Abstract
Earlier studies in patients with pulmonary TB have revealed a higher production of Th1 cell type cytokines in moderate TB, with predominant Th2-like responses in advanced disease. Given the influence of IL-12 in T cell differentiation, as well as the roles of transforming growth factor-beta (TGF-β), nitric oxide and tumour necrosis factor-alpha (TNF-α) in the immune response against intracellular pathogens, we decided to analyse the interferon-gamma (IFN-γ), IL-4, IL-12, TGF-β, TNF-α and nitrite concentrations in culture supernatants of PBMC from TB patients showing different degrees of lung involvement. The sample population comprised 18 untreated TB patients with either moderate (n = 9) or advanced (n = 9) disease and 12 age- and sex-matched healthy controls (total population (patients and controls) 12 women, 18 men, aged 37 ± 13 years (mean ±s.d.)). PBMC were stimulated with whole sonicate from Mycobacterium tuberculosis and the supernatants were collected on day 4 for measurement of cytokine and nitrite levels. Antigen-stimulated IFN-γ, TGF-β and TNF-α production was found to be significantly increased in TB patients, both moderate and advanced, compared with the controls. Levels of IFN-γ were significantly higher in moderate disease than advanced cases, whereas advanced cases showed significantly higher IL-12, TGF-β and TNF-α concentrations when compared with cases of moderate TB. Nitrite levels were also increased in TB patients and the increase was statistically significant when advanced cases were compared with controls. These findings may contribute to a clearer picture of the net effect of cytokine interactions in TB, essential for a better understanding of the immunopathological mechanisms underlying the distinct clinical forms of the disease.
Keywords: tuberculosis, disease severity, nitrite, cytokines, immunoregulation
INTRODUCTION
Pulmonary TB accounts for increasing morbidity and mortality across the world. Approximately 8–12 million new TB cases emerge and 3 million people die of this disease every year [1]. The clinical spectrum of TB ranges from a few foci affecting the upper parts of the lungs to intense tissue destruction and caseous necrosis, which usually disintegrates forming cavitary lesions. Such different disease outcomes are thought to be the result of complex interactions between Mycobacterium tuberculosis and elements of the specific immune response. Resistance to mycobacterial infections is known to be conferred by T cell-mediated immune mechanisms involving cytokines that ultimately lead to the recruitment and activation of monocyte/macrophage cells possessing an enhanced state of microbicidal activity. Evidence recorded in both mouse [2] and man [3] indicates that, in response to pathogens, T helper (Th) cells can be differentiated into mutually exclusive Th1 and Th2 subsets. Th1 cells secrete cytokines that are involved in cell-mediated immune responses (interferon-gamma (IFN-γ) and IL-2), whereas Th2 lymphocytes secrete mediators that provide optimal help for humoral immunity (in humans IL-4, IL-5, IL-13, and to a lesser degree, IL-10).
Given this background, in a recent study we analysed the in vitro cytokine production of PBMC from TB patients of different severity, upon in vitro stimulation with M. tuberculosis antigens. Patients with mild TB showed preferential production of IFN-γ over IL-4, whereas moderate patients revealed a mixed production of IFN-γ and IL-4, which coexisted with higher synthesis of transforming growth factor-beta (TGF-β) compared with the mild cases. Advanced disease showed the highest levels of IL-4 and TGF-β production, with IFN-γ synthesis, although readily detectable, decreased in comparison with the other patient groups [4].
Whether such a divergent profile of cytokine responses reflects differentiation of a selected T cell subset or simply an environmental control of T cell responses is not fully understood. Evidence from experimental studies suggests that non-T cell-derived signals such as cytokines or cell surface co-stimulatory molecules can direct the differentiation of an immune response. An important cytokine implicated in this regard is IL-12. This heterodimeric cytokine is produced by activated monocytes, macrophages, and B cells [5]. One of the most important activities of IL-12 in the immune system is to induce the production of IFN-γ, not only by natural killer (NK) cells but also by T cells [6]. Accumulating evidence indicates that IL-12 has the prominent capacity to induce the differentiation of naive CD4+ T cells into Th1 cells [5], whereas anti-IL-12 has been shown to decrease IFN-γ production, inducing a Th2-like cytokine response in vivo [7]. It follows that an assessment of the in vitro production of IL-12 in TB patients will help to elucidate whether differences in IFN-γ synthesis, according to the severity of lung involvement, are related to the former T cell differentiation-influencing cytokine.
