Abstract
Polyunsaturated fatty acids are known to affect the immune response and administration of the omega-6 fatty acid linoleic acid has been reported to be beneficial in multiple sclerosis (MS) and EAE. In this study we have investigated the effects of oral feeding of plant lipid rich in the omega-6 fatty acid gamma-linolenic acid from Borago officinalis on acute and relapse disease and the immune response in EAE using SJL mice. EAE was induced by an encephalitogenic peptide (92–106) of myelin oligodendrocyte glycoprotein (MOG), and mice were fed the plant lipid daily from 7 days after EAE induction to assess the effects on acute disease and from day 25 to assess the effects on disease relapse. The clinical incidence and histological manifestations of acute EAE, and the clinical relapse phase of chronic relapsing EAE (CREAE) were markedly inhibited by omega-6 fatty acid feeding. A significant increase in the production of TGF-β1 in response to concanavalin A (Con A) at day 13 and a significant increase in TGF-β1 and PGE2 to Con A, PPD and MOG peptide (92–106) at day 21 were detected in spleen mononuclear cells from fatty acid-fed mice. There was no difference in interferon-gamma, IL-4 and IL-2 production between the fatty acid‐fed and control groups. Significantly higher TGF-β mRNA expression was found in the spleens of omega-6 fatty acid-fed mice at day 21. There were no differences in spleen cell proliferative response to Con A, PPD and MOG peptide (92–106). Biochemical analysis of spleen cell membrane fatty acids revealed significant increases in the eicosanoid precursor fatty acids dihomo-γ-linolenic acid and arachidonic acid in response to gamma-linolenic acid feeding, indicating rapid metabolism to longer chain omega-6 fatty acids. These results show that oral feeding of gamma-linolenic acid-rich plant lipid markedly affects the disease course of acute EAE and CREAE and is associated with an increase in cell membrane long chain omega-6 fatty acids, production of PGE2 and gene transcription and, on activation, secretion of TGF-β1.
Keywords: experimental autoimmune encephalomyelitis, MOG peptide, omega-6 fatty acids, arachidonic acid, transforming growth factor-beta 1, prostaglandin E2
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) with an unknown aetiology, although, once established, the disease is considered to be immune-mediated [1,2]. The immune mechanisms in MS may be studied in EAE, which can be induced in susceptible animals by inoculation with neuroantigens such as myelin basic protein (MBP) and proteolipid protein (PLP) and their peptides [3,4]. We have previously described the induction of chronic relapsing EAE (CREAE) with myelin oligodendrocyte glycoprotein (MOG) and peptides of MOG in mice [5]. Such induction results in CNS lesions of demyelination and perivascular mononuclear cell infiltrates similar to those observed in MS [5].
Beneficial effects of polyunsaturated fatty acid administration have been reported in MS and EAE with the omega-6 fatty acid linoleic acid [6–8]. One metabolite of linoleic acid (18:2n-6), gamma-linolenic acid (18:3n-6) is a key molecule in omega-6 fatty acid metabolism leading to rapid (unlike linoleic acid) production of the longer chain omega-6 fatty acids dihomo-γ-linolenic acid (20:3n-6) and arachidonic acid (20:4n-6) and the prostaglandins PGE1 and PGE2, respectively [9–11]. The long chain oxygenated omega-6 fatty acids, e.g. PGE1 and PGE2 (eicosanoids), are dependent on dietary fatty acids, and have been shown to have physiological immunoregulatory functions [11–13]. PGEs can be potent anti-inflammatory mediators which inhibit interferon-gamma (IFN-γ) and IL-4 production in human T cell clones stimulated by antigens and mitogen [14,15]. Similar results have been shown for peripheral blood mononuclear cells (PBMC) from human volunteers taking an omega-6 fatty acid supplement and conversely, the production of TGF-β1, a potent anti-inflammatory cytokine, was significantly raised [16]. Mertin & Stackpoole [17,18] found that a high linoleic acid-rich oil partially suppressed Lewis rat EAE which could be abolished by indomethacin and splenectomy, and concluded that the partially suppressive effects of omega-6 fatty acids on EAE are derived from prostaglandins produced in the spleen. Our work has shown that oral administration of the omega-6 fatty acid gamma-linolenic acid can completely protect Lewis rats from spinal cord homogenate-induced EAE depending on dose, whereas linoleic acid has a dose-dependent action on the clinical severity of EAE, but not abolishing it [10]. The mechanisms by which omega-6 fatty acids modify the immune response in EAE are however, unclear. This study was therefore designed, first, to determine whether the acute and relapse phases of MOG peptide-induced EAE in SJL mice could be modified by feeding an omega-6 fatty acid-rich plant lipid containing gamma-linolenic acid. Second, it was designed to establish whether any such changes produced by omega-6 fatty acid feeding, in MOG peptide-induced EAE, could be related to effects on spleen mononuclear cell membrane fatty acid composition, response to specific antigens and to mitogen, cytokine gene expression and production of cytokines and eicosanoid.
MATERIALS AND METHODS
Animals and diet
Young adult male SJL mice (H-2s) aged 4–5 weeks (Harlan Olac, UK) were housed in the Biological Services Facility at the Rayne Institute, St Thomas' Hospital, and allowed access ad libitum to water and rat and mouse standard diet (Bantin & Kingman, Humberside, UK). The macronutrient composition of the diet (% w/w) was: 18% protein, 2·5% fat, 57% carbohydrate, 4% fibre, 14% moisture and 5% ash. The diet included the following micronutrients: dL-μ-tocopherol 104 IU/kg, selenium 0·36 mg/kg, copper 20 mg/kg, zinc 95 mg/kg, retinol 12 000 IU/kg; and the fat composition (% total diet) was: saturated fatty acids 0·68%, unsaturated fatty acids 1·37%, of which linoleic acid comprised 0·90%. Animals were used for experimental purposes following an acclimatization period of 7–10 days.
Induction of EAE
Mice were injected subcutaneously with 200 μg MOG peptide (residues 92–106, amino acid sequence DEGGYTCFFRDHSYQ) in PBS, emulsified with Freund's incomplete adjuvant (FIA) supplemented with 60 μg mycobacterium on days 0 and 7 as described previously [5]. In addition, animals were also injected intraperitoneally with 200 ng Bordetella pertussis toxin (Porton Products Ltd, Maidenhead, UK) in PBS, immediately and 24 h after immunization with the MOG peptide. Mice were weighed and examined daily from day 7 onward by at least two observers for the presence of clinical signs. Clinical signs were assessed as follows: 0 = normal; 1 = limp tail; 2 = impaired righting reflex; 3 = partial hind limb paralysis; and 4 = complete hind limb paralysis. Animals exhibiting signs of a lesser severity than typically observed were scored as 0·5 less than the indicated grade.
