Abstract
Two monoclonal antibodies (1H6.2 and 45.30) were raised against MBP purified from human brain under experimental conditions that allowed MBP to retain binding to surrounding myelin lipids (human lipid-bound MBP (hLB-MBP)). 1H6.2 and 45.30 MoAbs were selected on the basis of their different binding properties to: hLB-MBP, human lipid-free-MBP (hLF-MBP) and bovine lipid-free-MBP (bLF-MBP). Although the isotype of both MoAbs was IgM, their specificity, as tested in ELISA assays against chemical haptens and unrelated protein antigens, was restricted to MBP. 1H6.2 and 45.30 MoAbs stained MBP from human brain white matter tissue extracts, as well as bLF-MBP, in Western blot assays. Both MoAbs stained oligodendrocytes and myelin in immunohistochemical analysis of white matter from human brain. Tissue sections from human peripheral nerves were labelled by 1H6.2 only, however, demonstrating that the MoAbs recognize two different epitopes. Epitopes recognized by 1H6.2 and 45.30 MoAbs were also expressed by a wide array of human non-neural cells of either normal or pathological origin, as evidenced by cytofluorimetric assays. In particular, MBP epitopes (MEs) were expressed by lymphoid cells as well as by cells which play a pivotal role in immune homeostasis and in the immune response, such as thymic epithelial cells and professional antigen-presenting cells. Both MoAbs were efficiently internalized by cells from a human B cell line, suggesting trafficking of MEs along the endocytic pathways. These findings support hypotheses regarding the role of MEs expressed by non-neural cells in establishing self-tolerance and/or in triggering the immune response against MBP antigen.
Keywords: lipid-bound MBP, monoclonal antibodies, MBP epitopes, multiple sclerosis
INTRODUCTION
MBP is recognized as playing a role in autoimmune diseases such as multiple sclerosis (MS) [1], and its clinical potential as an autoantigen has been well established in animal models of allergic encephalomyelitis (EAE) and relapsing EAE (r-EAE) [1,2].
The MBP gene locus, also termed Golli-MBP, consists of a 105-kb DNA region which includes at least 11 exons with multiple transcriptional start sites [3], and mRNAs transcribed from the MBP gene have been detected in the brain and immune system [3–5]. These novel mRNAs were initially identified in bone marrow and were predicted to generate two new families of MBP proteins termed HMBPR (haematopoietic-MBP-related proteins) and MBP2. Antisera raised against the deduced amino acid sequence of HMBPR1 revealed the presence of multiple proteins in a range of tissue extracts from mice, including the brain and lymphoid tissues [6]. Overall, these findings demonstrate that MBP is not sequestered behind the blood–brain barrier, and also raise several questions regarding the role of these MBP proteins in self-tolerance and in the initiation of immune-associated demyelinating diseases. It remains to be elucidated in detail however, which non-neural cells can express MBP proteins or MBP epitopes (MEs) in humans and their specific pathogenic role.
MoAbs were generated using, as the antigen, a form of MBP extracted from human brain under experimental procedures which allowed the protein to be isolated bound to surrounding lipids (hLB-MBP) [7,8]. hLB-MBP appears to have greater similarity conformationally to native MBP, i.e. MBP expressed in myelin membranes, than MBP extracted using conventional procedures and freed of lipids (hLF-MBP [7]). LB-MBP is encephalitogenic in Lewis rats and its immunogenic activity is exerted at a concentration which is nearly two orders of magnitude lower than that of MBP in the lipid-free soluble form [9–11]. Two new IgM MoAbs (1H6.2 and 45.30) were selected for their different binding properties to hLB-, hLF- and bovine lipid-free-MBP (bLF-MBP).
Cytoplasmic and cell surface expression of MEs identified by the two MoAbs 1H6.2 and 45.30, as well as trafficking of MEs along endocytic pathways, has been investigated by cytofluorimetry using a wide array of non-neural cells including lymphoid and non-lymphoid cells, and in particular cells involved in the regulation of the immune response. Among these, thymic epithelial cells (TEC) and peripheral-blood dendritic cells (DC) are well known to play a pivotal role in the shaping of tolerance towards self-antigens [12,13]. Other non-lymphoid cells, such as non-professional antigen-presenting cells (APC), might interfere with the maintenance of peripheral tolerance via inducible expression of MHC class II molecules. Endothelial cells and CFPAC-1, a human pancreatic adenocarcinoma cell line, were chosen as examples of non-professional APC [14,15]. It should be considered however, that virtually all cells can interfere with self-tolerance by mechanisms of cross-tolerance or cross-priming [16,17]. We found that MBP epitopes were expressed by all the above cells, although to different extents. Moreover, both 1H6.2 and 45.30 MoAbs were rapidly internalized by human B lymphoma cells (Raji) which are known to behave as APC [18].
Taken together, our results demonstrate the expression of MEs in non-neural tissues and, in particular, in cells of fundamental importance for the homeostasis of the immune system, with implications for the triggering and modulation of an autoimmune response against neural tissues.
MATERIALS AND METHODS
Animals, cell lines and control hybridomas
BALB/c mice from our own breeding facilities were used. Raji (human B cell lymphoma), MOLT-4 (human T cell laeukemia) and Ag8 (non-secreting mouse myeloma) were purchased from the American Type Culture Collection (Rockville, MD). CFPAC-1 (human pancreatic adenocarcinoma) cells were a kind gift of Professor N. R. Lemoine (Imperial Cancer Research Funds, Oncology Unit, RPMS, Hammersmith Hospital, London, UK).
