negative neutrophils, whereas their eosinophils were all formazan-positive. The patients' mother had a mosaic of strongly and weakly positive monocytes (their sister's monocytes were not tested). The other family members had completely normal NBT tests. These results are summarized in Table 1. As in the previous family, the neutrophils of the patients bound MoAb 7D5 very poorly (MFI 54 and 51, control 639), whereas their eosinophils showed a normal to high binding (MFI 936 and 1413, control 640). Their mother and sister III-3 had two populations of neutrophils, with low and normal 7D5 binding capacity, respectively.
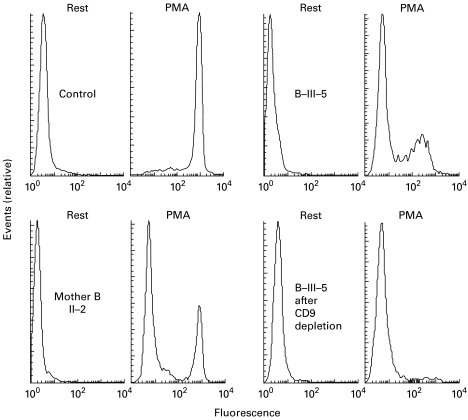
To confirm normal NADPH oxidase reactivity of the eosinophils in these patients, we performed a DHR oxidation assay before and after eosinophil depletion of granulocyte preparations. The results (Fig. 5) show that the granulocytes of patient III-5 in family B (morphologically 26% eosinophils) contained 34% DHR-reactive cells before depletion and 2% DHR-reactive cells after depletion (1% eosinophils). For patient III-4 these numbers were 23% eosinophils and 16% DHR-reactive cells before depletion, and 10% eosinophils and 6% DHR-reactive cells after depletion. Their mother had only 3% eosinophils in her granulocyte preparation, but her neutrophils proved to be a mixture of DHR-reactive and DHR-non-reactive cells, in accordance with the X-linked nature of the defect.
Fig. 5.
Hydrogen peroxide production by the granulocytes from members of family B. For experimental conditions, see legend of Fig. 4. Left top, control cells (2% eosinophils) with and without phorbol myristate acetate (PMA); no eosinophil depletion was attempted. Left bottom, cells from mother II-2 (3% eosinophils), no eosinophil depletion. Right, cells from patient III-5 (26% eosinophils) before (top) and after (bottom) eosinophil depletion. The mother clearly shows one active and one inactive population of neutrophils.
DISCUSSION
In two families five patients were diagnosed with a (partial) defect in the NADPH oxidase activity according to the NBT test, oxygen consumption and DHR activity. However, in the NBT test, some cells with a strong oxidase activity were found. The percentage of eosinophils in the cell preparations correlated with the percentage of oxidase-positive cells in the NBT test. All neutrophils were negative.
Treatment of the patients' cell suspensions with CD9-coated beads brought down the percentages of eosinophils as well as the DHR oxidase activity. Furthermore, the expression of cytochrome b558, as indicated by binding of MoAb 7D5, indicated that the neutrophils from the patients were virtually devoid of cytochrome b558, correlating with a total lack of oxidase activity (DHR assay), whereas their eosinophils showed a normal cytochrome b558 expression and oxidase activity. The mothers of the patients showed mosaics of oxidase-positive and -negative neutrophils. Therefore, we conclude that in these patients the granulocyte oxidase activities were not derived from the neutrophils, but can be ascribed to the eosinophils. Although the percentage of eosinophils in the granulocyte preparations from the patients was often elevated in comparison with that seen in healthy controls, this is a phenomenon not understood but often observed in CGD patients. Perhaps this constitutes some compensatory mechanism for the neutrophil defect in the patients described in the present study.
In the DNA analysis we found in both families a mutation in the promoter region of the CYBB gene. In family A substitution of C to T at position 52 (C-52T) had taken place, while in family B the same substitution was found at position 53 (C-53T). In the mothers the same mutations were found on one allele. Furthermore, these mutations were also found in family A in individuals I-2 and III-2, and in family B in individual III-3.
In 1995 Kuribayashi et al. [20] described a Japanese CGD patient with a lack of 7D5 binding to peripheral neutrophils, weak binding to monocytes and normal binding to eosinophils. This patient showed a normal NADPH oxidase activity in his eosinophils, but no activity in his neutrophils or monocytes. The mRNA for gp91-phox in the mononuclear cells had a normal size but the level was strongly depressed. Later, Suzuki et al. [21] showed that this patient had a C-53T mutation in the CYBB promoter region. This mutation is in a binding site for the transcription factor PU.1, and the authors showed that mutations at positions −50 to −53 strongly inhibited the binding of PU.1 to, and the promoter activity of, a gp91-phox promoter construct (positions −102 to +12). Thus, the mutations identified in our patients inhibit binding of PU.1 to the CYBB promoter region, leading to suppression of CYBB transcription. Apparently, PU.1 is an essential activator for the expression of gp91-phox in neutrophils but not in eosinophils. The compensatory mechanism in eosinophils is not known.
In family A, patient II-2 died from Nocardia infection at the age of 34 years, before the diagnosis of CGD was made. Until then he had no serious infections. The indicator patient III-4 was well until he too, acquired at the age of 1 year a Nocardia infection with the same sensitivity for antibiotics as in his uncle. Nocardia infections seldom occur in CGD [1], and infections with N. farcinica are extremely rare [22]. The third patient (II-1) in this family was diagnosed by chance at the age of 40 years by routine family analysis. He has no complaints at all and was surprised to hear the diagnosis. In family B, the two brothers III-4 and III-5 were well until they acquired at a relatively late age their first infection with an atypical M. avium. Also this microorganism is usually not the microorganism that causes infection in CGD patients. Nevertheless, these two cases show that CGD can be the cause of this infection.