Treatment
Ultra-refined omega-6 fatty acid-rich lipid from the plant Borago officinalis (Hoffmann-La Roche, Basel, Switzerland) was administered daily to mice by gavage. This refined plant lipid is rich in linoleic acid (38%) and gamma-linolenic acid (25%); other major fatty acids include the non-essential fatty acids palmitic (9·3%) and oleic acids (14·2%). The omega-6 fatty acid-rich lipid was stored under nitrogen at 4°C. In experiments to study the effects on the acute phase of EAE, animals were fed daily with 250–300 μl of the omega-6 fatty acid-rich lipid or saline-fed from 7 days after induction of EAE until killed on either day 13 (early acute-phase disease) or day 21 (recovery phase of the acute disease). In experiments designed to investigate the effects of omega-6 fatty acids on disease relapse mice were allowed to go through the acute phase and recover, then at day 25 they were fed daily with 250–300 µl of the omega-6 fatty acid-rich lipid or saline-fed until day 45.
Histology
Mice were killed using Enflurane anaesthetic. Brain and spinal cord were processed routinely for histology and 5 μm wax sections stained with either haematoxylin–eosin or luxol fast blue/cresyl fast violet for myelin evaluation. Pieces of CNS tissue were also snap-frozen in liquid nitrogen.
Lymphocyte proliferation
Mononuclear cells were isolated from spleens by sieving and density centrifugation on Lymphoprep (Sigma Chemical Co., Poole, UK) and cultured at 37°C in 5% CO2 atmosphere in 96-well microtitre culture plates (Nunc, Roskilde, Denmark) at a density of 2 × 105 cells/well in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS). Cells, >95% viable as judged by trypan blue exclusion, were cultured with 1 μg/ml concanavalin A (Con A; Sigma), 5 μg/ml MOG peptide (92–106), 5 μg/ml non-encephalitogenic peptide (residues 50–64: LYRNGKDQDAEQAPE), or 5 μl/ml PPD (Evans Medical Ltd, Leatherhead, UK). After 48 h, 3H-thymidine was added to each well (0·1 μCi/well) and the cells were incubated for a further 18 h. The cells were harvested and radioactive thymidine incorporation was determined by liquid scintillation counting. Results are expressed as a stimulation index (SI).
Cytokine and eicosanoid assays
Spleen mononuclear cells from omega-6-fed and control EAE mice were cultured as above in the presence of Con A (1 μg/ml), PPD (5 μl/ml), MOG peptide 92–106 (5 μg/ml) or MOG peptide 50–64 (5 μg/ml) for 24 h and supernatants removed and stored at −70°C until required. Mouse IL-2, IFN-γ and IL-4 were measured in supernatants using commercially available ELISA kits (Genzyme, Cambridge, MA) with sensitivities of 15–960 pg/ml, 125–8200 pg/ml and 5–540 pg/ml, respectively. Biologically active TGF-β1 was measured using a TGF-β1 ELISA system (Promega, Madison, WI) with a sensitivity of 25–1000 pg/ml. PGE2 was assayed by competitive immunoassay (Cayman, Ann Arbor, MI) with a sensitivity of 8–1000 pg/ml.
Lipid extraction and membrane fatty acid analysis
Total lipids from spleen cells were extracted by the method of Folch et al. [19]. Total membrane phospholipids from the total lipid extract were separated on precoated silica gel 60A plates (Whatman Ltd, Maidstone, UK) by development in petroleum ether–ether–acetic acid–methanol (85:15:2·5:1 by volume) containing 2,6-di-t-butyl-4-methyl phenol (0·1 g/l); this chromatographically removes the neutral lipids. The phospholipid band (which is stationary) was visualized under UV after spraying the dried plate with 2,7, dichorofluoroscein (0.1 g/l in methanol). Fatty acid separation and identification was by gas-liquid chromatography as described previously [10], except the chromatograph utilized was a HRGC Mega 2 series 8560 gas chromatograph (Fisons Instruments, Milan, Italy). Fatty acids are expressed as percent composition of total fatty acids measured.
Isolation of RNA and Northern blot analysis
Total RNA was extracted from liquid N2-frozen spleen tissue using the commercially available RNAzol™ B Method (RNAzol™ B; Biogenesis, Poole, UK). Briefly, this involves homogenization and RNA extraction using chloroform, removal of the aqueous phase containing RNA and precipitation with isopropanol and washing in 75% ethanol. The resultant RNA was quantified spectrophotometrically, and the ratio of 260/280 was >1·8. Samples (20 μg) of the RNA were loaded onto a 1% agarose gel containing formaldehyde and separated by electrophoresis at 120 mV for 2 h. The integrity of the RNA was confirmed by examination of an ethidium bromide stain under UV illumination. The RNA was then transferred to a nylon membrane (Hybond N+ Amersham Int., Aylesbury, UK) by capillary transfer overnight and cross-linked using a UV transilluminator (Hybaid, Ashford, UK). Membranes were prehybridized at 42°C in a hybridization oven (Hybaid) in a solution containing 50% formamide, 5× SSPE (1× SSPE is 0·3 m sodium chloride, 10 mm sodium dihydrogen orthophosphate, 1 mm ethylenediaminetetra-acetic acid pH 7·4), 10× Denhardt's solution (10× Denhardt's is 1 g Ficoll, 1 g polyvinylpyrrolidone and 1 g bovine serum albumin in 500 ml water), 1% SDS and 100 μg sonicated denatured salmon sperm DNA/ml. The TGF-β cDNA probe (American Type Culture Collection, Rockville, MD) was labelled with deoxycytidine 5′[μ32P]triphosphate (Amersham Int.) to a specific activity of approximately 108 ct/min per μg [20]. After hybridization, membranes were washed with 2× SSC (1× SSC is 0·3 m sodium chloride, 0·03 m sodium citrate, pH 7·0), 0·1% w/v SDS at room temperature and then twice with 0·1× SSC, 0·1% w/v SDS at 55°C. The membrane was then exposed to x-ray film for 3 days. The probe was stripped and rehybridized with a cDNA probe for 18s rRNA (a gift from D. Edwards, University of Calgary, Calgary, Alberta, Canada) to correct for loading differences. The autoradiographs were scanned using a laser densitometer (Molecular Dynamics, Sunnyvale, CA) and measured in absorbance units. Results are expressed as the ratio of TGF-β to 18s RNA.