The approach of studying TB patients with a different degree of pulmonary affectation is also suitable for analysing two additional mediators, which are likely to be involved both in immune protection as well as immunopathology, namely tumour necrosis factor-alpha (TNF-α) and nitric oxide (NO). TNF-α is a monocyte-activating cytokine, stimulating anti-mycobacterial activity [8], that also exerts a role in granuloma formation [9]. Nevertheless, it may also be responsible for the toxic syndrome and tissue necrosis accompanying TB, as it displays important proinflammatory activities [10,11]. With regard to NO, although this compound is known to be involved in the macrophage clearance of mycobacteria [12,13], there is evidence that NO plays an important role in the inflammation and immunosuppression induced by this kind of pathogen as well [12,14].
In view of these considerations, a study was undertaken to analyse IFN-γ, IL-4, IL-12, TNF-α and nitrite concentrations in culture supernatants of M. tuberculosis-stimulated PBMC from pulmonary TB patients with different disease severities. In addition, TGF-β values were also measured given the influence of this cytokine in T cell and macrophage activities [15]. Simultaneous assessment of cytokine production by PBMC, as a mixed population of immunocompetent cells, will help us to understand the complexity of cytokine–cytokine regulatory influences and the ultimate fate of the immune response resulting from such interactions.
PATIENTS AND METHODS
Study groups
The study included 18 TB patients attending the Carrasco Hospital, Rosario, Argentina, whose diagnosis was based on clinical and radiological data together with the identification of tubercle bacilli in sputum. The patients (six women and 12 men) ranged in age from 15 to 67 years with a mean age of 36·6 years (s.d. = 15). Pulmonary disease was classified, according to the extent and type of disease as assessed by x-ray, into two groups: moderate (n = 9) patients presenting unilateral involvement of two or more lobes with cavities, if present, reaching a total diameter no greater than 4 cm; advanced (n = 9) bilateral disease with massive affectation and multiple cavities. Healthy volunteers (n = 12), not in contact with TB patients but living in the same area, were included as controls; four of them had a bacille Calmette–Guerin (BCG) scar. The controls consisted of six women and six men (mean age 36 ± 10 years), who had no history of prior clinical TB but revealed a positive tuberculin reaction upon challenge with PPD, shortly after blood samples were collected. All participants had negative HIV serology. Patients were bled before the initiation of anti-tuberculous treatment, provided informed consent had been obtained.
Isolation and stimulation of PBMC
For preparation of PBMC, fresh heparinized blood was diluted 1:1 in RPMI 1640 (Gibco) containing standard concentrations of l-glutamine, penicillin, and streptomycin (culture medium (CM)), layered over a Ficoll–Triyosom gradient (density 1·077) and centrifuged at 400 g for 30 min at room temperature (19–22°C). PBMC recovered from the interface were washed three times in CM by centrifuging at 300 g for 10 min. Following washing, PBMC were resuspended in CM containing 10% heat-inactivated pooled normal AB human sera (CMS). Cells were cultured in quadruplicate in flat-bottomed microculture plates. Each well contained 100 μl of cell suspension (2 × 105 cells/well) plus 100 μl CMS plus either whole sonicated antigen from M. tuberculosis (WSA; 10 μg/ml, kindly given by Dr J. L. Stanford, London, UK) or concanavalin A (Con A; 2·5 μg/ml). The cultures were incubated for 5 days at 37°C, 5% CO2 in a humidified atmosphere and pulsed with 3H-thymidine for 18 h prior to termination of culture. Proliferative results were expressed as the average ct/min of wells with antigen or mitogen stimulus minus the average ct/min of wells without stimulation. To measure the levels of cytokines, culture supernatants were collected on day 4 (before adding 3H-thymidine) and stored at −20°C until testing. Both patient groups revealed no significant differences in the relative numbers of lymphocytes and monocytes in fresh peripheral blood.
Cytokine assays
Levels of IFN-γ, IL-4, IL-12, TGF-β and TNF-α in supernatants from cultures of PBMC stimulated with or without mycobacterial antigens were determined by ELISA with commercial kits, as described by the manufacturer (IFN-γ, IL-4, and TGF-β Genzyme Diagnostics, Boston, MA; IL-12 and TNF-α R&D Systems). Samples were assayed in duplicate and results expressed as the average of the two readings in an ELISA reader at 450 nm. Cytokines were quantified with reference to standard curves generated using human recombinant cytokines. The sensitivity of the assays were 3 pg/ml for IFN-γ, 45 pg/ml for IL-4, 50 pg/ml for TGF-β, 5 pg/ml for IL-12, and 4·4 pg/ml for TNF-α.
Nitrite evaluation
Nitrite accumulation, an indicator of NO synthesis, in the supernatant of 4-day cultured PBMC was assayed by the Griess reaction. Briefly, equal amounts of the cell-free supernatant from each sample were added to an equal volume of freshly prepared Griess reagent. The Griess reagent was prepared by mixing equal volumes of 1% sulfanilamide in 2·5% H3PO4, and 0·1% naphthylethylene diamine dihydrochloride in 2·5% H3PO4, and incubating for 10 min at room temperature. Absorbance was measured at 560 nm. Nitrite concentration was quantified using various NaNO2 concentrations in culture medium as the standard and data were expressed as μm.