Treatment of patient III-4 from family A with IFN-γ for 2 months had hardly any effect on the capacity of his neutrophils to reduce NBT (not shown). Thus, IFN-γ is apparently incapable of enhancing CYBB transcription in the absence of PU.1 binding to the promoter region. Nevertheless, this treatment may be beneficial, because it also ameliorates the clinical course of classical CGD patients without enhancing NADPH oxidase activity [23].
In conclusion, we found a relatively mild clinical course in five patients with CGD. The findings of a mutation in the promoter region of CYBB and NADPH oxidase-deficient neutrophils but normally functioning eosinophils indicate that eosinophils have a different mechanism to express gp91-phox. Most probably, the normal oxidase activity of the eosinophils is the reason for the milder clinical course in these patients than in most CGD patients, who lack NADPH oxidase activity in all phagocytic leucocytes. Although neutrophils form the first line of defence against microorganisms, normal functioning eosinophils are apparently able to compensate to a certain extent for non-functioning neutrophils.
Acknowledgments
We thank Rob van Zwieten, Christa Homburg and Erik Mul for excellent technical assistance.
REFERENCES
- 1.Roos D, Curnutte JT. Chronic granulomatous disease. In: Ochs HD, Smith CIE, Puck JM, editors. Primary immunodeficiency diseases A molecular and genetic approach. Oxford: Oxford University Press; 1999. pp. 353–74. [Google Scholar]
- 2.Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1968;46:668–79. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curnutte JT, Kipnes RS, Babior BM. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the granulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975;293:628–32. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal AW. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987;326:88–92. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- 6.Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987;80:732–42. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal AW, West I, Wientjes F, et al. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992;284:781–8. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn MT, Evans T, Loetterle LR, Jesaitas AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993;268:20983–7. [PubMed] [Google Scholar]
- 9.Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of Rac with p67-phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;267:531–3. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 10.Dusi S, Donini M, Rossi F. Mechanism of NADPH oxidase activation: translocation of p40-phox, Rac-1 and Rac-2 from the cytosol to the membranes of human neutrophils lacking p47-phox or p67-phox. Biochem J. 1996;314:409–12. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos D, de Boer M, Kuribayashi F, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–81. [PubMed] [Google Scholar]
- 12.Weening RS, Adriaansz LH, Weemaes CMR, Lutter R, Roos D. Clinical differences in chronic granulomatous disease patients with cytochrome-b-negative neutrophils and patients with cytochrome-b-positive neutrophils. J Pediatr. 1985;107:102–4. doi: 10.1016/s0022-3476(85)80626-0. [DOI] [PubMed] [Google Scholar]
- 13.Cross AR, Curnutte JT. The cytosolic activating factors p47-phox and p67-phox have distinct roles in the regulation of electron flow within NADPH oxidase. J Biol Chem. 1995;270:6543–8. doi: 10.1074/jbc.270.12.6543. [DOI] [PubMed] [Google Scholar]
- 14.Roos D, de Boer M. Purification and cryopreservation of phagocytes from human blood. In: Disabato G, Everse J, editors. Methods Enzymol. Vol. 132. New York: Academic Press; 1986. pp. 225–43. [DOI] [PubMed] [Google Scholar]
- 15.Roos D, Weening RS, Voetman AA, van Schaik MLJ, Bot AAM, Meerhof LJ, Loos JA. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979;53:851–66. [PubMed] [Google Scholar]
- 16.Weening RS, Roos D, Loos J. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974;83:570–6. [PubMed] [Google Scholar]
- 17.Meerhof LJ, Roos D. Heterogeneity in chronic granulomatous disease detected with an improved nitroblue tetrazolium slide test. J Leuk Biol. 1986;329:699–711. doi: 10.1002/jlb.39.6.699. [DOI] [PubMed] [Google Scholar]
- 18.Rothe G, Emmendörfer A, Oser A, et al. Flow cytometric measurement of the respiratory burst activity of phagocytes using dihydrorhodamine 123. J Immunol Methods. 1991;138:133–5. doi: 10.1016/0022-1759(91)90074-p. [DOI] [PubMed] [Google Scholar]
- 19.Hamers MN, Bot AAM, Weening RS, Sips HJ, Roos D. Kinetics and mechanism of the bactericidal action of human neutrophils against Escherichia coli. Blood. 1984;64:635–41. [PubMed] [Google Scholar]
- 20.Kuribayashi F, Kumatori A, Suzuki S, Nakamura M, Matsumoto T, Tsuji Y. Human peripheral eosinophils have a specific mechanism to express gp91-phox, the large subunit of cytochrome b558. Biochem Biophys Res Commun. 1995;209:146–52. doi: 10.1006/bbrc.1995.1482. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Kumatori A, Haagen IA, et al. PU.1 as an essential activator for the expression of gp91phox gene in human peripheral neutrophils, monocytes and B lymphocytes. Proc Natl Acad Sci USA. 1998;95:6085–90. doi: 10.1073/pnas.95.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fijen CAP, Schrama J, Kuijper EJ, Boiron P, Gerritsen W, Speelman P. Infection due to Nocardia farcinica in a woman with chronic granulomatous disease. Clin Infect Dis. 1998;26:222–4. doi: 10.1086/517033. [DOI] [PubMed] [Google Scholar]
- 23.The International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon-gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–16. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]