Statistical analysis
Differences in the incidence of clinical (score 1 and above) and histological disease between the groups were assessed using the χ2 test. Clinical severity differences between the groups were assessed by Wilcoxon's sum of ranks test. The mean day of clinical onset was compared using unpaired Student's t-test. Assessment of differences in cytokine production, proliferative responses, fatty acids and the ratio of TGF-β to 18s RNA between the groups was by the Mann–Whitney U-test or Student's t-test.
RESULTS
Clinical and histological findings
The effects of oral feeding with gamma-linolenic acid-rich lipid from B. officinalis on the course of EAE was investigated in MOG peptide (92–106)-induced disease in SJL mice. In the first experiment, 11 mice were fed from day 7 with the omega-6 fatty acid-rich lipid and eight mice were saline gavaged. The clinical and histological findings for mice inoculated with MOG peptide (92–106) and either omega-6-fed or saline-fed are shown in Table 1 for acute EAE. Clinical signs for acute EAE were observed in 3/11 omega-6-fed mice compared with 7/8 control mice (P < 0·02). The omega-6-fed group had a markedly reduced mean group score (P < 0·01) which reflects the finding that most animals did not develop acute EAE (Table 1). The mean severity score of mice in the control group that displayed EAE was 3·1 (range 2–4) and the mean day of onset was 15·5 ± 0·9. The three mice in the omega-6-fed group that exhibited EAE had a mean severity score of 2·3 (range 1–4) with a mean day of clinical disease onset of 18·6 ± 0·9 (P < 0·002), demonstrating a delay in disease onset but no effect on clinical severity in those omega-6-fed mice that developed clinical disease. Histological signs of CNS perivascular mononuclear leucocyte-infiltrated sites were present in 7/8 mice in the control EAE group compared with 2/11 omega-6-fed mice (P < 0·02) at day 21 when the animals were killed (Table 1). All but one of the control mice with EAE had weight loss (> 2 g) whereas only 5/11 omega-6-fed mice experienced weight loss (P < 0·002).
Table 1.
Effect of oral feeding of omega-6 fatty acids on the clinical and histopathological manifestations of acute EAE in SJL mice
| Controls | Omega-6-fed | |
|---|---|---|
| Clinical incidence | 7/8 | 3/11* |
| No. with CNS lesions | 7/8 | 2/11* |
| Mean group score† | 2·75 ± 1·2 | 0·6 ± 1·2** |
| Mean EAE score‡ | 3·1 ± 0·7 | 2·3 ± 1·5§ |
| Mean day of onset | 15·5 ± 0·9 | 18·6 ± 0·9§*** |
| No. with weight-loss (> 2 g) | 7/8 | 5/11*** |
Mice were gavaged daily with 250–300 μl ultra-refined lipid from Borago officinalis or saline-fed from day 7 onwards after induction of EAE with myelin oligodendrocyte glycoprotein (MOG) peptide (92–106) and histology undertaken at day 21.
Mean group score was calculated from all the animals in the group.
Mean EAE score was calculated from the maximum scores of animals that had clinical signs of EAE.
Only three (n = 3) mice exhibited acute EAE.
P < 0·02
P < 0·01
P < 0·002.
In subsequent experiments undertaken to examine the effects of omega-6 feeding on the disease relapse in EAE (CREAE), two groups of nine mice were allowed to recover from the acute phase of EAE and then fed from day 25 with either omega-6 or saline by gavage (Table 2). The incidence of clinical disease relapse in the omega-6-fed mice was markedly reduced: omega-6-fed mice exhibited 2/9 mice with clinical signs of CREAE compared with 8/9 control CREAE mice (P < 0·02). The significantly lower mean (0·55) group score for the omega-6-fed mice compared with the control CREAE (2·55) reflects the number of animals not exhibiting any clinical disease in the treated group (Table 2). The mean relapse score of animals in the control group exhibiting CREAE was 2·8 (range 1–3·5) and the mean day of onset was 30·7 ± 2·4 compared with the two (n = 2) only omega-6-fed mice who developed clinical disease who had a relapse score of 2·5 and day of onset of 34·5, indicating a delayed onset with no effect on severity (Table 2), a similar finding to that for the omega-6-fed mice that exhibited clinical disease in the acute disease experiments.
Table 2.
Effect of oral feeding of omega-6 fatty acids on the clinical manifestations of relapsing EAE (CREAE) in SJL mice
| Controls | Omega-6-fed | |
|---|---|---|
| Mean acute score† | 3·5 ± 0·5 | 3·4 ± 0·8 |
| No. of relapses/total | 8/9 | 2/9* |
| Mean group relapse score‡ | 2·55 ± 1·4 | 0·55 ± 1·1** |
| Mean relapse score† | 2·8 ± 1·2 | 2·5§ |
| Mean day of relapse onset | 30·7 ± 2·4 | 34·5 ± 3·5§* |
| No. with weight-loss (> 2 g) | 6/9 | 2/9 |
Mice were gavaged daily with 250–300 μl ultra-refined lipid from Borago officinalis or saline-fed from day 25 (after recovery from the acute phase of EAE) onwards.
Mean acute and relapse score was calculated from the maximum scores of animals that had clinical signs of EAE.
Mean group relapse score was calculated from all the animals in the group.
Only two (n = 2) mice exhibited CREAE.
P < 0·02
P < 0·01.
