Abstract
To study the regulation of the human cellular immune response to HBsAg we produced a series of HBsAg-specific T cell lines from good and poor responders to the hepatitis B vaccine. All T cell lines expressed CD4 on their membrane and could therefore be considered of the helper/inducer phenotype. The different HBsAg-specific T cell lines were restricted by HLA-DRB5*0101, DRB1*1201, -DRB1*0701, -DRB1*0301, -DPB1*0201, -DPB1*0402, and -DPB1*0901. In good responders to the hepatitis B vaccine different HLA molecules could act as restricting element. In poor responders the diversity of HLA class II restriction determinants was more limited. This leads us to conclude that the immune response to HBsAg is multispecific and polyclonal in good responders and paucispecific and oligoclonal in poor responders to the hepatitis B vaccine. By using a panel of synthetic peptides representing selected sequences of the HBsAg, the fine specificities of each of these T cell lines could be determined. Strikingly, the majority of the identified T cell epitopes was located in and around the first hydrophobic transmembranous region of the HBsAg. This was observed in T cell lines from good and poor vaccine responders, without distinction. The remarkable T cell immunogenicity of this region may reside in its richness in binding motifs for a variety of HLA class II determinants.
Keywords: T cell epitopes, HBsAg, synthetic peptides, HLA restriction
INTRODUCTION
The immune response to HBsAg, the major component of the viral envelope of the hepatitis B virus (HBV), plays an important role in the immunity against HBV infections [1,2]. Humoral and cellular responses to the HBsAg have been detected after recovery from acute hepatitis B and in hepatitis B (HB) vaccine recipients. Soon it was demonstrated that antibodies to HBsAg (anti-HBs) protect against reinfection in convalescent patients and against primary infection in vaccinees [3].
It was shown, in mice and man [4,5], that synthesis of these antibodies is regulated by T lymphocytes (T-dependent HBsAg). Furthermore, a close correlation between the in vivo humoral and the in vitro cellular responses to HBsAg has been demonstrated in HB vaccine recipients [6] and in patients with acute HBV infections [7]. For these reasons an adequate T cell response to HBsAg seems indispensable for a proper recognition of the HBV by the immune system and is in consequence essential in resistance to viral infections and may represent an important mechanism of protection induced by the HBsAg vaccine [8]. To explore further the T cell immunogenicity of HBsAg we generated a series of HBsAg-specific T cell lines from good and poor responders to HBsAg vaccine and determined their fine specificity and HLA restriction.
Using hepatitis B envelope proteins purified from plasma of chronic HBsAg carriers and HBsAg produced by recombinant DNA technology, very efficient anti-HBV vaccines have been produced in the past two decades. The licensed yeast-derived vaccine is composed of a non-glycosylated recombinant protein of 226 amino acids (aa) corresponding to the complete HBsAg sequence [9].
T cells do not bind protein antigen directly, but recognize a bimolecular complex consisting of a MHC molecule (class I or class II) and a peptide fragment of a protein antigen [10,11]. The finding that small synthetic peptides can bind directly to MHC class II molecules has made it possible to mimic this phenomenon by incubating the antigen-presenting cells (APC) with different peptides representing selected regions of the native antigen. This experimental set-up was used in the present study.
The aa sequences recognized by T cells within the hepatitis B envelope proteins were initially investigated in mice [12]. However, epitopes for mouse T cells need not necessarily represent aa sequences recognized by human T cells, as shown by the study of the fine specificity of the T cell responses to S and core antigen [1,13,14]. The fine specificity of the human T cell response to the HBsAg has been analysed mostly in vaccine recipients. Celis et al. [15] reported that aa sequence 193–202 within the N-terminus of the S region contains an immunodominant epitope for CD4+, HBsAg-specific T cells, which is recognized in association with the HLA class-II DPw4 molecule. Rao and coworkers demonstrated that peptide 298–321 of the S region encodes a dominant, conformational, group-specific epitope. This epitope is recognized by human anti-HBs [16]. Further experiments revealed that this peptide also contains at least two T helper epitopes, one located between residues 298 and 311 and the other between residues 313 and 321 [17]. This is consistent with previous results from other research groups [18]. Min et al. [19] and Honorati et al. [20] recently described one (aa 255–273) and three (aa 310–329, aa 339–346, and aa 389–397) new T epitopes, respectively. Deulofeut et al. [21] further identified aa 313–320 as a major immunodominant peptide that is HLA-DR-restricted. However, the library of HBsAg-identified T cell epitopes and their HLA restriction is still expanding.
Approximately 5–10% of healthy vaccine recipients fail to produce protective levels of antibodies to the hepatitis B vaccine after standard immunization [22]. This phenomenon has been observed in all vaccine evaluation studies, irrespective of the HBsAg vaccine used [23,24], and its cause remains unknown. The lymphocytes from most good responders to hepatitis B vaccine proliferated in vitro upon stimulation with HBsAg particles, whereas the lymphocytes from the majority of intermediate or poor/non-responders (NR) generally do not react upon stimulation with HBsAg. Only recently HBsAg-specific lymphocyte proliferation was demonstrated in two groups of former vaccine NR. In the first study a preS1-preS2-S vaccine (‘Hepagene’) was used. Contrary to previously published ‘S’ vaccination data, Hepagene-stimulated T cell responses showed a lack of correlation with the humoral responses. Limiting dilution analyses demonstrated that the cellular immune response is associated with the kinetics of exposure to Hepagene rather than magnitude of anti-HBs responses [25,26]. A second study demonstrated that in seven out of nine (intradermal (i.d.)) revaccinated former vaccine NR a HBsAg-specific proliferative T cell response could be detected. It was concluded that i.d. HBsAg vaccination elicited stronger humoral and cellular immune responses than the conventional intramuscular (i.m.) vaccine and that former i.m. vaccine NR should be treated this way [27].
The absence of a humoral immune response to HBsAg vaccines is associated with certain HLA types. HLA-DR3 and -DR7 occurred more frequently in hyporesponders and NR than in the general population [28–31]. Alper et al. [32] speculated that the response to HBsAg was governed by a dominant immune response gene located in the MHC and that low or non-responsiveness was caused by the absence of this gene and the presence on both chromosomes of extended MHC haplotypes such as HLA-B8, DR3, SCO1 and HLA-B44, DR7, FC31 that are associated with non-response. It is tempting to speculate that non-responders are defective in the presentation of HBsAg, which leads to a deficient activation of the HBsAg-specific CD4+ T lymphocytes. In a previous paper [33] we addressed this issue and concluded that NR APC were not defective in HBsAg presentation to haplo-identical HBsAg-specific T cell lines.
Because the generation of a successful anti-HBs response requires cooperation between HBsAg-specific T and B lymphocytes, one can speculate that a reduction or absence of one or both of these components may lead to a defective or absent anti-HBs response. A physical or functional elimination of HBsAg-specific T cells, occurring during thymic or post-thymic repertoire maturation, may lead to a selective non-responsiveness to HBsAg at the T cell level. In this study we addressed the question whether DR3- and DR7-restricted CD4+ T lymphocytes are present in the circulation of NR and whether these T cell lines recognize HBsAg-derived T cell epitopes.