Epstein–Barr virus (EBV)-transformed human B cells were generated in our laboratory as follows. B lymphocytes from healthy human donors were separated from peripheral blood. EBV was obtained from the supernatant of confluent cultures of marmoset infected cells (B95-8) by filtration through 0·45-μm porous filters. One millilitre of these virus-enriched samples was added to 5–10 × 106 cells and the cells were incubated overnight at 37°C. EBV-transformed B cells were then selected on the basis of their continuous proliferation in tissue culture flasks.
All cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 40 mg/l folic acid and grown in a humidified 5% CO2 atmosphere.
Cell preparations
Monocyte-derived human DC were obtained from the peripheral blood of donors following described procedures [19]. Peripheral blood mononuclear cells (PBMC) were separated on an isopycnic gradient of Ficoll. PBMC were washed twice with RPMI medium and incubated with neuraminidase-treated sheep erythrocytes for 2 h at 37°C to remove T lymphocytes. Cells were then plated in Petri dishes and incubated for a further 2 h at 37°C. At this point, non-adherent cells contained mostly B lymphocytes which were removed by gentle washing with RPMI. Adherent cells were then incubated overnight at 37°C. After overnight incubation, the cells in the supernatant were stimulated by adding 10 μg/ml IL-4 and 0·5 mg/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and incubated for 1 week to obtain mature DC cells. The adherent cells, on the other hand, formed a population enriched in macrophages. At all stages during DC preparation, the cell phenotype of each cell population was monitored by cytofluorimetric assays using MoAbs directed towards standard markers (i.e. CD3, CD19, CD14, CD1, DR, CD83).
Thymic epithelial cells were obtained from normal human thymus explants following the procedure described by Riviera et al. [20]. Briefly, thymic specimens were minced into small pieces and treated with a 0·05% trypsin and 0·01% EDTA solution for 90 min at 37°C. The resultant cell suspension was plated onto lethally irradiated 3T3-J2 cells (a kind gift of Professor H. Green, Harvard Medical School, Boston, MA) in 2:1 Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 medium supplemented with 10% FBS, 5 μg/ml insulin, 5 μg/ml transferrin, 0·18 mm adenine, 0·4 μg/ml hydrocortisone, 0·1 nm cholera toxin, 20 pm triiodothyronine, 10 ng/ml epidermal growth factor, 4 mm glutamine and 50 U/ml penicillin–streptomycin. The epithelial nature of the primary cultures was monitored by immunostaining with a monoclonal anti-keratin antibody (Becton Dickinson, Bedford, MA).
Human endothelial cells from umbilical veins were prepared following standard procedures [21] by collagenase digestion of human specimens. Human fibroblasts from primary explants were a kind gift of Dr G. Moretto (Division of Neurology, Hospital of Belluno, Italy) and were prepared following previously described procedures [22].
Mice hyperimmunization and production of MoAbs
hLB-MBP was purified from human brain following previously described procedures [7,8]. Purified hLB-MBP (500 μg) was emulsified in Freund's complete adjuvant (FCA) and injected intraperitoneally into 8–10-week-old mice. At 2-week intervals, mice were injected subcutaneously and into footpad with either 500 μg hLB-MBP in FCA or 50 μg hLB-MBP in Freund's incomplete adjuvant. The last immunization with 10 μg hLB-MBP was administered intravenously 4 days before the animals were killed and their spleens collected. Splenocytes were plated in 24-well culture plates and incubated at a 10:1 ratio with Ag8 cells in the presence of 50% (w/v) polyethylene glycol (PEG) 1500. Hybridomas were selected in HAT minimal medium and the supernatants were screened by ELISA to test their different reactivities against hLF- or hLB-MBP. Positive wells containing hybridomas (6/48 wells) were harvested and cells cloned at three cells/well in 96-well culture plates filled with HAT medium supplemented with 20% FBS. Supernatants were further screened for their different reactivities against the two MBP forms (number of positive wells: 174/768). Hybridoma-secreting anti-MBP antibodies were further cloned at 0·3 cells/well in HAT + FBS medium and screened once again against hLF- and hLB-MBP (number of positive wells: 18/288). Finally, the cells were expanded in HT + FBS medium and then in RPMI +FBS medium.
MoAbs were purified from culture supernatants by (NH4)2SO4 precipitation followed by ion-exchange chromatography onto DEAE columns. The purity of different preparations was assessed by size-exclusion fast performance liquid chromatography (FPLC). The isotype of selected antibodies was determined using commercially available kits following the manufacturer's procedures (IsoStrip mouse monoclonal antibody isotyping kit; Roche Diagnostic GmbH, Mannheim, Germany).
Control IgM MoAbs were purified using the above protocol from the supernatant of SP6 hybridomas (specific for 2,4,6-trinitrobenzene sulphonic acid (TNP)) and S13.11 hybridoma (anti-H2Kd).
ELISA
The following antigens were used in ELISA: hLB-MBP, hLF-MBP, bLF-MBP, bovine serum albumin (BSA), keyhole limpet haemocyanin (KLH), ovalbumin (OVA), lysozyme (Ly) and human transferrin (Tfn). Proteins were purchased from Sigma except for hLB- and hLF-MBP. Haptens were coupled to BSA using spontaneously coupling derivatives of TNP and FITC following conventional procedures (see also below). Proteins and haptenized proteins were coated onto microtitre plates (Nunc-Immuno plates, Life Technologies, Paisley, UK) at 20 μg/ml in 50 mm carbonate buffer pH 9·6 at 4°C for 12 h. Bound MoAbs were detected with goat anti-mouse alkaline phophatase-conjugated antibodies for 30 min at room temperature followed by the addition of p‐nitrophenyl phosphate (PNPP) substrate. The enzyme activity was assayed using a Titertek Multiscan Spectrophotometer (ICN Biomedicals, Milan, Italy).