Statistical analysis
Comparisons among groups were performed using the Kruskall–Wallis analysis of variance and the Mann–Whitney U-test. Data were considered statistically significant when P ≤ 0·05.
RESULTS
Lymphoproliferative responses from WSA-stimulated PBMC of both TB patient groups (moderate and advanced) were lower than those recorded in the controls, but the trend was not statistically significant. The magnitude of the response to Con A of patients with moderate or advanced TB was also found to be depressed in relation to the controls, with comparisons yielding a significant difference (P < 0·025, data not shown).
In vitro IL-4 and IFN-γ production and their relationship with IL-12 and TGF-β synthesis
Supernatants from 96-h cultured PBMC of patients and controls were assessed for IL-4, IFN-γ, IL-12 and TGF-β concentrations. The data were presented as both crude and relative values, i.e. the levels recorded in WSA-stimulated cultures and those obtained after subtracting the concentrations detected in unstimulated cultures, respectively.
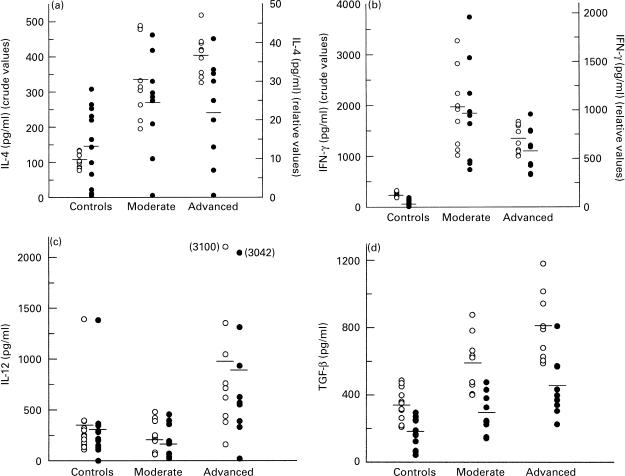
With regard to IL-4, the results from Fig. 1a show that both groups of TB patients had increased antigen-dependent IL-4 concentrations in relation to the controls (P < 0·0005 crude value); IL-4 levels were highest in those patients with advanced disease, but this increase was not statistically significant in comparison with the moderate cases. Because of the substantial secretion of IL-4 from PBMC in the absence of stimulant, the relative IL-4 levels were well below the absolute values; however, the relative values showed the same pattern of IL-4 expression in the different groups, although the trend did not reach the level of statistical significance. Antigen-stimulated IFN-γ production was also found to be significantly increased in TB patients compared with controls (P < 0·0005 crude value), being more evident in patients with moderate disease who produced significantly higher levels of IFN-γ than the advanced cases (P < 0·05 crude value, Fig. 1b). Further comparisons using the relative IFN-γ concentrations showed a similar pattern of statistical difference (Fig. 1b).
Fig. 1.
(a) IL-4, (b) IFN-γ, (c) IL-12, and (d) transforming growth factor-beta (TGF-β) production by PBMC from TB patients and controls. Cells (2 × 105/well) were cultured either in the presence or absence of whole sonicated antigen from Mycobacterium tuberculosis (WSA, 10 μg/ml). Supernatants were collected on day 4 following stimulation. Each point represents an individual result (pg/ml), with the horizontal bar indicating mean levels. ○, Crude values. •, Relative values corresponding to cytokine concentrations in supernatants from WSA-stimulated cells minus those in supernatants of unstimulated cells. Positional crude and relative values for (a) IL-4 and (b) IFN-γ are indicated by the left and right ordinates, respectively. Both values are shown on the single abscissa for (c) IL-12 and (d) TGF-β. (a) IL-4, (b) IFN-γ and (d) TGF-β concentrations were significantly lower in the controls compared with the TB patients (○, P < 0·0005 for (a,b,d); •, P < 0·005 for (b,d). (b) IFN-γ concentrations in advanced TB patients were significantly lower than the moderate cases (○, P < 0·05; •, P = 0·05). (c) Synthesis of IL-12 in patients with advanced TB was significantly higher than controls and moderate TB patients (○, P < 0·01; •, P < 0·028). (d) TGF-β levels were significantly lower in moderate compared with advanced TB patients using both crude (○, P < 0·04) and relative (•, P = 0·05) values.