SUBJECTS AND METHODS
Subjects
Two good and three poor responders to the HB vaccine, all negative for HBsAg, antibodies to the hepatitis B core antigen (anti-HBc) and HBV-DNA, were selected from previous vaccination studies [6,34]. A vaccinee was considered to be a poor responder if his/her anti-HBs titre did not exceed 10 U/l 1 month after the third vaccine dose. Good responders had anti-HBs titres of ≥1000 U/l after the fourth vaccine dose. Subjects TW and AD were selected from a study in which 50 individuals were vaccinated with either the plasma-derived hepatitis B vaccine Hevac-B or the recombinant hepatitis B vaccine Engerix-B [6]. Subjects VA, DO and RE were selected from a vaccination study [34] where 40 poor/non-responders were revaccinated with three supplementary doses of either Engerix-B [35] or an experimental hepatitis B vaccine (SL*) [36] (both from Smith-Kline Beecham Biologicals, Rixensart, Belgium). Peripheral blood mononuclear cells (PBMC) from good responder vaccine recipients, used in the presentation experiments with allogeneic APC, were selected from another vaccination study [37], in which 100 healthy young adults were vaccinated with either Engerix-B or SL*. In the primovaccination studies the four vaccine doses were administered in the deltoid muscle according to a 0-, 1-, 2-, 12-month schedule. In the revaccination study the three doses were administered at monthly intervals.
Assessment of hepatitis B serology
Venous blood samples for serological and cellular assays were collected from each subject and the in vivo production of anti-HBs was measured in serum with the AUSAB RIA from Abbott Laboratories (North Chicago, IL). Anti-HBs titres were expressed in U/l using the Hollinger equation [38]. The AUSRIA II-125 and the CORAB RIA (Abbott Labs) were used for the detection of HBsAg and anti-HBc, respectively.
HLA typing
The HLA class II DR-, DP- and DQ-type of all subjects were determined using INNO-LiPA strips (Innogenetics N.V., Ghent, Belgium). The polymerase chain reaction (PCR) typing method is based on a technique developed by Cassiman and collaborators [39]. The HLA alleles were designated using a consensus nomenclature adapted by the World Health Organization in 1996 [40]. DRB1*XXXX and DPB1*XXXX refer to the genetic HLA typing. It was agreed that serologically identified products of the DRB1 locus would be known simply by the allele name, omitting the B1*.
Antigens
A recombinant, yeast-derived HBV envelope preparation was used for in vitro lymphocyte stimulation. It contained the major protein (rHBsAg or S particles) (subtype ad).
Fifteen synthetic peptides (Table 1), representing selected sequences from the S protein of HBV of subtype ad were synthesized by the solid-phase method developed by Merrifield [41] and used for the specificity testing of the different T cell lines.
Table 1.
Amino acid (aa) sequences and position of HBsAg-derived peptides
| NH2-….COOH | aa sequence |
|---|---|
| 175–184 | MENITSGFLG |
| 180–198 | SGFLGPLLVLQAGFFLLTR |
| 193–207 | FFLLTRILTIPQSLD |
| 197–215 | TRILTIPQSLDSWWTSLNF |
| 211–222 | TSLNFLGGSPVC |
| 226–244 | NSQSPTSNHSPTSCPPICP |
| 241–258 | PICPGYRWMCLRRFIIFL |
| 276–293 | GMLPVCPLIPGSTTTNTG |
| 289–304 | TTNTGPCKTCTTPAQG |
| 316–333 | PTDGNCTCIPIPSSWAFA |
| 341–357 | SVRFSWLSLLVPFVQWF |
| 351–368 | VPFVQWFVGLSPTVWLSA |
| 361–378 | SPTVWLSAIWMMWYWGPS |
| 374–389 | YWGPSLYSIVSPFIPL |
| 384–400 | SPFIPLLPIFFCLWVYI |
Peptide design was based on published sequences of HBsAg (subtype ad). Since the aa numbering refers to the sequence of the whole HB-envelope (preS1 + preS2 + S), aa no. 175 corresponds to the first aa of the S-region.
Tetanus toxoid (TT), obtained from Statens Seruminstitut (WHO, Copenhagen, Denmark) served as control antigen in lymphoproliferative assays.
Lymphoproliferation assays
The in vitro cellular immune response of the vaccinees was measured using a HBsAg-specific lymphoproliferation assay as described previously [6]. Unfractionated PBMC were suspended in RPMI 1640 medium supplemented with 25 mm HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mm l-glutamine (all from Gibco, Grand Island, NY), 5 × 10−5 m β-mercaptoethanol (β-ME; Sigma, St Louis, MO), and 10% heat-inactivated human AB+ serum (complete medium). For the proliferation assays with freshly isolated unfractionated PBMC this complete medium was supplemented with 5 × 10−5 m indomethacine (Sigma). PBMC (4 × 105/well) from the vaccine recipients were cultured for 6 days (37°C in 5% CO2) in 96-well round-bottomed microtitre plates containing HBsAg particles at a final concentration of 3 μg/ml. TT at 6 μg/ml was used as positive control antigen. Unstimulated control cultures (blanks) consisted of PBMC that were kept in culture medium without antigen.
In the proliferation assays with T cell lines, 2 × 104 T cells/well were incubated in complete medium in flat-bottomed plates, for 4 days. Either 1 × 105 autologous or allogeneic, irradiated (25 Gy, 60Co source) PBMC as APC were added, in the presence or absence of 3 μg/ml HBsAg.
For the epitope analysis of the T cell lines we used HLA class II transfected L cells. In these proliferation assays, 2 × 104 mitomycin C-treated L cells (APC) were cultured in flat-bottomed microplates (= day 1). After overnight incubation, peptides were added in 10% AB-Dulbecco's modified Eagles' medium (DMEM). After a 2-h peptide pulse, the cells were washed three times and cultures were set up in 200 μl of cRPMI 1640 with 10% AB. T cells (2 × 104) were added (= day 2). On day 6 the cultures were harvested.
All proliferation assays were performed in triplicate and 3H-TdR (0·5 μCi/well; Amersham Int., Aylesbury, UK) was added 18 h before harvesting. The cultures were harvested using an automated harvesting device and assayed for 3H-TdR incorporation by liquid scintillation counting in an LKB-Wallac 8100 Counter (LKB, Bromma, Sweden). Data are expressed as x¯ ± s.d. (mean of triplicate cultures ± s.d.), Δct/min (mean ct/min of antigen stimulated cultures – mean ct/min of control cultures) or as stimulation index (SI), which was calculated by the equation SI = (mean experimental ct/minwith antigen)/(mean control ct/minwithout antigen). SI was considered positive when ≥3. Peptide recognition by a T cell line was only considered positive when at least two different peptide concentrations induced a proliferative response in the T cell line.