Lymphocyte stimulation
To study the effects of omega-6 feeding on cytokine production and T cell mitogen and antigen responses in EAE, two groups of eight mice were either omega-6-fed or saline-fed and four mice in each group killed at either day 13 or day 21. Clinical data (mice killed at days 13 and 21 combined) for these mice confirmed the previous results, with omega-6-fed mice exhibiting no clinical disease (0/8), whereas all of the control mice (8/8) had clinical signs of EAE (mean severity score day 13 was 1·6, range 1–2·5; mean severity score day 21 was 2·8, range 2–4). Control acute EAE mice had weight loss of >2 g (7/8) but omega-6-fed mice showed no weight loss. Lymphoproliferative responses to Con A, PPD and the encephalitogenic MOG peptide (92–106) after 13 and 21 days for omega-6-fed and control EAE mice are shown in Table 3. Statistically significant changes were not observed between the lymphoproliferative responses of omega-6-fed and control EAE mice at either day 13 or day 21. There was no lymphoproliferative response observed to the control non-encephalitogenic MOG peptide residues 50–64 (Table 3).
Table 3.
Stimulation indices of spleen mononuclear cells from omega-6-fed and control acute EAE in SJL mice at day 13 and day 21
| Day 13 | Day 21 | |||
|---|---|---|---|---|
| Controls | Omega-6-fed | Controls | Omega-6-fed | |
| Con A | 14·6 ± 1·3 | 13·7 ± 1·8 | 19·8 ± 6·8 | 24·5 ± 8·7 |
| MOG (92–106) | 4·7 ± 0·8 | 5·1 ± 2·0 | 5·0 ± 1·1 | 5·5 ± 1·1 |
| MOG (50–64) | < 1·5 | < 1·5 | < 1·5 | < 1·5 |
| PPD | 17·2 ± 10 | 14·4 ± 3·7 | 20 ± 7·3 | 14·2 ± 6·7 |
Mice were immunized with myelin oligodendrocyte glycoprotein (MOG) peptide (92–106) and spleen cells removed at day 13 or day 21 and cultured with 1 μg/ml concanavalin A (Con A), 5 μg/ml MOG peptide (92–106) or 5 μl/ml PPD. Values are mean ±s.d. from n = 4 animals per group. No responses were recorded in the presence of the non-encephalitogenic MOG peptide (50–64). The stimulation index is the mean ct/min of Con A, MOG or PPD-stimulated cultures divided by the mean ct/min without Con A, MOG or PPD. Background ct/min in cultures without Con A, MOG or PPD for controls and fatty acid-feeding experiments were 581 ± 287 and 467 ± 177 ct/min, respectively.
Cytokine and eicosanoid production
IL-2, IFN-γ, IL-4, TGF-β1 and PGE2 were measured in culture supernatants from cells stimulated with the encephalitogenic MOG peptide (92–106), the non-encephalitogenic MOG peptide (50–64), PPD or Con A. At day 13, spleen mononuclear cells from omega-6-fed mice produced significantly more TGF-β1 (P < 0·05) in Con A-stimulated cell culture supernatants compared with control EAE cultures (Table 4). There were no significant differences in TGF-β1 production in MOG peptide (92–106) and PPD-stimulated cell culture supernatants between the omega-6-fed and control EAE groups at day 13. At day 21, TGF-β1 and PGE2 production was significantly higher in Con A, PPD and MOG peptide (92–106)-stimulated cell culture supernatants from omega-6-fed mice compared with controls (P < 0·05 and P < 0·01, respectively (Table 5)).
Table 4.
Cytokines and eicosanoid produced from spleen mononuclear cells in the presence of concanavalin A (Con A), myelin oligodendrocyte glycoprotein (MOG) peptide (92–106) and PPD from omega-6-fed and control acute EAE at day 13
| Controls | Omega-6-fed | ||||||
|---|---|---|---|---|---|---|---|
| Con A | MOG | PPD | Con A | MOG | PPD | ||
| TGF-β1 | (pg/ml) | 226 ± 25 | 234 ± 37 | 247 ± 47 | 377 ± 95† | 280 ± 67 | 302 ± 29 |
| IL-2 | (pg/ml) | 160 ± 48 | 18 ± 0·8 | 57 ± 10 | 138 ± 46 | 18·5 ± 1·5 | 55 ± 17 |
| IFN-γ | (pg/ml) | 1753 ± 636 | 70 ± 35 | 405 ± 99 | 1812 ± 433 | 168 ± 190 | 427 ± 37 |
| IL-4 | (pg/ml) | 21 ± 7·8 | 4·7 ± 1·7 | 16 ± 7·1 | 14 ± 5 | 4 ± 0·9 | 12 ± 6 |
| PGE2 | (pg/ml) | 494 ± 210 | 176 ± 31 | 279 ± 151 | 515 ± 269 | 184 ± 65 | 223 ± 11 |
Cells were stimulated with 1 μg/ml Con A, 5 μg/ml MOG peptide (92–106), or 5 μl/ml PPD for 24 h, and culture supernatants assayed for cytokines and eicosanoid by ELISA. Significance of difference compared with day 13 control EAE
P < 0·05; values are mean ±s.d. from n = 3–4 animals per group.
No detectable cytokines and eicosanoid were observed in response to the non-encephalitogenic MOG peptide (50–64).
Table 5.
Cytokines and eicosanoid produced from spleen mononuclear cells in the presence of concanavalin A (Con A), myelin oligodendrocyte glycoprotein (MOG) peptide (92–106) and PPD from omega-6-fed and control acute EAE at day 21
| Controls | Omega-6-fed | ||||||
|---|---|---|---|---|---|---|---|
| Con A | MOG | PPD | Con A | MOG | PPD | ||
| TGF-β1 | (pg/ml) | 115 ± 32†† | 82 ± 31††† | 42 ± 4†† | 207 ± 22* | 194 ± 28* | 165 ± 23* |
| IL-2 | (pg/ml) | 155 ± 45 | 19 ± 3·8 | 45 ± 23 | 126 ± 40 | 22·5 ± 6 | 64 ± 23 |
| IFN-γ | (pg/ml) | 1156 ± 378 | ND | 275 ± 68 | 1377 ± 364 | 51 ± 37 | 421 ± 218 |
| IL-4 | (pg/ml) | 16 ± 7 | 7 ± 2·6 | 11 ± 4 | 23 ± 7·5 | ND | 14 ± 3 |
| PGE2 | (pg/ml) | 149 ± 59 | 59 ± 15††† | 146 ± 43 | 790 ± 208** | 244 ± 101** | 451 ± 80** |
Cells were stimulated with 1 μg/ml Con A, 5 μg/ml MOG peptide (92–106), or 5 μl/ml PPD for 24 h, and culture supernatants assayed for cytokines and eicosanoid by ELISA. Significance of difference compared with control EAE at day 21
P < 0·05
P < 0·01, and compared with control EAE day 13
P < 0·01
P < 0·001. ND, Not detected. Values are mean ±s.d. from n = 4 animals per group. There were no detectable cytokines or eicosanoid in response to non-encephalitogenic MOG peptide (50–64).