Flow cytometry
Cell surface and intracellular staining
Cell surface and intracellular staining were carried out by indirect immuno‐fluorescence. Cell-associated fluorescence was analysed by flow cytometry using an Epics XL cytofluorimeter (Coulter, Hialeah, FL). For cell surface staining, 15 μg/ml (16 nm) of 1H6.2 or 45.30 MoAbs were added to 5 × 105−106 cells in 1 ml RPMI. Cells were incubated on ice for 1 h and bound MoAbs were revealed using a fluoresceinated goat anti-mouse antibody. For each sample, 10 000 events were acquired. Gating was performed on the basis of the light scattering properties of the cells and set for viable cells. For intracellular staining, 3 × 105 cells were washed once with PBS and 1 ml of 70% chilled MetOH was added to the pellets under mild vortexing conditions to permeabilize the cell membranes. Cells were then incubated on ice for 1 h, washed three times with PBS + 5% FBS and subjected to the staining procedure described above. Controls were obtained by incubating cells with either the secondary antibody only or irrelevant IgM MoAbs (S13.11 and/or SP6).
Binding and internalization assays
The two MoAbs 1H6.2 and 45.30 were labelled with FITC following procedures described elsewhere [23]. The FITC/protein ratio of the conjugates was determined spectrophotometrically and was approximately 3 μg/mg for both 1H6.2–FITC and 45.30–FITC.
To test whether the fluoresceination procedure could have altered the binding specificities of the two MoAbs, binding experiments were carried out using EBV-transformed B lymphocytes. Pellets of 106 cells were resuspended in 0·1 ml PBS containing different amounts of 1H6.2–FITC or 45.30–FITC in the presence or absence of a 100-fold excess of unlabelled MoAb. Cells were incubated on ice in the dark for 1 h, washed twice with cold PBS and cell surface-bound MoAb–FITC were estimated by cytofluorimetry.
Internalization assays were carried out by incubating Raji cells at 37°C with 0·03 mg/ml 1H6.2–FITC, 45.30–FITC or S13.11–FITC in the presence of 200 μm leupeptin (Sigma). Leupeptin was used to block possible proteolysis of FITC-labelled MoAbs which would result in an increase in fluorescence emission [23]. To prevent MoAb–FITC intracellular degradation, leupeptin was used at a concentration that does not alter receptor-mediated endocytosis [23]. At the indicated time points in Fig. 6, cells were placed on ice to block internalization, and all samples were post-treated for 30 min with 50 μm monensin and 50 μl of an anti-FITC MoAb solution (clone FL-D6; Sigma) in a final volume of 500 μl cold medium. Cells were then analysed using a cytofluorimeter. Cells were post-treated with monensin to neutralize the acid-pH-mediated quenching of fluorescence emission that could occur during internalization into endocytic vesicles [23]. Treatment with 1:100 solution of anti-FITC MoAb quenched 95% of cell surface-associated fluorescence, allowing the measurement of internalized MoAbs only. Binding of MoAb–FITC at the cell surface at 37°C could also be estimated in the same assays by subtracting mean fluorescence intensity (MFI) values measured in the presence of anti-FITC MoAb (i.e. fluorescence intensity due to internalized molecules only) from the total MFI values (i.e. fluorescence intensity due to internalized + surface-associated molecules).
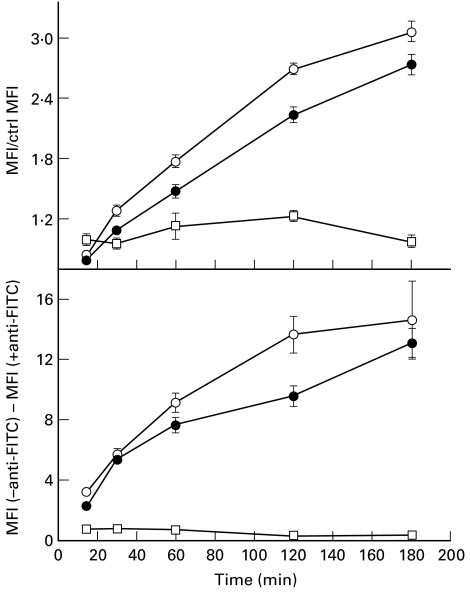
Fig. 6.
Internalization (top panel) and binding (bottom panel) kinetics at 37°C of 1H6–FITC (○) and 45.30–FITC (•) in Raji cells. Top panel: data are expressed as the ratio between values of mean fluorescence intensity (MFI) measured for samples incubated at 37°C for the indicated time periods and control samples kept at 4°C to block internalization. All samples were treated with an anti-FITC MoAb to quench the cell surface-associated fluorescence. Bottom panel: data are expressed as the difference between MFI values measured for cells untreated (total cell fluorescence) and treated (intracellular fluorescence) with anti-FITC MoAb. This difference is therefore an estimate of the amount of MoAb–FITC bound at the cell surface at each time point. For comparison, the internalization and the binding kinetics of an irrelevant S13.11–FITC MoAb is also shown (□).