No proliferation was observed when antigen was added to the T cell lines in the absence of PBMC (data not shown). The observed responses thus reflect antigen presentation by the added PBMC and not by the T cell lines themselves or residual monocytes/macrophages present in these T cell cultures. Background proliferation in the absence of antigen (SI = 1) was negligible, indicating the absence of alloreactivity.
Generation of HB envelope-specific T cell lines
Envelope-specific T cell lines were generated from vaccinees TW, AD, VA, DO and RE. Generation of T cell lines from TW and VA was described previously [33]. All T cell lines were generated in the same way: heparinized, fresh venous blood was obtained from the vaccinees several months after the last vaccine dose. Ninety-six microcultures were set up with 2500 T cells, isolated by rosetting, to which 105 irradiated (25 Gy), autologous PBMC and S particles (3 μg/ml) were added in 200 μl complete medium. After 5 days rIL-2 (human, 5 U/ml; Eurocetus, Leiden, The Netherlands) was added triweekly to each culture. Every 2 weeks the cultures were restimulated with antigen and autologous, irradiated PBMC as APC. After 8 weeks the HBsAg specificity of the viable T cell cultures was examined and S-specific T cell cultures were identified.
All T cell lines, tested monthly, were free of Mycoplasma contamination (tested with the Mycoplasma Detection Kit from Boehringer Mannheim).
Estimation of T cell precursor frequency
Minimal estimates of the proliferating HBsAg-specific T lymphocyte precursors were obtained by the minimum χ2 method from the Poisson distribution relationship between the responding cell number and the logarithm of the percentage of non-responding (negative) microcultures [42].
Flow cytometry of T cell lines
Phenotypic analysis of T cell lines was performed on a fluorescence-activated cell sorter (FACScan; Becton Dickinson Immunocytometry Systems, Erembodegem, Belgium) using a set of MoAbs against lymphocyte surface markers. The following MoAbs were used: anti-human CD3–PE-labelled, anti-human CD4–FITC-labelled and anti-human CD8–FITC-labelled (all isotype IgG1 from Becton Dickinson). Cells (1 × 105) were incubated in 100 μl of appropriate monoclonal reagents at 4°C for 30 min. After extensive washing with PBS containing 1% bovine serum albumin (BSA; Sigma) and 0·1% azide, an appropriate conjugate was added for another 30 min at 4°C. After washing with PBS–BSA–azide, the cells were analysed. A propidium iodide solution (50 μg/ml) was used to exclude dead cells.
Monoclonal antibodies
Blocking assays were performed in which mouse MoAb, specific for the human HLA class II antigen, or an irrelevant MoAb with the same isotype were added (at a final concentration of 0·5 μg/ml) to cultures consisting of autologous PBMC as APC, T cells and antigen. The degree of inhibition of the proliferative response of the T cell lines was then measured. For the blocking of T cell lines HBL-TW and HBL-AD we employed mouse MoAb recognizing human HLA-DP (isotype IgG1/κ), HLA-DQ (isotype IgG1/κ) or HLA-DR (isotype IgG2a/κ), all obtained from Becton Dickinson. For the blocking experiments with HBL-VA we used the same HLA-DP and -DQ antibodies and an anti-DR (isotype IgG1/κ) obtained from Serotec (Oxford, UK). For the blocking experiments with HBL-DO and HBL-RE we used the same HLA-DR and -DP antibodies as described for HBL-VA and an anti-DQ (isotype IgG2a/κ) obtained from PharMingen (San Diego, CA). Isotypic control antibodies consisted of mouse IgG1/κ (from ICN ImmunoBiologicals, Costa Mesa, CA) and mouse IgG2a/κ (from Sigma Immunochemicals). The inhibiting effect of the antibodies was calculated by the formula % inhibition = (Δct/minwithout MoAb −Δct/minwith MoAb/Δct/minwithout MoAb) ×100.
RESULTS
Generation of HBsAg-specific T cell lines (CD3+, CD4+)
HBsAg-specific T cell lines were generated from good responding vaccinees TW and AD, and from poor responders VA, DO and RE (Table 2). The HLA class II phenotype of the vaccinees is shown in Table 2. The generation of T cell lines from subjects TW (HBL- -TW) and VA (HBL- -VA) has been described previously [33]. HBL-2-TW, HBL-3-TW, HBL-16-TW and HBL-19-TW were derived from vaccinee TW 5 months after the fourth vaccine dose when the anti-HBs was 60 000 U/l. HBsAg-specific T cell precursor frequency was estimated to be 1/12 000. HLA-DR, -DP, and -DQ specificity was determined from all 37 generated T cell lines. Out of these 37 T cell lines, HBL-2-TW, HBL-3-TW, HBL-16-TW and HBL-19-TW were randomly selected for further determination of fine HLA restriction. HBL-37(1)-AD was derived from subject AD 1 month after the fourth vaccine dose when the anti-HBs titre was 43 000 U/l.
Table 2.
Origin and T cell precursor frequency of HBsAg-specific T cell lines
| T cell lines | Vaccinees' HLA class II type | Vaccine received | Vaccine doses (anti-HBs (U/l)) | Moment cloning | Estimated precursor frequency |
|---|---|---|---|---|---|
| HBL-TW | DRB1*1501/3 DRB5*0101 | Hevac-B | P1 = 118 | ||
| DRB1*0402 DRB4*0101 | P2 = 324 | 5mP4 | 1/12 000 | ||
| DPB1*0402 | P3 = 1011 | 60 000 | |||
| DPB1*0301 | P4 = 60 000 | ||||
| DQB1*0602 | |||||
| DQB1*0302 | |||||
| HBL-AD | DRB1*1302 DRB3*0301 | Engerix-B | P1 = 210 | ||
| DRB1*1201 DRB3*0202 | P2 = 1950 | 1mP4 | ND | ||
| DPB1*0401 | P3 = 1221 | 43 000 | |||
| DPB1*0402 | P4 = 43 000 | ||||
| DQB1*0301 | |||||
| DQB1*0604 | |||||
| HBL-VA | DRB1*0701 DRB4*0101 | Engerix-B | P1 = 0 | ||
| DRB1*1401 DRB3*020* | SL* | P2 = 0 | 5mP7 | ND | |
| DPB1*1401 | P3 = 2 | 1500 | |||
| DPB1*1401 | P4 = 75 | ||||
| DQB1*03032 | P5 = 94 | ||||
| DQB1*05031 | P6 = 1490 | ||||
| P7 = 2341 | |||||
| HBL-DO | DRB1*0301 DRB3*0101 | Hevac-B | P1 = 0 | ||
| DRB1*0301 DRB3*020* | Engerix-B | P2 = 0 | 20mP9 | 1/35 000 | |
| DPB1*02012 | P3 = 0 | 3000 | |||
| DPB1*0301 | P4 = 2 | ||||
| DQB1*020* | P5 = 22 | ||||
| DQB1*020* | P6 = 2800 | ||||
| P7 = 4300 | |||||
| P8 = 900 | |||||
| P9 = 25 371 | |||||
| HBL-RE | DRB1*0301 DRB3*0101 | Hevac-B | P1 = 0 | ||
| DRB1*1401 DRB3*020* | Engerix-B | P2 = 0 | 11mP10 | < 1/50 000 | |
| DPB1*0101 | SL* | P3 = 0 | 372 | ||
| DPB1*0901 | P4 = 0 | ||||
| DQB1*020* | P5 = 0 | ||||
| DQB1*05031 | P6 = 6 | ||||
| P7 = 37 | |||||
| P8 = 600 | |||||
| P9 = 3000 | |||||
| P10 = 4398 |
The two last capitals in the name of the T cell lines (HBL-––) refer to the vaccinee whose peripheral blood mononuclear cells were used for the generation of the cell lines.