There was no significant difference in IL-2 production between omega-6-fed and control EAE mice in Con A, PPD and MOG peptide (92–106) supernatants at both day 13 and day 21 (Tables 4 and 5). No significant differences between the groups were observed in IFN-γ production for Con A, PPD and MOG peptide (92–106) supernatants at day 13 (Table 4). At day 21 no differences in the Con A- and PPD-stimulated cultures between the groups were observed for IFN-γ production, although production in the control group in MOG peptide (92–106)-stimulated culture supernatants was undetectable. Results obtained for IL-4 production, although generally in the lower range of the detection sensitivities for the assay, suggest a trend at day 13 for Con A- and PPD-stimulated cell cultures from the omega-6-fed group to produce less IL-4. At day 21 there was an apparent reversal of the day 13 findings, which could indicate trafficking of IL-4-producing cells.
Control EAE mice at day 21 had significantly decreased levelsof TGF-β1 in Con A (P < 0·01), MOG peptide 92–106 (P < 0·001) and PPD (P < 0·01) cultures compared with their corresponding day 13 levels. A similar pattern was also observed for PGE2 at day 21 in Con A (3·3-fold), MOG peptide 92–106 (P < 0·001) and PPD (1·9-fold)-stimulated cultures, although the Con A and PPD changes were not statistically significant. No detectable cytokines were observed in response to the non-encephalitogenic MOG peptide (50–64).
Membrane phospholipid fatty acids
The fatty acid composition of spleen cell membranes from omega-6-fed and control EAE mice at day 21 are summarized in Table 6. Mice fed the gamma-linolenic acid-rich lipid had significant increases in the proportion of dihomo-γ- linolenic acid (20:3n-6) (P < 0·02) and arachidonic acid (20:4n-6) (P < 0·05) compared with control EAE mice. There was also a significant increase (P < 0·05) in the 20:3n-6/20:4n-6 ratio in the omega-6-fed animals. These metabolic changes indicate that gamma-linolenic acid, which bypasses the rate-limiting Δ-6 desaturase, is rapidly converted to dihomo-γ- linolenic and arachidonic acids. Furthermore, the increase in the dihomo-γ-linolenic acid to arachidonic acid ratio and the lack of change in very long chain omega-6 fatty acids (data not shown) suggests that Δ-5 desaturase is also rate-limited and that retroconversion may be taking place. These metabolic changes are consistent with those reported by us previously [9,10].
Table 6.
Spleen cell membrane phospholipid fatty acids from omega-6-fed and control acute EAE at day 21
| Fatty acids | Controls | Omega-6-fed | |
|---|---|---|---|
| 18:2n-6 | (Linoleic) | 3·7 ± 0·75 | 4·7 ± 0·9 |
| 18:3n-6 | (Gamma-linolenic) | 0·6 ± 0·24 | 0·4 ± 0·02 |
| 20:2n-6 | 0·5 ± 0·15 | 0·6 ± 0·12 | |
| 20:3n-6 | (Dihomo-γ-linolenic acid) | 0·79 ± 0·07 | 1·8 ± 0·5** |
| 20:4n-6 | (Arachidonic) | 6·3 ± 1·4 | 10·4 ± 1·7* |
| 20:3n-6/20:4n-6 | 0·12 ± 0·02 | 0·18 ± 0·01* |
Spleen cell membrane fatty acid analysis in omega-6 and saline-fed myelin oligodendrocyte glycoprotein (MOG) peptide-induced (residues 92–106) acute EAE in SJL mice at day 21. Results are expressed as mean percent composition ±s.d., n = 4 animals per group. Omega-6-fed animals were gavaged daily with 250–300 μl ultra-refined Borago officinalis from day 7 onwards. Significance of difference
P < 0·05
P < 0·02 compared with control EAE group.
TGF-β1 cytokine gene expression
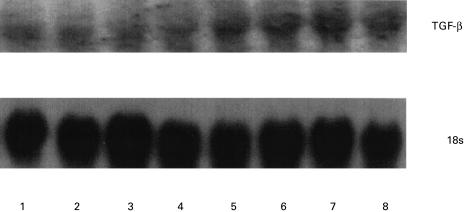
Total RNA was extracted from spleens of four omega-6-fed and four control EAE mice at day 21 and were hybridized with a TGF-β cDNA probe. This revealed a higher expression of TGF-β in the spleens of omega-6-fed animals compared with the control EAE group (Fig. 1). The density of each of the autoradiographic bands was semiquantified by scanning using a laser densitometer and normalized to that of 18s and expressed as a ratio. Expression of TGF-β was significantly (P < 0·02) higher in the omega-6-fed group compared with the control EAE group (Table 7). The differences observed in the level of TGF-β expression were not due to variation in RNA loading, as confirmed by the 18s levels.
Fig. 1.
Effect of omega-6 feeding on spleen TGF-β mRNA expression in EAE. Northern blots were prepared from 20 μg of total RNA from spleens of omega-6-fed or control EAE mice. Total RNA was electrophoresed, electroblotted, prehybridized, hybridized and autoradiographed as described in Materials and methods. Lanes 1–4, Control EAE; lanes 5–8, omega-6 fatty acid-fed EAE mice.
Table 7.
Densitometric analysis of Northern blot
| Controls | Omega-6-fed | |
|---|---|---|
| TGF-β | 0·353 ± 0·124 | 0·828 ± 0·178* |
Spleen cell TGF-β mRNA expression in omega-6- and saline-fed myelin oligodendrocyte glycoprotein (MOG) peptide-induced (residues 92–106) acute EAE in SJL mice at day 21. The autoradiographic bands in Fig. 1 are semiquantified by laser densitometer (Molecular Dynamics, Sunnyvale, CA). Values are mean ±s.d. from n = 4 animals per group and are represented as ratio of TGF-β expression to that of 18s. Significance of difference
P < 0·02 compared with control EAE.