RESULTS
Characterization of 1H6.2 and 45.30 MoAbs
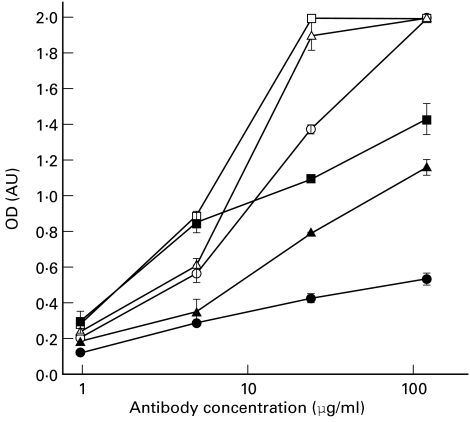
Purified 1H6.2 and 45.30 MoAbs were tested for their ability to bind hLB-, hLF- and bLF-MBP antigens by solid-phase ELISA (Fig. 1). MoAb 1H6.2 bound all antigens to almost the same extent, whereas 45.30 MoAb bound hLF- and bLF-MBP less efficiently than hLB-MBP (Fig. 1). These results suggest that the 1H6.2 and 45.30 MoAbs recognize two different epitopes on MBP antigen.
Fig. 1.
Reactivity of 1H6.2 (open symbols) and 45.30 (closed symbols) MoAbs against human lipid-bound (hLB)-MBP (squares), human lipid-free (hLF)-MBP (triangles) and bovine lipid-free (bLF)-MBP (circles). Data are expressed as mean optical density (OD) values ±s.d. of triplicates.
MoAbs 1H6.2 and 45.30 were both of the IgM isotype. Since IgM antibodies with a broad specificity towards antigens are naturally found in the serum, the spectrum of reactivity of 1H6.2 and 45.30 MoAbs was tested by ELISA using a panel of non-related protein antigens or protein–hapten conjugates (Table 1). 1H6.2 and 45.30 MoAbs reacted against MBP only. Two control IgM MoAbs were also used in the same assays: one reacting against TNP hapten (SP6) but showing a broad cross-reactivity against unrelated haptens, and the other specific for H2Kd molecules (S13.11). As expected, SP6 MoAb showed some cross-reactivity against FITC-conjugated proteins (Table 1). The reactivity of 1H6.2 and 45.30 MoAbs was also tested against intact erythrocytes and erythrocyte membrane components. Reactivity towards intact erythrocytes was measured by haemagglutination assays performed on human cells and compared with that measured for control anti-D antibodies; reactivity toward erythrocyte membrane components was assayed by Western blot analysis performed on erythrocyte lysates. No reactivity of 1H6.2 and 45.30 MoAbs against erythrocytes or erythrocyte components was observed.
Table 1.
Reactivity of 1H6.2, 45.30 and control MoAbs against different protein and protein–hapten antigens (ELISA)
| Antigen | 1H6.2* | 45.30 | S13.11 | SP6 |
|---|---|---|---|---|
| bLF-MBP | 1·950 | 0·545 | 0·106 | 0·153 |
| BSA | 0·150 | 0·030 | 0·113 | 0·020 |
| KLH | 0·030 | 0·027 | 0·428 | 0·450 |
| BSA–FITC | 0·022 | 0·036 | 0·123 | 0·206 |
| KLH–FITC | 0·017 | 0·039 | 0·332 | 2·0 |
| BSA–TNP | 0·265 | 0·020 | 0·174 | 2·0 |
| Ly | 0·027 | 0·033 | 0·073 | 0·046 |
| Tfn | 0·012 | 0·024 | 0·099 | 0·025 |
| OVA | 0·013 | 0·021 | 0·086 | 0·114 |
Data are expressed as mean optical density (OD) values of triplicates. For the sake of clarity s.d. values (s.d. < 15%) are not shown.
All MoAbs were used at a final concentration of 300 μg/ml. Reactivity of secondary goat anti-mouse antibodies was measured and subtracted from the data reported in the table.
bLF, Bovine lipid-free; BSA, bovine serum albumin; KLH, keyhole limpet haemocyanin; TNP, trinitrobenzene sulphonic acid; Ly, lysozyme; Tfn, human transferrin; OVA, ovalbumin.
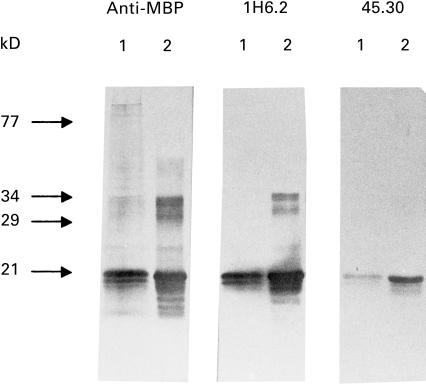
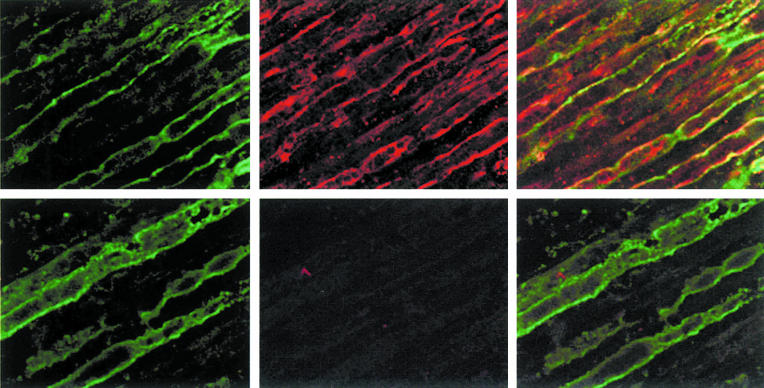
1H6.2 and 45.30 reacted with MBP from human brain white matter tissue extracts as well as bLF-MBP in Western blot assays (Fig. 2). Both MoAbs displayed a similar staining pattern against oligodendrocytes and myelin in the white matter of normal human brain cryosection (data not shown); however, 1H6.2, but not 45.30, MoAb stained myelin sheets in cryosections of human peripheral nerves (Fig. 3).