P1, 1 month after the first vaccine dose; P2, 1 month after the second dose; …; 5mP4, 5 months after the fourth vaccine dose; 1mP4, 1 month after the fourth vaccine dose; …; ND, not determined.
DR0301- and/or DR0701-restricted HBsAg-specific T cell lines were generated with great difficulty from PBMC obtained from poor/non-responding vaccine recipients. HBL-1-VA and HBL-19-VA were derived from subject VA 5 months after the seventh vaccine dose (anti-HBs: 1500 U/l) as described [30]. HBL-14-DO, HBL-23-DO, HBL-50-DO and HBL-50(1)-DO were derived from subject DO, 20 months after the ninth vaccine dose (anti-HBs: 3000 U/l). HBsAg-specific T cell precursor frequency was estimated at 1/35 000. HBL-10-RE was derived from vaccinee RE, 11 months after the tenth vaccine dose (anti-HBs: 372 U/l). HBsAg-specific T cell precursor frequency was estimated to be <1/50 000.
All T cell lines were CD3+ and CD4+ (FACS analysis, data not shown) and free of Mycoplasma contamination as shown by monthly testing.
HBsAg specificity and HLA restriction of the T cell lines
The antigen specificity and HLA restriction of the T cell lines was studied by examining the proliferative response of the lines following stimulation with HBsAg. Table 3 summarizes the results of assays performed with autologous PBMC as APC. All tested cell lines proliferated in a dose-dependent manner (data not shown) upon stimulation with HBsAg and not upon stimulation with TT. The lines can therefore be considered antigen-specific.
Table 3.
HLA restriction pattern of HBsAg-specific T cell lines
| Proliferative response without MoAb | Percentage of inhibition | ||||||
|---|---|---|---|---|---|---|---|
| T cell line | x¯ ± s.d. (ct/min) | Blank | Anti-DR | Anti-DP | Anti-DQ | Control | Fine HLA restriction* |
| HBL-2-TW | 40 449 ± 1036 | 501 | 78·7 | 3·2 | 0 | 0/0 | DR2 |
| HBL-3-TW | 7012 ± 760 | 948 | 98·8 | 0 | 0 | 5·2/0 | DR2 |
| HBL-16-TW | 7281 ± 928 | 1132 | 8·5 | 100 | 2·9 | 0/3 | DP0402 |
| HBL-19-TW | 11 234 ± 544 | 111 | 83·7 | 94·2 | 0 | 0/2 | DP0402/DR0402 ? |
| HBL-37(1)-AD | 29 610 ± 1244 | 2850 | 94·1 | 0 | 2·2 | 5·3/ND | DR1201 |
| HBL-1-VA | 17 020 ± 1295 | 810 | 95·7 | 0 | 6·5 | 0/ND | DR0701 |
| HBL-19-VA | 14 931 ± 1475 | 834 | 97·1 | 11·9 | 19·9 | 25·8/ND | DR0701/DR1401 |
| HBL-14-DO | 43 072 ± 952 | 668 | 73·4 | 28·0 | 1·1 | 3/6·8 | DR0301/DP02012 |
| HBL-23-DO | 22 699 ± 1968 | 1603 | 5·3 | 86·1 | 4·6 | 13·2/15·6 | DP02012 |
| HBL-50-DO | 55 643 ± 1911 | 396 | 69·5 | 0 | 5·1 | 6·3/4 | DR0301 |
| HBL-50(1)-DO | 8236 ± 527 | 136 | 97·6 | 10·6 | 18·4 | 17·9/26·4 | DR0301 |
| HBL-10-RE | 51 509 ± 2469 | 787 | 45·6 | 31·1 | 0 | 0/3 | DP0901/DR ? |
T cells (2 × 104) were cultured with irradiated autologous peripheral blood mononuclear cells (105) and HBsAg (3 μg/ml) in the presence of MoAb to DP, DQ, DR or isotype control antibodies. T cell proliferation was measured by the incorporation of 3H-TdR during the final 18 h of a 92-h culture period. Data are expressed as the percentage of inhibition of the proliferative response observed in the presence of the different anti-class II MoAbs. Mean T cell proliferation (x¯ ± s.d.) stimulated by HBsAg in the absence of MoAb is shown. The values in the control column represent the percentage inhibition for the IgG1 and IgG2a isotypic controls, respectively.
Fine HLA restriction was determined as described in text.
Addition of anti-DR, -DP and -DQ to co-cultures of the T cell lines and autologous PBMC, showed that 10 cell lines were predominantly DR-restricted. Lines HBL-16-TW and HBL-23-DO, on the other hand, were almost exclusively DP-restricted. Other cell lines consisted of a mixed population of HLA-DR and -DP restricted T cells.
To corroborate the results of these HLA restriction studies we performed additional experiments where HBsAg was presented by HLA-typed, allogeneic PBMC displaying different haplotypes. These experiments are described in detail in a previous publication [33] and the final results are presented in the right column of Table 3. Briefly, in the presence of APC from unrelated individuals, T lymphocyte clones were effectively restimulated only in the presence of antigen and APC bearing the HLA molecule that restricted their HBsAg response. By doing so, HBL-2-TW and HBL-3-TW turned out to be DR2 (DRB1*1501/DRB5*0101) restricted. HBL-16-TW, on the other hand, was DP0402-restricted, and HBL-19-TW was DP0402- and probably also DR0402-restricted. Presentation experiments with HBL-37(1)-AD revealed the DR1201 restriction of this T cell line. HBL-1-VA and HBL-19-VA were both DR0701-restricted, but HBL-19-VA was also DR1401-restricted. HBL-14-DO, HBL-50-DO and HBL-50(1)-DO were all DR0301-restricted, with HBL-14-DO being also DP02012-restricted. HBL-23-DO, on the other hand, was exclusively DP02012-restricted. HBL-10-RE turned out to be DP0901-restricted (Table 3).