DISCUSSION
Oral administration of plant lipid rich in the omega-6 fatty acid gamma-linolenic acid protects SJL mice against the clinical and histological manifestations of MOG peptide (92–106)-induced acute EAE when fed daily from 7 days after disease induction. Moreover, when fed after recovery from acute EAE the treatment protected against disease relapse (CREAE), demonstrating that these fatty acids can alter the progression of an established and ongoing autoimmune disease. There was a non-specific increase on activation with the encephalitogenic MOG peptide (92–106) (but not the non-encephalitogenic MOG peptide residues 50–64), Con A and PPD in the production of TGF-β1 and PGE2 from spleen mononuclear cells of gamma-linolenic acid-fed mice compared with the control EAE mice ex vivo. No effect on the production of IL-2 and IFN-γ was observed. Furthermore, there was increased membrane eicosanoid (PGE1 and PGE2) precursor omega-6 fatty acids and higher TGF-β mRNA expression in spleen cells of gamma-linolenic acid-fed mice, indicating a direct effect of the fatty acids or their metabolites on gene transcription. The earlier (day 13) increase in Con A TGF-β1 production, but not PGE2, further suggests it is the fatty acids that affect gene transcription. In contrast to the precursor product relationship between omega-6 fatty acids and their oxygenated metabolites the PGEs, which explain our findings of increased PGE2, it is not known how TGF-β1 is increased by omega-6 fatty acid feeding. TGF-β1 is an early response gene [21], and there is evidence that the omega-6 fatty acid arachidonic acid can induce early response genes [22] and may therefore be an important regulating signal for TGF-β1. The decrease in PGE2 and TGF-β1 in the spleen cells of the control EAE group at day 21, when the animals were recovering, may reflect cell trafficking to the CNS and involvement of PGE2- and TGF-β1-producing cells in the natural recovery from EAE. It could be argued that the apparent increase in PGE2 and TGF-β1 in the gamma-linolenic acid-fed group compared with untreated EAE is simply the consequence of a decrease in these molecules due to cell trafficking in the latter, or that the fatty acid treatment affects the ability of cells to recirculate. However, because there was higher TGF-β mRNA expression and increased membrane eicosanoid precursor omega-6 fatty acids in the gamma-linolenic acid-fed group, the effect of gamma-linolenic acid feeding is the most likely explanation for the increased production of PGE2 and TGF-β1 by spleen cells ex vivo. This is also supported by similar findings in human volunteer studies [16]. Although the cell source of PGE2 is probably monocytes [23] activated by T cells, we have not identified the TGF-β1-producing cell type in this study—it could be either T cells or monocytes or both. Lymphoproliferative responses to Con A, MOG peptide (92–106) and PPD were unaltered between gamma-linolenic acid-fed and control EAE, indicating no gross immunosuppression by the fatty acid treatment. However, in some experiments we have observed a significantly lower MOG peptide (92–106) lymphoproliferative and IL-2 response, with no effect on Con A and PPD by omega-6 feeding in MOG peptide (92–106)-induced EAE [24]. This suggests that the level of PGE2 and TGF-β1 produced by omega-6 fatty acid feeding has a low threshold for suppression and will only have suppressional effects on a weakly lymphoproliferative response, such as that induced by MOG peptide (92–106), but not stronger Con A and PPD lymphoproliferative responses. Studies in vitro have shown that adding omega-6 fatty acids directly to mononuclear cells suppress proliferative responses to mitogens [25,26]. Although PGEs can suppress lymphoproliferation, eicosanoid-independent mechanisms for the inhibitory effects of their precursor polyunsaturated fatty acids have been shown [ 27–29]. It is possible that high level TGF-β1 induction may be behind this observation. The disparity in our results, compared with in vitro findings, is most likely due to their ex vivo nature, therefore cellular membrane fatty acid balance, free and bound (esterified) fatty acid concentration, level of unsaturation, fatty acid compartmentalization and antioxidant status will be different [27,30].
Our findings in EAE are consistent with reports that neutralization of TGF-β1 with specific antibodies enhances EAE, indicating that this cytokine is involved in the natural regulation of EAE [31] and administration of TGF-β1 protects in acute and relapsing EAE [32,33]. In addition, TGF-β knock-out mice survive for only a few weeks before widespread activation of autoimmune T cells leads to death [34] and targeting TGF-β gene deletion to T cells has a similar effect, demonstrating that T cell homeostasis requires TGF-β signalling [35]. Prostaglandin inhibitors such as indomethacin augment EAE [36] and prostaglandins of the E series have potent effects on cell-mediated immunity and EAE [37]. Additionally during the natural recovery phase from EAE, TGF-β-secreting T cells can inhibit EAE effector cells, TGF-β is expressed in the CNS and, in oral tolerance-induced protection in EAE, TGF-β and PGE2 are expressed in the brain [38–40]. TGF-β has also been shown to suppress the activation and proliferation of microglia which have important inflammatory and immunoregulatory functions within the CNS [41]. Our preliminary immunohistochemical findings also indicate that the gamma-linolenic acid-fed mice in this study have increased TGF-β1 staining in the CNS. Adoptive transfer experiments have shown EAE to be a T cell-mediated autoimmune disease in which Th1-type cytokine (IL-2 and IFN-γ)-producing cells cause pathology [42]. Feeding gamma-linolenic acid can increase PGE2 (as shown here) and PGE1 production [11] and PGEs can inhibit the production of Th1 but not Th2 cytokines in vitro [12]. On this basis we could propose that dietary gamma-linolenic acid prevents T cell-mediated autoimmune disease by altering the balance of cytokines from a Th1 to a Th2-type response. However, our data suggest that the effects of dietary gamma-linolenic acid on MOG peptide (92–106)-induced EAE are mediated through PGE2 and TGF-β1-producing cells or Th3-like mechanisms. It is not clear if PGE2 or its precursor fatty acids (or another metabolite of these acids) can induce TGF-β1 directly or vice versa, and whether they act individually or synergistically in EAE. Fernandes et al. [43] reported that feeding long chain omega-3 fatty acids to autoimmune disease in lupus-prone NZB/NZW mice, which delays the onset and progression of the disease, is associated with increased TGF-β1 mRNA expression in the spleen. It is possible that one of the common mechanisms by which the long chain omega-6 and omega-3 fatty acids could regulate autoimmune disease is via an effect on TGF-β1. Vidard et al. [44] suggested from studies of T cell tolerance and site-specific lymphokine profiles that the type of Th cytokine subset pattern produced depends on the environment they are found in. Pond [45] has shown that adipose tissues around lymph nodes preferentially incorporate or selectively retain polyunsaturated fatty acids. This could therefore be a significant mechanism by which local fatty acids affect Th subset cytokines. In view of our findings and the observation that under conditions of omega-6 fatty acid deficiency EAE is severely potentiated [46,47], omega-6 fatty acids may be important in maintaining the functional integrity of the immune system in relation to T cell-mediated autoimmunity.