Fig. 2.
Western blot identification of human (lane 1) and bovine (lane 2) MBP using 1H6.2 and 45.30 MoAbs. To compare the reactivity of 1H6.2 and 45.30, a commercially available anti-MBP MoAb was used (anti-MBP recognizing epitope 84-89; Serotec, Oxford, UK). The typical major 18·5-kD MBP band is recognized by both 1H6.2 and 45.30 MoAbs. MBP has a reduced electrophoretic mobility due to its positive charge and runs at approximately the 21 kD level under these experimental conditions [24,25].
Fig. 3.
Immunohistochemical analysis of the reactivity of 1H6.2 and 45.30 MoAbs against human sciatic nerve cryosections by indirect immunofluorescence confocal microscopy. Immunoreactivity of 1H6.2 and 45.30 is compared with that measured for human anti-MAG serum (from a patient with IgM myeloma with specificity towards MAG antigen) used as positive control. Top panels: anti-MAG (left), 1H6.2 (middle), double staining with anti-MAG and 1H6.2 (right). Bottom panels: anti-MAG (left), 45.30 (middle), double staining with anti-MAG and 45.30 (right). MoAb 1H6.2 labels myelin sheets with a scattered pattern. Ranvier nodes are not labelled by 1H6.2 MoAb. Anti-MAG and 1H6.2 show non-overlapping reactivity. MoAb 45.30 does not label myelin of peripheral nerves in spite of the fact that the concentration used in this assay was 10 times higher than that of 1H6.2 MoAb.
Overall, these findings demonstrate that the specificity of 1H6.2 and 45.30 MoAbs is restricted to MBP. In addition, Western blot and immunohistological assays demonstrate that 1H6.2 and 45.30 MoAbs bind two different epitopes.
Expression of epitopes recognized by 1H6.2 and 45.30 in non-neural cells
Normal and pathological cells of different histotypes were tested for surface and intracellular staining with 1H6.2 and 45.30 MoAbs in cytofluorimetric assays (Tables 2 and 3). Overall, 1H6.2 MoAb showed a higher reactivity than 45.30 MoAb. Intracellular fluorescence was higher than cell surface fluorescence.
Table 2.
Cell surface expression of MBP epitopes revealed by 1H6.2 and 45.30 MoAbs (cytofluorimetric indirect assays)
| Cells | Ctrl* | 1H6.2 | 45.30 | SP6 | S13.11 |
|---|---|---|---|---|---|
| Macrophages | 0·128 | 0·630 | 0·578 | 0·370 | |
| Endothelial | 0·412 | 0·472 | 0·414 | 0·394 | 0·405 |
| Fibroblasts | 0·522 | 0·544 | 0·525 | 0·534 | |
| DC | 0·165 | 0·227 | 0·225 | 0·218 | |
| PBMC | 0·134 | 0·139 | 0·145 | 0·130 | 0·147 |
| TEC | 0·290 | 0·255 | 0·191 | 0·242 | 0·190 |
| Thymocytes | 0·116 | 0·119 | 0·119 | 0·117 | |
| CFPAC-1 | 0·654 | 2·42 | 0·716 | 0·673 | |
| MOLT-4 | 0·402 | 0·414 | 0·402 | 0·441 | |
| B-EBV | 0·155 | 0·281 | 0·336 | 0·178 | |
| Raji | 0·187 | 0·212 | 0·210 | 0·186 |
Data are expressed as mean fluorescence intensity (MFI). MFI values of cells labelled with irrelevant SP6 and/or S13.11 IgM MoAb are also shown.
In this column MFI values of cells labelled with secondary antibody only are reported.
DC, Dendritic cells; PBMC, peripheral blood mononuclear cells; TEC, thymic epithelial cells.
Table 3.
Intracellular expression of MBP-epitopes revealed by 1H6.2 and 45.30 mAbs (Cytofluorimetric indirect assays)
| Cells | Ctrl* | 1H6.2 | 45.30 | SP6 | S13.11 |
|---|---|---|---|---|---|
| Macrophages | 0·705 | 5·55 | 1·05 | 0·814 | |
| Endothelial | 0·649 | 6·89 | 2·80 | 0·840 | 0·963 |
| Fibroblasts | 0·470 | 1·37 | 0·485 | 0·533 | |
| DC | 0·488 | 0·760 | 0·947 | 0·507 | |
| PBMC | 0·172 | 3·09 | 0·585 | 0·245 | 0·176 |
| TEC | 0·176 | 3·19 | 1·04 | 0·357 | |
| Thymocytes | 0·190 | 3·59 | 0·321 | 0·236 | |
| CFPAC-1 | 1·21 | 42·7 | 6·78 | 2·37 | |
| MOLT-4 | 1·26 | 7·02 | 1·66 | 2·05 | |
| B-EBV | 0·487 | 21·1 | 1·44 | 1·57 | |
| Raji | 0·526 | 3·00 | 0·750 | 0·501 |
Data are expressed as mean fluorescence intensity (MFI). MFI values of cells labelled with irrelevant SP6 and/or S13.11 IgM MoAb are also shown.
In this column MFI values of cells labelled with secondary antibody only are reported.
DC, Dendritic cells; PBMC, peripheral blood mononuclear cells; TEC, thymic epithelial cells.
No cell types within the set considered were found to be negative for intracellular expression of both 1H6.2 and 45.30 epitopes. A slight positivity for cell surface expression of MEs was observed for most cell types. Among cell surface-positive cells, absolute MFI values varied in the positive window between different experiments, suggesting the dependence of ME expression at the cell surface on some cell physiological parameter.