Diversity of HLA class II restriction determinants involved in HBsAg presentation
We have examined the restriction pattern of 35 cell lines derived from two ‘good responders’, one ‘intermediate responder’, and two ‘poor responders’ after vaccination. This was done by means of blocking assays with mouse MoAb specific for the human HLA class II antigens. Since some T cell lines had mixed DR/DP/DQ restriction patterns, the total number of individual identified T cell clones is more than 35. The diversity of HLA class II restriction determinants involved in HBsAg presentation is demonstrated in Table 4.
Table 4.
Diversity of HLA class II restriction elements involved in HBsAg presentation
| HLA-DR | HLA-DP | HLA-DQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Good | TW | HLA phenotype TW | DR1501/3 | DR0402 | DP0301 | DP0402 | DQ0302 | DQ0602 |
| No. of lines restricted by | 9 | 2 | 1 | 4 | 1 | 1 | ||
| AD | HLA phenotype AD | DR1302 | DR1201 | DP0401 | DP0402 | DQ0301 | DQ0604 | |
| No. of lines restricted by | 1 | 5 | 4 | 3 | 0 | |||
| Intermediate | VA | HLA phenotype VA | DR0701 | DR1401 | DP1401 | DQ0303 | DQ0503 | |
| No. of lines restricted by | 7 | 2 | 4 | |||||
| Poor | DO | HLA phenotype DO | DR0301$ | DR0301$$ | DP02012 | DP0301 | DQ020† | |
| No. of lines restricted by | 2 | 3 | 4 | 0 | 0 | 0 | ||
| RE | HLA phenotype RE | DR0301$ | DR1401 | DP0101 | DP0901 | DQ020† | DQ0503 | |
| No. of lines restricted by | 1 | 0 | 4 | 0 | 0 |
T cell lines from good (TW, AD), intermediate (VA) and poor (DO, RE) responders were analysed and their restriction elements are shown. For HBL-TW, six different HLA class II determinants are involved (two different HLA-DR, two different HLA-DP, and two different HLA-DQ responses). For HBL-RE, two different HLA class II determinants are involved (HLA-DP0901 and an undefined HLA-DR molecule). Strength of HBsAg-vaccine response correlates with the diversity of HLA class II restriction elements involved.
Refers to all possible subtypes.
Refers to HLA-DRB1*0301, -DRB3*0101.
Refers to HLA-DRB1*0301, -DRB3*020*.
For vaccinee TW, for example, it can be concluded that at least 60% of the precursor HBsAg-specific T cells were DR-, 27% were DP- and 11% were DQ-restricted. Fine restriction analysis revealed that 50% (9/18) were DR1501-restricted and 22% were DP0402-restricted. DQ3- and DQ1-restriction were found in 5% of T cells. The T cell response in this good responding vaccinee is clearly multispecific and polyclonal (at least six different HLA class II determinants are involved).
The same analyses were performed for the T cell lines generated from the other vaccinees. Remarkably, for DO and RE, no DQ-restricted T cell lines were found. All HBsAg-specific T cell lines derived from poor responder RE were DP0901-restricted. This suggests that in ‘poor responders’, only a few HLA determinants are involved and contribute to the vaccine-induced T cell response.
Peptide specificity of HBsAg-specific T cell lines
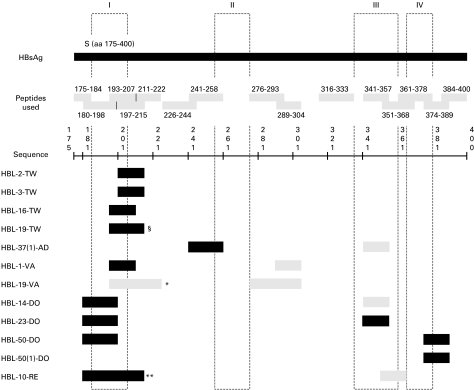
To unravel the epitope specificity (= peptide recognition) of the 12 lines, the T cells were incubated with autologous, irradiated PBMC and a panel of 15 synthetic HB envelope-derived peptides. Based on the proliferation of the T cell lines, the peptide specificity of each T cell line could be determined (Fig. 1). HBL-2-TW and HBL-3-TW, both HLA-DR2-restricted, recognized peptide 197–215. Experiments with transfected L cells (data not shown) revealed that sequence 197–215 is presented by HLA-DRB5*0101. Since HBL-19-TW also recognized this peptide, we can assume that it most probably also binds in DR0402. HBL-16-TW and HBL-19-TW, both DP0402-restricted, recognized peptide 193–207. The same peptide was also recognized by two DR0701-restricted T cell lines. Experiments with transfected L-cells revealed that sequence 193–207 bound in DP0402- as well as in DR07-molecules. This peptide thus seems to contain at least two overlapping T cell epitopes. Results from experiments performed with HBL-37(1)-AD reveal that sequence 241–258 most probably binds to DR1201-molecules. Peptide 180–198, which was recognized by four T cell lines, contains the DP02012 and/or the DR0301 epitope. Peptide 374–389 contains also a DR0301 epitope.
Fig. 1.
Peptide restriction pattern of HBsAg-specific T cell lines. The columns I, II, III and IV represent the four transmembrane regions of the HBsAg, as predicted by Stirk et al. [43]. Peptides that are recognized by HBL-19-TW in §: 193–207 and 197–215; by HBL-19-VA in *: 193–207 and 211–222; by HBL-10-RE in **: 180–198, 193–207, and 197–215. Dark shading refers to a strong peptide recognition and light shading refers to a weak peptide recognition.
The fact that certain T cell lines are able to recognize more than one peptide should be attributed here to the non-clonal character of the cell lines rather than to a promiscuity of the T cells.
It is striking that 10 out of 12 T cell lines recognized sequences within the region 180–215. A part of this sequence (aa 182–202) was described by Stirk as the first hydrophobic region of the HBsAg [43].
DISCUSSION
As part of a larger programme aimed at understanding the human immune response towards HBsAg we have generated numerous HBsAg-specific T cell lines derived from two good responder and three poor responder hepatitis B vaccine recipients. The precursor frequency of HBsAg-specific T cells was 1/12 000 in one good responder and 1/35 000 and <1/50 000 in two poor responders. Similar frequencies of envelope-specific T cells in vaccinees have been reported by Ferrari et al. [44]. The presence of HBsAg-specific T cells in vaccine NR was recently also demonstrated by two other groups [25–27]. In the first study, former vaccine NR were revaccinated with a preS-containing vaccine (‘Hepagene’). These Hepagene-stimulated T cell responses showed a lack of correlation with the humoral responses. This agrees with the previous observation of Degrassi et al. [45], where T and B cells, responsive to HBsAg, displayed different kinetics of recirculation in the peripheral blood. HBsAg-specific T cells could also be demonstrated (in vitro) in the circulation of intradermally revaccinated vaccine NR [27].