The decreased omega-6 fatty acids in cellular membranes of blood cells in MS patients [48–51] may have important implications for immune regulation in MS. Interestingly, several reports indicate lower TGF-β during the MS relapse and conversely, higher levels during remission as well as correlation between magnetic resonance imaging disease activity and TGF-β1, suggesting it may be an important endogenous remission agent in MS [52–55]. Omega-6 fatty acid supplementation in MS could therefore have a physiological and/or pharmacological basis. We are currently undertaking phase I/II clinical trials in MS with specific omega-6 fatty acids and investigating further how these fatty acids may exert effects on the immune response in EAE and MS.
Acknowledgments
The authors would like to thank the Henry Smith Charity and the Multiple Sclerosis Society of Great Britain and Northern Ireland for financial support of this research. L.S.H. would also like to thank Mr William Jefferson for help and advice with the Northern blots.
REFERENCES
- 1.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399(Suppl. 24):A40–A46. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 2.Brosnan C, Raine C. Mechanisms of immune injury in multiple sclerosis. Brain Pathol. 1996;6:243–57. doi: 10.1111/j.1750-3639.1996.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakai K, Zamvil GS, Mitchell JD, et al. Characterisation of a major T cell epitope in SJL/J mice with synthetic oligopeptides of myelin basic protein. J Neuroimmunol. 1988;19:21–32. doi: 10.1016/0165-5728(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 4.Amor S, Baker D, Groome N, et al. Identification of major encephalitogenic epitope (residues 56–70) of proteolipid protein for the induction of experimental allergic encephalomyelitis in Biozzi AB/H and non-obese diabetic mice. J Immunol. 1993;150:5666–72. [PubMed] [Google Scholar]
- 5.Amor S, Groome N, Linnington C, et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994;153:4349–56. [PubMed] [Google Scholar]
- 6.Dworkin RH, Bates D, Millar JHD, et al. Linoleic acid and multiple sclerosis: a reanalysis of three double blind trials. Neurology. 1984;34:1441–5. doi: 10.1212/wnl.34.11.1441. [DOI] [PubMed] [Google Scholar]
- 7.Meade CJ, Mertin J, Sheena J, et al. Reduction by linoleic acid of the severity of experimental allergic encephalomyelitis in the guinea-pig. J Neuro Sci. 1978;35:291–308. doi: 10.1016/0022-510x(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 8.Hughes D, Keith AB, Mertin J, et al. Linoleic acid therapy in severe experimental allergic encephalomyelitis in the guinea pig. Clin Exp Immunol. 1980;41:523–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Phylactos AC, Harbige LS, Crawford MA. Essential fatty acids alter the activity of manganese superoxide dismutase in rat heart. Lipids. 1994;29:111–5. doi: 10.1007/BF02537150. [DOI] [PubMed] [Google Scholar]
- 10.Harbige LS, Yeatman N, Amor S, et al. Prevention of experimental autoimmune encephalomyelitis in Lewis rats by a novel fungal source of γ-linolenic acid. Br J Nutr. 1995;74:701–15. doi: 10.1079/bjn19950173. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Chapkin RS. Mouse peritoneal macrophage prostaglandin E1 synthesis is altered by dietary gamma-linolenic acid. J Nutr. 1992;122:1600–6. doi: 10.1093/jn/122.8.1600. [DOI] [PubMed] [Google Scholar]
- 12.Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–52. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, An S, Smith WL. Specificity of expression and effects of eicosanoid mediators in normal physiology and human disease. FASEB J. 1995;9:1051–8. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 14.Harbige LS, Layward L, Morris M, et al. Cytokine secretion by human T cell clones is differentially regulated by eicosanoids. Trans Biochem Soc. 1997;25:347S. doi: 10.1042/bst025347s. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Yssel H, Harada Y, et al. Effects of prostaglandin E2 on Th0-type human T cell clones: modulation of functions of nuclear proteins involved in cytokine production. Int Immunol. 1994;6:523–32. doi: 10.1093/intimm/6.4.523. [DOI] [PubMed] [Google Scholar]
- 16.Fisher BAC, Harbige LS. Effect of omega-6 lipid-rich borage oil feeding on immune function in healthy volunteers. Trans Biochem Soc. 1997;25:343S. doi: 10.1042/bst025343s. [DOI] [PubMed] [Google Scholar]
- 17.Mertin J, Stackpoole A. Suppression by essential fatty acids of experimental allergic encephalomyelitis is abolished by indomethacin. Prostaglandins Med. 1978;1:283–91. doi: 10.1016/0161-4630(78)90047-2. [DOI] [PubMed] [Google Scholar]
- 18.Mertin J, Stackpoole A. The spleen is required for the suppression of experimental allergic encephalomyelitis by prostaglandin precursors. Clin Exp Immunol. 1979;36:449–55. [PMC free article] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animals tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Feinberg AP, Vogelstein B. Addendum: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–7. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 21.Martin P, Dickson MC, Millan FA, et al. Rapid induction and clearance of TGFβ1 is an early response to wounding in the mouse embryo. Dev Genet. 1993;14:225–38. doi: 10.1002/dvg.1020140309. [DOI] [PubMed] [Google Scholar]
- 22.Sellmayer A, Weber PC, Bonventre JV. Modulation of cell growth and expression of early response genes by endogenous arachidonic acid metabolites. In: Sinclair A, Gibson R, editors. Essential fatty acids and eicosanoids. Third International Congress. Champaign, IL: American Oil Chemists Society; 1993. pp. 450–5. [Google Scholar]
- 23.Goldyne ME, Stobo JD. Immunoregulatory role of prostaglandins and related lipids. CRC Crit Rev Immunol. 1981;2:189–223. [Google Scholar]
- 24.Harbige LS, Layward L, Amor S, et al. Immunomodulatory effects of linoleic and gamma-linolenic acid-rich oil in experimental autoimmune encephalomyelitis. Immunology. 1994;83(Suppl. 1):55. [Google Scholar]