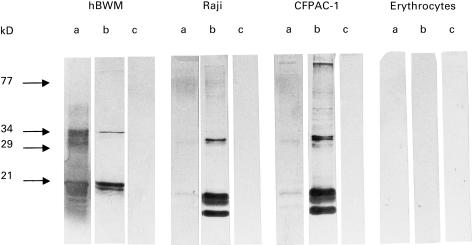
MEs identified by 1H6.2 MoAb (whose reactivity against non-neural cells was higher than that of 45.30) were also analysed by SDS–PAGE and Western blotting using total cell lysates from CFPAC-1 and Raji cells (Fig. 4). Major bands identified by 1H6.2 MoAb were approximately 14·8, 15·5 and 16·2 kD in weight. Similar bands were observed when other cell types were used (e.g. TEC, T lymphocytes; data not shown). These proteins are different from the classical major 18·5-kD MBP form expressed in brain white matter but of comparable size with minor MBP isoforms expressed at the CNS level [25].
Fig. 4.
Western blot identification of MBP epitopes expressed by two non-neural cells using 1H6.2 MoAb. The reactivity of 1H6.2 MoAb against total cell lysates from Raji and CFPAC-1 cells is compared with that observed for 1H6.2 reacting against human brain white matter tissue extracts (hBWM). As a control, reactivity of 1H6.2 against total cell lysates of erythrocytes is also shown. Lane a, control anti-MBP MoAb (Serotec) recognizing the 84-89 MBP epitope; lane b, 1H6.2 MoAb; lane c, secondary anti-mouse IgM MoAb (Amersham, Aylesbury, UK). MBP has a reduced electrophoretic mobility due to its positive charge and runs at approximately the 21 kD level under these experimental conditions [24,25]. Bands at approximately 30 kD or at higher molecular weights are also stained by 1H6.2 and by anti-MBP MoAbs. These proteins might correspond to multimers of MBP and/or MBP epitopes.
Uptake of 1H6.2 and 45.30 MoAbs by APC
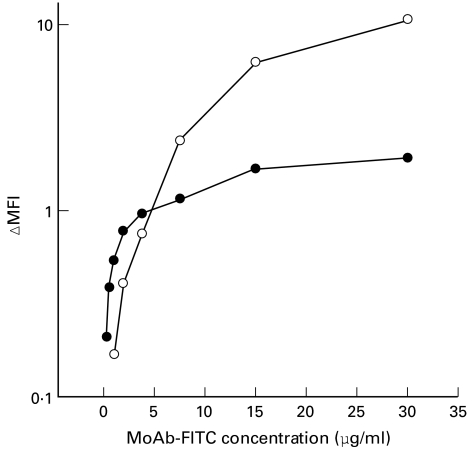
Uptake of 1H6.2 and 45.30 MoAbs was demonstrated in APC using cytofluorimetric techniques. Both MoAbs were directly fluorescinated and MoAb–FITC conjugate specificity was measured in binding assays (Fig. 5); binding of both MoAbs was displaced by a 100-fold excess of unlabelled MoAb, demonstrating that 1H6.2– and 45.30–FITC bound specifically to the surface of target cells. The binding curve of 45.30–FITC plateaud at concentrations >15 μg/ml MoAb, indicating saturation of binding sites (Fig. 5). No clear-cut saturation was observed for 1H6.2–FITC at the maximum concentration used in the assays, suggesting differences in the affinity between 1H6.2 and 45.30 and/or in the number of 1H6.2 and 45.30 epitopes expressed at the cell surface.
Fig. 5.
Binding of 1H6.2–FITC (○) and 45.30–FITC (•) MoAbs to Epstein–Barr virus (EBV)-transformed human B cells. Values are reported as the difference between mean fluorescence intensity (MFI) values measured at the indicated concentrations of MoAb–FITC, minus MFI values of unspecific binding. Unspecific binding was measured by incubating cells with MoAb–FITC in the presence of a 100-fold molar excess of unlabelled MoAb.
Receptor-mediated endocytosis was demonstrated using Raji cells which represent a well-known experimental model of professional APC [18]. Both 1H6.2–FITC and 45.30–FITC MoAbs were rapidly endocytosed (Fig. 6, top panel) in spite of the low cell surface expression of MEs by Raji cells (see also Table 2). Internalization started within 20 min after exposure of cells to saturating amounts of fluoresceinated MoAbs (Fig. 6, top panel). Internalization kinetics appeared similar for 1H6.2 and 45.30. As a control, the internalization kinetics of S13.11–FITC were studied in parallel. A smaller amount of S13.11–FITC was internalized per unit time with respect to MoAb–FITC, demonstrating that both 1H6.2 and 45.30 were actively internalized by Raji cells via ME-mediated endocytosis. Interestingly, cell surface-associated fluorescence measured in the same assays increased with time for both 1H6.2–FITC and 45.30–FITC (Fig. 6, bottom panel). Thus at 37°C, expression of MEs at the cell surface of Raji cells is positively modulated by MoAbs. This in turn would explain why active internalization of MoAbs can occur in Raji cells in spite of the low initial surface expression of MEs.
DISCUSSION
Myelin basic protein is considered to be one of the self-antigens involved in the pathogenesis of MS, and its pathogenic role has been investigated in experimental models of EAE and r-EAE [1,2]. Both MS and r-EAE are characterized by a relapsing/remitting course, which implies the existence of recurrent peaks of immune system stimulation [26].