The HLA restriction pattern of a panel of T cell lines derived from the five vaccinees was examined. This analysis showed that the diversity of HLA class II restriction elements involved in HBsAg presentation clearly differed between good and poor responding individuals. In good responders, T cells were found that recognized HBsAg in the context of most of the available class II molecules. In poor responders, fewer HLA class II molecules seemed to participate in the HBsAg-specific T cell response. One obvious reason for this is the fact that poor responders are frequently homozygous for certain HLA-DR, -DP and -DQ molecules, some of which are known to be associated with poor responsiveness. The phenomenon of oligospecificity probably contributes to the reduced anti-HBs response observed in these individuals. Whether this is due to the inability of certain class II molecules to interact stably with HBsAg-derived peptides or whether certain HLA-peptide complexes are not ‘seen’ by T cells remains unsolved. Furthermore, this phenomenon of oligospecificity can contribute to the deficient cytokine secretion profile observed in T cells from non-responders [46].
Twelve cell lines, five derived from good responders and seven from poor responders, were analysed in great detail. A thorough analysis of their HLA restriction resulted in the identification of DRB5*0101, DRB1*0301, DRB1*0701, DRB1*1201, DPB1*02012, DPB1*0402, and DPB1*0901 as restricting elements for HBsAg recognition. With these lines at hand, we demonstrate here that various regions of the HBsAg sequence, presented by different MHC class II molecules, can function as T cell recognition sites. To investigate whether the envelope-specific T cells recognize a single or numerous epitopes of the particle and to see whether these T cell epitopes corresponded to those reported in other human studies, we further examined the fine specificity of these T cells. The two DRB5*0101-restricted T cell lines recognized peptide 197–215. This sequence has been reported by Barnaba et al., who found it DR2-restricted [47]. The two DP0402-restricted T cell lines recognized peptide 193–207. Celis et al. [15] were the first to demonstrate that aa 193–202 are presented through DPw4 in responder vaccinees. We demonstrate here that the same peptide sequence (aa 193–207) is also recognized in a DR7 context. Honorati et al. [20] reported that the minimal binding epitope of this peptide is 195–202 (LLTRILTI). Since these authors did not perform a HLA-DP typing, the precise HLA restriction of this minimal epitope remains unknown. HBL-37(1)-AD, which is DR1201-restricted, predominantly recognizes sequence 241–258. The two DR0701-restricted T cell lines both recognize 193–207 and 289–304. Peptide 289–304 is very likely to contain one or more T cell epitopes. Indeed, Milich et al. [48] found a proliferative response in PBMC of a vaccine recipient following stimulation with peptide 284–311 that encompasses the entire 289–304 sequence. From the T cell lines derived from subject DO we learn that peptides 180–198 and 341–357 are recognized in a DP02012-restricted way, whereas peptides 180–198 and 374–389 are probably recognized in a DR0301 context. Other regions of the HBVenv proteins able to induce proliferative responses in recipients of the HBsAg vaccine have previously been identified within the sequences 211–222, 255–273, 298–321, 310–329, 339–346, 361–378 and 389–397 of HBsAg [17,19,20].
It is striking to see that 10 of our 12 T cell lines recognized sequences located between aa 180 and 222 of the S region. This region, encompassing the first hydrophobic region, seems to be a potent T cell immunogen [43]. The importance of this region (hydrophobic α-helical domain) has already been highlighted by Celis et al. [15] and other investigators [19,20,47]. In an attempt to explain the remarkable T cell antigenicity of the first hydrophobic region of HBsAg, we screened (data not shown) this sequence for the presence of known (published) HLA class II binding motifs [49–53] and revealed numerous binding motifs that have been proposed for the different HLA class II molecules. This may explain its prominent recognition by HBsAg-specific T lymphocytes.
It has been suggested that the inadequate anti-HBs production in non/poor responders is caused by a lack of antigen-specific T cell help [33,54,55]. Since HBsAg-non-responsiveness was observed mainly in HLA-DR0301 or HLA-DR0701 subjects, the question whether DR0301- and DR0701-restricted T cells occur in these individuals becomes relevant. Therefore we tried very hard to obtain DR0301- and DR0701-restricted T cell lines. With much difficulty, we ultimately succeeded in generating such cell lines from poor responders to the hepatitis B vaccine and thus demonstrated the presence of HBsAg-specific T cells in the circulation, albeit at very low frequency. The hyporesponsiveness of DR3+ and DR7+ individuals may be caused by a defective T cell recognition of HBsAg, but the hole in the T cell repertoire is certainly not absolute. Since non-responsiveness to HBsAg vaccines is clearly HLA-linked, it was suggested that the lack of the appropriate haplotype could cause non-responsiveness to HBV vaccine in some subjects. In this and previous studies we demonstrate that DR3, DR7 and DP2 molecules, known to be associated with non-responsiveness [31,32], are able to present selected regions of the HBsAg. Peptide 193–207, which is known to bind in a DP4 molecule, a marker of good responsiveness, remarkably also binds in the DR7 molecule, a marker associated with poor responsiveness. This T cell epitope is thus ‘selected’ in good, as well as in poor HBsAg responders. Whether both class II molecules compete for the same peptide at some point of the antigen presentation process remains to be determined.
Recently it was demonstrated that DQ2 might be involved in HBsAg non-responsiveness, and all DQ2 homozygotes turn out to be poor responders [31,56]. It remains to be established whether DQ2-restricted HBsAg-specific T cells exist and whether they can be isolated and propagated as T cell lines or clones. In our survey, we did not find a single DQ2-restricted T cell. To examine whether DQ2 can interact with HBsAg-derived peptides, we searched for DQ2 binding motifs within the HBsAg sequence. The DQ2 motif published by Sollid et al. [57,58] occurred only once (aa 386–394) within the entire HBsAg sequence, whereas other published DQ2 binding motifs [59] were totally lacking. This finding, in combination with the observed pauciclonality of the T cell response in poor responders, may explain why DQ2+ individuals are unable to mount a vigourous response to HBsAg. Only after numerous vaccines did these individuals ultimately become responders [34], and, where examined, this response lacked the multispecificity observed in a good responding individuals.
Acknowledgments
We thank Dr P. Hauser and Dr P. Voet of SmithKline Beecham Biologicals for supplying the hepatitis B vaccines and peptides. We are grateful to Dr R. Rossau from Innogenetics N.V., Ghent, and Dr B. Vandekerckhove from the Blood Transfusion Centre of Oost-Vlaanderen (BRC) for initial HLA typings. We thank A. Willems and A. Vandeputte for excellent technical assistance. The authors are especially grateful to TW, DO, VA, AD, and RE for the many blood donations. We thank Eurocetus (The Netherlands) for the kind gift of rIL-2. This work was supported in part by grants by the National Fund for Scientific Research (Belgium; Grants 7.0013.90 and 3.0024.90), by the Special Research Fund of the Ghent University (OZF 01174591), and by SmithKline Beecham Biologicals.