- 25.Mertin J, Huges D. Specific inhibitory action of polyunsaturated fatty acids on lymphocyte transformation induced by PHA and PPD. Inter Archs Allergy Appl Immunol. 1975;48:203–10. doi: 10.1159/000231306. [DOI] [PubMed] [Google Scholar]
- 26.Calder PC. Fatty acids, dietary lipids and lymphocyte functions. Trans Biochem Soc. 1995;23:302–9. doi: 10.1042/bst0230302. [DOI] [PubMed] [Google Scholar]
- 27.Calder PC. Dietary fatty acids and lymphocyte functions. Proc Nutr Soc. 1998;57:487–502. doi: 10.1079/pns19980073. [DOI] [PubMed] [Google Scholar]
- 28.Santoli D, Zurier RB. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol. 1989;143:1303–9. [PubMed] [Google Scholar]
- 29.Calder PC, Bevans SJ, Newsholme EA. The inhibition of T lymphocyte proliferation by fatty acids is via an eicosanoid independent mechanism. Immunology. 1992;75:108–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Harbige LS. Dietary n-6 and n-3 fatty acids in immunity and autoimmune disease. Proc Nutr Soc. 1998;57:555–62. doi: 10.1079/pns19980081. [DOI] [PubMed] [Google Scholar]
- 31.Racke MK, Cannella B, Albert P, et al. Evidence of endogenous regulatory function of transforming growth factor-β1 in experimental allergic encephalomyelitis. Int Immunol. 1992;4:615–20. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- 32.Racke MK, Dhib-Jalbut S, Cannella B, et al. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-β1. J Immunol. 1991;37:75–84. [PubMed] [Google Scholar]
- 33.Santambrogio L, Hochwald GM, Saxena B, et al. Studies on the mechanisms by which transforming growth factor-β protects against allergic encephalomyelitis. J Immunol. 1993;151:1116–27. [PubMed] [Google Scholar]
- 34.Schull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;395:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L, Flavell RA. Abrogation of TGFβ signalling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 36.Ovadia H, Paterson PY. Effect of indomethecin treatment upon actively induced and transferred experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol. 1982;49:386–92. [PMC free article] [PubMed] [Google Scholar]
- 37.Mertin J, Stackpoole A, Shumay SJ. Nutrition and immunity: the immunoregulatory effect of n-6 essential fatty acids is mediated through prostaglandin E. Int Archs Allergy Appl Immunol. 1985;77:390–5. doi: 10.1159/000233814. [DOI] [PubMed] [Google Scholar]
- 38.Karpus WJ, Swanborg RH. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-β. J Immunol. 1991;146:1163–8. [PubMed] [Google Scholar]
- 39.Issazadeh S, Mustafa M, Ljungdahl A, et al. Interferon gamma, interleukin 4 and transforming growth factor β in experimental autoimmune encephalomyelitis in Lewis rats: dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J Neurosci Res. 1995;40:579–90. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- 40.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to the myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzumura A, Sawada M, Yamamoto H, et al. Transforming growth factor-β suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–8. [PubMed] [Google Scholar]
- 42.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes G, Bysani C, Venkatraman JT, et al. Increased TGFβ and decreased oncogene expression by ω-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J Immunol. 1994;152:5979–87. [PubMed] [Google Scholar]
- 44.Vidard L, Colarusso LJ, Benacerraf B. Specific T-cell tolerance may reflect selective activation of lymphokine synthesis. Proc Natl Acad Sci USA. 1995;92:2259–62. doi: 10.1073/pnas.92.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pond CM. Interactions between adipose tissue and the immune system. Proc Nutr Soc. 1996;55:111–26. doi: 10.1079/pns19960014. [DOI] [PubMed] [Google Scholar]
- 46.Clausen J, Moller J. Allergic encephalomyelitis induced by brain antigen after deficiency in polyunsaturated fatty acids during myelination. Acta Neurol Scand. 1967;43:375–88. doi: 10.1111/j.1600-0404.1967.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 47.Selivonchick DP, Johnston PV. Fat deficiency in rats during development of the central nervous system and susceptibility to experimental allergic encephalomyelitis. J Nutr. 1975;105:288–300. doi: 10.1093/jn/105.3.288. [DOI] [PubMed] [Google Scholar]
- 48.Gul S, Smith AD, Thompson RHS, et al. Fatty acid composition of phospholipids from platelets and erythrocytes in multiple sclerosis. J Neurol Neurosurg Psychiat. 1970;33:506–10. doi: 10.1136/jnnp.33.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Homa ST, Belin J, Smith AD, et al. Levels of linoleate and arachidonate in red blood cells of healthy individuals and patients with multiple sclerosis. J Neurol Neurosurg Psychiat. 1980;43:106–10. doi: 10.1136/jnnp.43.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher M, Johnson MH, Natale AM, et al. Linoleic acid levels in white blood cells, platelets and serum of multiple sclerosis patients. Acta Neurol Scand. 1987;76:241–5. doi: 10.1111/j.1600-0404.1987.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 51.Navarro X, Segura R. Red blood cell fatty acids in multiple sclerosis. Acta Neurol Scand. 1989;79:32–37. doi: 10.1111/j.1600-0404.1989.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 52.Beck J, Rondot P, Jullien P, et al. TGF-β-like activity produced during regression of exacerbations in multiple sclerosis. Acta Neurol Scand. 84:452–5. doi: 10.1111/j.1600-0404.1991.tb04988.x. [DOI] [PubMed] [Google Scholar]
- 53.Rieckmann P, Albrecht M, Kitze B, et al. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology. 1994;44:1523–6. doi: 10.1212/wnl.44.8.1523. [DOI] [PubMed] [Google Scholar]
- 54.Mokhtarian F, Shi Y, Shirazian D, et al. Defective production of anti-inflammatory cytokine, TGF-β by T cell lines of patients with active multiple sclerosis. J Immunol. 1994;152:6003–10. [PubMed] [Google Scholar]
- 55.Bertolotto A, Capobianco M, Malucchi S, et al. Transforming growth factor β1 (TGF-β1) mRNA level correlates with magnetic resonance imaging disease activity in multiple sclerosis patients. Neuroscience Lett. 1999;263:21–24. doi: 10.1016/s0304-3940(99)00102-0. [DOI] [PubMed] [Google Scholar]