Until recently, MBP was considered to be expressed in CNS tissues only and sequestered behind the blood–brain barrier. In this light, it appeared difficult to understand how central and peripheral tolerance to MBP could be established. On the other hand, in the case of MS, the above assumption directly implied the hypothesis of an infection of the CNS as the triggering event responsible for immune stimulation; however, to date such a neurotropic agent remains elusive. Furthermore, epidemiological data appear more consistent with the hypothesis that the primary immune stimulus lies outside the CNS [27]. Thus, the question whether MBP or MBP-antigenic epitopes (MEs) are expressed outside neural tissue becomes crucial to clarify the dynamics of immune stimulation as well as the mechanisms leading to central and peripheral tolerance.
Several reports have shown that mRNAs transcribed from the Golli-MBP gene locus are present in neural, as well as non-neural, tissues [3–5], and some of the expected MBP proteins have been identified in tissue extracts from primary lymphoid organs [6]. The cellular localization of MBP epitopes in human immune cells, as well as in cells important for immune homeostasis, requires detailed investigation.
Two anti-MBP MoAbs were generated and selected on the basis of their different binding properties to hLB-MBP, hLF-MBP and bLF-MBP. MoAbs 1H6.2 and 45.30 were found to stain the cell surface and/or cytoplasm of a wide array of cells of lymphoid and non-lymphoid origin. The epitopes expressed by non-neural cells analysed so far are proteins whose molecular weight (approx. 14–16 kD) resembles that of MBP isoforms expressed at low levels in the CNS [25]. The major 18·5-kD CNS MBP form was not found to be expressed in these cells (or was expressed at non-detectable levels).
Some of the cells labelled by MoAbs, such as DC, B cells, thymic epithelial cells, are known to exert a pivotal role either in establishing self-tolerance or in triggering the immune response. The presence of MEs in the cytoplasm of human thymic epithelial cells supports the hypothesis that MBP antigen could be recognized at the thymic level, therefore partcipating in the processes leading to central tolerance. This result might also explain the observed presence of MBP-reactive T cells in healthy individuals. MBP-reactive T cells could be positively selected at the thymic level owing to ME expression by TEC cells.
DC and Raji cells are examples of professional APC. They both express MEs either at the cell surface or in their cytoplasm. Moreover, Raji cells actively internalize 1H6.2 and 45.30 MoAbs in spite of the low initial surface expression of MEs. This result, together with the observation that surface expression of MEs in Raji cells is modulated at 37°C by the addition of MoAbs, indicates trafficking, following an appropriate stimulus, of MEs along the endocytic/secretory pathways. Dendritic cells and B cells might therefore present MEs in the context of MHC class II molecules, thus activating CD4+ encephalitogenic T cell clones.
MEs are also expressed in normal non-lymphoid cells, such as fibroblasts and endothelial cells and in a tumour cell line (CFPAC-1). Recently, it has been shown that non-immune cells may contribute to immune tolerance or autoimmunity by mechanisms of cross-tolerance and cross-priming, respectively [16,17], whereby self-antigens are transferred to professional APC. Thus, even non-specialized non-immune cells may contribute to turning the immune response against self-antigens. Furthermore, endothelial cells can express MHC class II molecules under a suitable stimulus (e.g. interferon-gamma), so acquiring an APC phenotype [14]. Endothelial cells from normal guinea pig brain can present autologous MBP to lymphocytes [28]. Thus, endothelial cells expressing MBP epitopes could trigger MBP-specific T cells on their own, ultimately contributing to impaire the blood–brain barrier.
Overall, MBP proteins appear to be widespread outside the CNS and to be expressed by cells playing a pivotal role in either triggering an immune response or establishing self-tolerance. The presence of MEs in professional and non-professional APC and the findings showing MEs trafficking along the endocytic pathways in B cells support the hypothesis that the primary immune stimulus leading to MS lies outside the CNS [29], without involving molecular mimicry of myelin by viral or bacterial components.
Acknowledgments
This work was supported in part by grants from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Cofinanziamento MURST A.F. 1997 Prof. G. Tridente, MURST 60%), from the Fondazione Cassa di Risparmio Progetto Sanità ‘Anticorpi monoclonali e forme native della proteina basica della mielina nella patogenesi delle malattie demielinizzanti’ and from the Associazione Italiana Sclerosi Multipla (AISM). We wish to thank Dr M. Tommasi and Dr U. Garbin for their help in culturing EBV-transformed and endothelial cells. Dr E. Nardelli and Dr T. Cavallaro are gratefully acknowledged for their assistance during hystological analysis. Dr I. Ferro is gratefully acknowledged for providing buffy coats. Dr R. Roncarati is gratefully acknowledged for her assistance during confocal analysis.