REFERENCES
- 1.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 2.Milich DR. Immune response to the hepatitis B virus: infection, animal models, vaccination. Viral Hepatitis Rev. 1997;3:63–103. [Google Scholar]
- 3.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833–41. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 4.Roberts IM, Bernard CC, Vyas GN, Mackay IR. T-cell dependence of immune response to hepatitis B surface antigen in mice. Nature. 1975;254:606–7. doi: 10.1038/254606a0. [DOI] [PubMed] [Google Scholar]
- 5.Chang TW, Celis E, Miller RW, Zurawski VR, Kung PC. In vitro response to HBsAg of peripheral blood lymphocytes from vaccine recipients of hepatitis B vaccine. Hepatology. 1984;4:824–9. doi: 10.1002/hep.1840040504. [DOI] [PubMed] [Google Scholar]
- 6.Leroux-Roels G, Van Hecke E, Michielsen W, Voet P, Hauser P, Petre J. Correlation between in vivo humoral and in vitro cellular immune responses following immunization with hepatitis B surface antigen (HBsAg) vaccines. Vaccine. 1994;12:812–8. doi: 10.1016/0264-410x(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 7.Cupps TR, Hoofnagle JH, Ellis RW, Miller WJ, Gerin JL, Volkman DJ, Haas-Smith SA. In vitro immune responses to hepatitis B surface antigens S and preS2 during acute infection by hepatitis B virus in humans. J Infect Dis. 1990;161:412–9. doi: 10.1093/infdis/161.3.412. [DOI] [PubMed] [Google Scholar]
- 8.Iwarson S, Wahl M, Ruttiman E, Snoy P, Seto B, Gerety RJ. Successful postexposure vaccination against hepatitis B in chimpanzees. J Med Virol. 1988;25:433–9. doi: 10.1002/jmv.1890250407. [DOI] [PubMed] [Google Scholar]
- 9.Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the preS sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–61. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 11.Buus S, Sette A, Colon SM, Miles C, Grey HM. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987;235:1353–8. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- 12.Milich DR, Leroux-Roels GG, Louie RE, Chisari FV. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). IV. Distinct H-2-linked Ir genes control antibody responses to different HBsAg determinants on the same molecule and map to the I-A and I-C sub-regions. J Exp Med. 1984;159:41–56. doi: 10.1084/jem.159.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celis E, Ou D, Otvos L. Recognition of hepatitis B surface antigen by human T lymphocytes: proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J Immunol. 1988;140:1808–15. [PubMed] [Google Scholar]
- 14.Ferrari C, Cavalli A, Penna A, et al. Fine specificity of the human T-cell response to the hepatitis B virus preS1 antigen. Gastroenterology. 1992;103:255–63. doi: 10.1016/0016-5085(92)91121-j. [DOI] [PubMed] [Google Scholar]
- 15.Celis E, Karr RW. Presentation of an immunodominant T-cell epitope of hepatitis B surface antigen by the HLA-DPw4 molecule. J Virol. 1989;63:747–52. doi: 10.1128/jvi.63.2.747-752.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manivel V, Ramesh R, Panda SK, Rao KV. A synthetic peptide spontaneously self-assembles to reconstruct a group-specific, conformational determinant of hepatitis B surface antigen. J Immunol. 1992;148:4006–11. [PubMed] [Google Scholar]
- 17.Mishra A, Rao KVS, Durgapal H, Manivel V, Panda SK. Human T-helper cell responses to a synthetic peptide derived from the hepatitis B surface antigen. Immunology. 1993;79:362–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Steward MW, Sisley BM, Stanley C, Brown SE, Howard CR. Immunity to hepatitis B: analysis of antibody and cellular responses in recipients of a plasma-derived vaccine using synthetic peptides mimicking S and preS regions. Clin Exp Immunol. 1988;71:19–27. [PMC free article] [PubMed] [Google Scholar]
- 19.Min WP, Kamikawaji N, Mineta M, Tana T, Kashiwagi S, Sasazuki T. Identification of an epitope for T cells correlated with antibody response to hepatitis B surface antigen in vaccinated humans. Hum Immunol. 1996;46:93–99. doi: 10.1016/0198-8859(96)00009-2. [DOI] [PubMed] [Google Scholar]
- 20.Honorati MC, Dolzani P, Mariani E, Piacentini A, Lisignoli G, Ferrari C, Facchini A. Epitope specificity of Th0/Th2 CD4+ T lymphocyte clones induced by vaccination with rHBsAg vaccine. Gastroenterology. 1997;112:2017–27. doi: 10.1053/gast.1997.v112.pm9178695. [DOI] [PubMed] [Google Scholar]
- 21.Deulofeut H, Iglesias A, Mikael N, et al. Cellular recognition and HLA restriction of a midsequence HBsAg peptide in hepatitis B vaccinated individuals. Mol Immunol. 1993;30:941–8. doi: 10.1016/0161-5890(93)90019-8. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman JN. Nonresponse to hepatitis B vaccines and the kinetics of anti-HBs production. J Med Virol. 1996;50:283–8. doi: 10.1002/(SICI)1096-9071(199612)50:4<283::AID-JMV1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Grob PJ, Jilg W, Milne A, et al. Viral hepatitis and liver disease. Baltimore, MD: Williams & Wilkins; 1990. Unresolved issues in hepatitis B immunization; pp. 856–60. [Google Scholar]
- 24.Katkov WN, Dienstag JL. Prevention and therapy of viral hepatitis. Semin Liver Dis. 1991;11:165–74. doi: 10.1055/s-2008-1040433. [DOI] [PubMed] [Google Scholar]
- 25.McDermott AB, Cohen SB, Zuckerman JN, Madrigal JA. Human leukocyte antigens influence the immune response to a pre-S/S hepatitis B vaccine. Vaccine. 1999;17:330–9. doi: 10.1016/s0264-410x(98)00203-5. [DOI] [PubMed] [Google Scholar]
- 26.McDermott AB, Madrigal JA, Sabin CA, Zuckerman JN, Cohen SB. The influence of host factors and immunogenetics on lymphocyte responses to Hepagene vaccination. Vaccine. 1999;17:1329–37. doi: 10.1016/s0264-410x(98)00389-2. [DOI] [PubMed] [Google Scholar]
- 27.Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–7. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 28.Walker M, Szmuness W, Stevens CE, et al. Genetics of anti-HBs responsiveness: I. HLA DR7 and nonresponsiveness to hepatitis vaccination. Transfusion. 1981;21:601. [Google Scholar]
- 29.Usonis V, Kühnl P, Brede HD, Doerr HW. Humoral immune response after hepatitis-B-vaccination: kinetics of anti-HBs antibodies and demonstration of HLA antigens. Zbl Bakt Hyg A. 1986;262:377–84. doi: 10.1016/s0176-6724(86)80011-6. [DOI] [PubMed] [Google Scholar]
- 30.Craven DE, Awdeh ZL, Kunches LM, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med. 1986;105:356–60. doi: 10.7326/0003-4819-105-3-356. [DOI] [PubMed] [Google Scholar]
- 31.Desombere I, Willems A, Leroux-Roels G. Response to hepatitis B vaccine: multiple HLA-genes are involved. Tissue Antigens. 1998;51:593–604. doi: 10.1111/j.1399-0039.1998.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 32.Alper CA, Kruskall MS, Marcus-Bagley D, et al. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med. 1989;321:708–12. doi: 10.1056/NEJM198909143211103. [DOI] [PubMed] [Google Scholar]
- 33.Desombere I, Hauser P, Rossau R, Paradijs J, Leroux-Roels GG. Nonresponders to hepatitis B vaccine can present envelope particles to T lymphocytes. J Immunol. 1995;154:520–9. [PubMed] [Google Scholar]
- 34.Leroux-Roels G, Desombere I, Cobbaut L, et al. Hepatitis B vaccine containing surface antigen and selected preS1 and preS2 sequences. 2. Immunogenicity in poor responders to hepatitis B vaccines. Vaccine. 1997;15:1732–6. doi: 10.1016/s0264-410x(97)00118-7. [DOI] [PubMed] [Google Scholar]
- 35.Andre FE, Safary A. Summary of clinical findings on Engerix-B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad Med J. 1987;63:169–77. [PubMed] [Google Scholar]
- 36.Cabezon T, Rutgers T, Biemans Q, et al. Vaccines 90: modern approaches to new vaccines including the prevention of AIDS. New York: Cold Spring Harbor Laboratory Press; 1990. A new hepatitis B vaccine containing preS1 and preS2 epitopes from S. cerevisiae; pp. 199–203. [Google Scholar]
- 37.Leroux-Roels G, Desombere I, De Tollenaere G, et al. Hepatitis B vaccine containing surface antigen and selected preS1 and preS2 sequences. 1. Safety and immunogenicity in young, healthy adults. Vaccine. 1997;15:1724–31. doi: 10.1016/s0264-410x(97)00117-5. [DOI] [PubMed] [Google Scholar]
- 38.Hollinger BL, Adam E, Heiberg D, et al. Viral hepatitis: 1981 International Symposium. Philadelphia: Franklin Institute Press; 1982. Responses to hepatitis B vaccine in a young adult population; pp. 451–66. [Google Scholar]
- 39.Buyse I, Decorte R, Baens M, Cuppens H, Semana G, Emonds MP, Marynen P, Cassiman JJ. Rapid DNA typing of class II HLA antigens using the polymerase chain reaction and reverse dot blot hybridization. Tissue Antigens. 1993;41:1–14. doi: 10.1111/j.1399-0039.1993.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 40.Bodmer JG, Marsh SGE, Albert ED, et al. Nomenclature for factors of the HLA system, 1996. Tissue Antigens. 1997;49:297–321. doi: 10.1111/j.1399-0039.1997.tb02759.x. [DOI] [PubMed] [Google Scholar]
- 41.Merrifield B. Solid phase synthesis. Science. 1986;232:341–7. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- 42.Lefkovits I, Waldmann H. Limiting dilution analysis of the cells of immune system I. The clonal basis of the immune response. Immunol Today. 1984;5:265–8. doi: 10.1016/0167-5699(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 43.Stirk HJ, Thornton JM, Howard CR. A topological model for hepatitis B surface antigen. Intervirology. 1992;33:148–58. doi: 10.1159/000150244. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari C, Penna A, Bertoletti A, Cavalli A, Valli A, Schianchi C, Fiaccadori E. The preS1 antigen of hepatitis B virus is highly immunogenic at the T cell level in man. J Clin Invest. 1989;84:1314–9. doi: 10.1172/JCI114299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degrassi A, Mariani E, Honorati MC, Roda P, Miniero R, Capelli M, Facchini A. Cellular response and anti-HBs synthesis in vitro after vaccination with yeast-derived recombinant hepatitis vaccine. Vaccine. 1992;10:617–22. doi: 10.1016/0264-410x(92)90443-n. [DOI] [PubMed] [Google Scholar]
- 46.Vingerhoets J, Vanham G, Kestens L, Penne G, Leroux-Roels G, Gigase P. Deficient T-cell responses in non-responders to hepatitis B vaccination: absence of Th1 cytokine production. Immunol Letters. 1994;39:163–8. doi: 10.1016/0165-2478(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 47.Barnaba V, Franco A, Paroli M, et al. Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with hepatitis B virus. J Immunol. 1994;152:3074–85. [PubMed] [Google Scholar]
- 48.Milich DR, Peterson DL, Leroux-Roels GG, Lerner RA, Chisari FV. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg) VI. T cell fine specificity. J Immunol. 1985;134:4203–11. [PubMed] [Google Scholar]
- 49.Kropshofer H, Max H, Muller CA, Hesse F, Stevanovic S, Jung G, Kalbacher H. Self-peptide released from class II HLA-DR1 exhibits a hydrophobic two-residue contact motif. J Exp Med. 1992;175:1799–803. doi: 10.1084/jem.175.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Pool sequencing of natural HLA-DR, DQ, and DP ligands reveals detailed peptide motifs, constraints of processing, and general rules. Immunogenetics. 1994;39:230–42. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]
- 51.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rammensee H-G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 53.Hammond SA, Obah E, Stanhope P, et al. Characterization of a conserved T cell epitope in HIV-1 gp41 recognized by vaccine-induced human cytolytic T cells. J Immunol. 1991;146:1470–7. [PubMed] [Google Scholar]
- 54.Salazar M, Deulofeut H, Granja C, et al. Normal HBsAg presentation and T-cell defect in the immune response of nonresponders. Immunogenetics. 1995;41:366–74. doi: 10.1007/BF00163994. [DOI] [PubMed] [Google Scholar]
- 55.Egea E, Iglesias A, Salazar M, et al. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991;173:531–8. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDermott AB, Zuckerman JN, Sabin CA, Marsh SG, Madrigal JA. Contribution of human leukocyte antigens to the antibody response to hepatitis B vaccination. Tissue Antigens. 1997;50:8–14. doi: 10.1111/j.1399-0039.1997.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 57.Johansen BH, Vartdal F, Eriksen JA, Thorsby E, Sollid LM. Identification of a putative motif for binding of peptides to HLA-DQ2. Int Immunol. 1996;8:177–82. doi: 10.1093/intimm/8.2.177. [DOI] [PubMed] [Google Scholar]
- 58.Vartdal F, Johansen BH, Friede T, et al. The peptide binding motif of the disease associated HLA-DQ (alpha 1*0501, beta 1*0201) molecule. Eur J Immunol. 1996;26:2764–72. doi: 10.1002/eji.1830261132. [DOI] [PubMed] [Google Scholar]
- 59.Verreck FA, Van de Poel A, Termijtelen A, Amons R, Drijfhout JW, Koning F. Identification of an HLA-DQ2 peptide binding motif and HLA-DPw3-bound self-peptide by pool sequencing. Eur J Immunol. 1994;24:375–9. doi: 10.1002/eji.1830240216. [DOI] [PubMed] [Google Scholar]