REFERENCES
- 1.Sun JB. Autoreactive T and B cells in nervous system diseases. Acta Neurol Scandin. 1993;87(Suppl.):142. [PubMed] [Google Scholar]
- 2.Mac-Kenzie-Graham AJ, Pribyl TM, Kim S, et al. Myelin protein expression is increased in lymph nodes of mice with relapsing experimental autoimmune encephalomyelitis. J Immunol. 1997;159:4602–10. [PubMed] [Google Scholar]
- 3.Campagnoni AT, Pribyl TM, Campagnoni CW, et al. Structural and developmental regulation of golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocytes lineage in the brain. J Biol Chem. 1993;286:4930–8. [PubMed] [Google Scholar]
- 4.Grima B, Zelenika D, Pessac B. A novel transcript overlapping the myelin basic protein gene. J Neurochem. 1992;59:2318–23. doi: 10.1111/j.1471-4159.1992.tb10126.x. [DOI] [PubMed] [Google Scholar]
- 5.Zelenika D, Grima B, Pessac B. A new family of transcripts of the myelin basic protein gene: expression in brain and in immune system. J Neurochem. 1993;60:1574–7. doi: 10.1111/j.1471-4159.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalwy S, Marty MC, Bausero P, et al. Myelin basic protein-related proteins in mouse brain and immune tissues. J Neurochem. 1998;70:435–8. doi: 10.1046/j.1471-4159.1998.70010435.x. [DOI] [PubMed] [Google Scholar]
- 7.Riccio P, Liuzzi MG, Quagliarello E. Lipid-bound, native-like myelin basic protein: batch-wise preparation and perspectives for use in demyelinating diseases. Mol Chem Neuropathol. 1990;13:185–94. doi: 10.1007/BF03159921. [DOI] [PubMed] [Google Scholar]
- 8.Riccio P, Bobba A, Romito E, et al. A new detergent to purify CNS myelin basic protein isoforms in lipid-bound form. Neuroreport. 1994;5:689–92. doi: 10.1097/00001756-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Riccio P, Dal Canto MC. Antigenic properties of myelin and the role of myelin in pathology. J Neuroimmunol. 1991;32:185–6. doi: 10.1016/0165-5728(91)90009-v. [DOI] [PubMed] [Google Scholar]
- 10.Lolli F, Liuzzi MG, Vergelli M, et al. Antibodies specific for the lipid-bound form of myelin basic protein during experimental autoimmune encephalomyelitis. J Neuroimmunol. 1993;44:69–76. doi: 10.1016/0165-5728(93)90269-5. [DOI] [PubMed] [Google Scholar]
- 11.Massacesi L, Vergelli M, Zehetbaurer B, et al. Induction of experimental autoimmune encephalomyelitis in rats and immune response to myelin basic protein in lipid bound form. J Neurol Sci. 1993;119:91–98. doi: 10.1016/0022-510x(93)90196-6. [DOI] [PubMed] [Google Scholar]
- 12.Picker LJ, Siegelman MH. Lymphoid tissues and organs. In: Paul WE, editor. Fundamental immunology. 4. Philadelphia: Lippincott-Raven; 1999. pp. 479–531. [Google Scholar]
- 13.Steinman RM. Dendritic cells. In: Paul WE, editor. Fundamental immunology. 4. Philadelphia: Lippincott-Raven; 1999. pp. 547–73. [Google Scholar]
- 14.Leeuwenberg JF, Van Damme J, Meager T, et al. Effects of tumor necrosis factor on the interferon-gamma-induced major histocompatibility complex class II antigen expression by human endothelial cells. Eur J Immunol. 1988;18:1469–72. doi: 10.1002/eji.1830180925. [DOI] [PubMed] [Google Scholar]
- 15.Scupoli MT, Sartoris S, Tosi G, et al. Expression of MHC class I and class II antigens in pancreatic adenocarcinomas. Tissue Antigens. 1996;48:301–11. doi: 10.1111/j.1399-0039.1996.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 16.Heath WR, Kurts C, Miller JFAP, et al. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–53. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 18.Tomai MA, Beachey EH, Majumdar G, et al. Metabolically active antigen presenting cells are required for human T cell proliferation in response to the superantigen streptococcal M protein. FEMS Microbiol Immunol. 1992;4:155–64. doi: 10.1111/j.1574-6968.1992.tb04982.x. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alfa. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riviera AP, Scuderi F, Provenzano C, et al. Epithelial tumours of the thymus. New York: Plenum Press; 1997. Estrogens modulate IL-6 production by cultured normal and pathological thymic epithelial cells; pp. 187–94. [Google Scholar]
- 21.Jaffe EA, Nachman RL, Becker CG, et al. Culture of human endothelial cells derived from umbelical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moretto G, Walker DG, Lanteri P, et al. Expression and regulation of glial-cell-line-derived neurotropic factor (GDNF) mRNA in human astrocytes in vitro. Cell Tissue Res. 1996;286:257–62. doi: 10.1007/s004410050695. [DOI] [PubMed] [Google Scholar]
- 23.Chignola R, Anselmi C, Franceschi A, et al. Sensitivity of human leukemia cells in exponential or stationary growth phase to anti-CD5 immunotoxins. J Immunol. 1994;152:2333–43. [PubMed] [Google Scholar]
- 24.Cheifetz S, Moscarello MA. Effect of bovine basic protein charge microheterogeneity on protein-induced aggregation of unilamellar vescicles containing a mixture of acidic and neutral phospholipids. Biochemistry. 1985;24:1909–14. doi: 10.1021/bi00329a016. [DOI] [PubMed] [Google Scholar]
- 25.Määttä JA, Coffey ET, Hermonen JA, et al. Detection of myelin basic protein isoforms by organic concentration. Biochem Biophys Res Commun. 1997;238:498–502. doi: 10.1006/bbrc.1997.7318. [DOI] [PubMed] [Google Scholar]
- 26.McDonald WI. The pathological and clinical dynamics of multiple sclerosis. J Neuropathol Exp Neurol. 1994;53:338–43. doi: 10.1097/00005072-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman MD. Do microbes with peptides mimicking myelin cause multiple sclerosis if the T cell response to their unique peptides is limited? J Theor Biol. 1998;193:691–708. doi: 10.1006/jtbi.1998.0734. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CE, Healey DG, Baker D, et al. Presentation of myelin basic protein by normal guinea-pig brain endothelial cells and its relevance to experimental allergic encephalomyelitis. Immunology. 1989;67:435–40. [PMC free article] [PubMed] [Google Scholar]
- 29.Voskuhl RR. Myelin protein expression in lymphoid tissues: implications for peripheral tolerance. Immunol Rev. 1998;164:81–92. doi: 10.1111/j.1600-065x.1998.tb01210.x. [DOI] [PubMed] [Google Scholar]