In view of the fact that IL-12 and TGF-β are known to influence the profile of Th-cell-secreted cytokines, attempts were made to analyse whether differences in the above results bore any relationship to the production of IL-12 and TGF-β. Data depicted in Fig. 1c indicate that IL-12 was easily detected in culture supernatants from patients and controls. Patients with advanced disease showed the highest IL-12 concentrations, reflected in significant statistical differences when compared with moderate cases and controls, no matter whether the data were analysed for crude (P < 0·01) or relative (P < 0·028) values (Fig. 1c). In line with the IFN-γ results, M. tuberculosis-driven TGF-β production by TB patients was found to be comparatively higher than that recorded in the controls, regardless of whether analysis utilized crude (P < 0·0005) or relative (P < 0·005) values (Fig. 1d). Within the TB patient groups, TGF-β production was highest in the advanced cases, yielding a significant difference when compared with moderate patients (P < 0·04 crude, P = 0·05 relative values, Fig. 1d).
Nitrite and TNF-α measurements
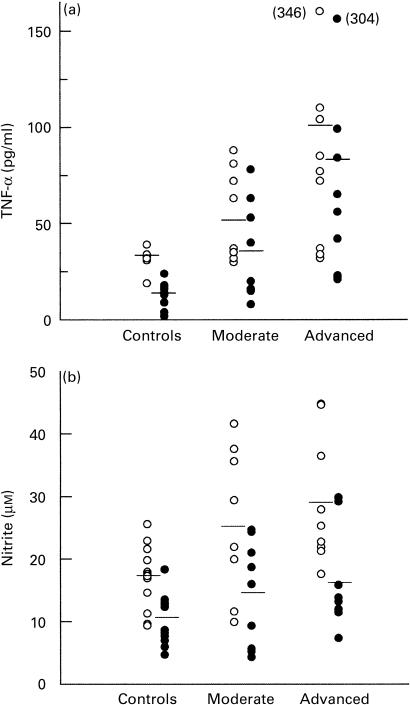
The levels of TNF-α present in culture supernatants from patients and controls in response to WSA stimulation are shown in Fig. 2a. Higher levels of TNF-α were produced by mononuclear cells from TB patients, being most evident in those with advanced disease. Analysis of crude values revealed a significant difference between patients and controls (P < 0·002), whereas in the case of relative levels comparisons between patient groups (P < 0·05 advanced versus moderate), and both groups as a whole against the controls (P < 0·004) were significant. Crude levels of nitrite accumulation in supernatants from controls were significantly lower than the values recorded in TB patients (P < 0·015), with the difference also being significant when comparing the relative values of controls and advanced cases (P = 0·05, Fig. 2).
Fig. 2.
(a) Tumour necrosis factor-alpha (TNF-α) and (b) nitrite production by PBMC from TB patients and controls. Cells (2 × 105/well) were cultured in the presence of whole sonicated antigen from Mycobacterium tuberculosis (WSA, 10 μg/ml). Supernatants were collected on day 4 following stimulation. Each point represents an individual result (pg/ml for TNF-α or μm for nitrite), with the horizontal bar indicating mean levels. ○, Crude values; •, relative values, corresponding to concentrations in supernatants from WSA-stimulated cells minus those in supernatants of unstimulated cells. (a) TNF-α and (b) nitrite concentrations were significantly lower in the controls compared with the TB patients ((a) ○, P < 0·002, •, P < 0·004; (b) ○, P < 0·015). (a) TNF-α levels were significantly lower in the moderate versus advanced cases (•, P < 0·05). (b) Relative nitrite concentrations were significantly increased in advanced cases versus controls (•, P = 0·05).
DISCUSSION
It is becoming clear that an appropriate balance of cell-mediated immune responses is of central importance for the development of protective immunity against intracellular pathogens, with an alteration of such a balance being likely to influence the establishment of tissue damage. It follows that the identification of cytokine production is crucial for a better understanding of disease pathogenesis due to microbes with an intracellular habitat, such as mycobacteria.
Based on a previous demonstration, in which in vitro M. tuberculosis-driven IFN-γ production by patients with pulmonary TB was found to deteriorate with disease aggravation [4], one of the objectives of the present study was to determine whether this alteration could be linked to changes in IL-12 production. IL-12 is the major regulatory stimulus for Th1-type immunity, triggering the development of Th1-type cells from precursor cells and IFN-γ production [5]. Both IL-12 and IFN-γ are essential for the development of protective responses during infection with M. tuberculosis in mice [16–18]; IFN-γ is required in humans [19]. Strikingly in our study, the lower IFN-γ levels in patients with severe disease were accompanied not by reduced but by increased IL-12 levels in culture supernatants.
Recent studies suggest that development of Th1 and Th2 responses may be controlled by the β-receptor subunit of IL-12 (IL-12Rβ), since a down-regulation of this receptor leads to a loss of IL-12 responsiveness in CD4+ T cells [20]. Because of this, the augmented levels of IL-12 in advanced TB cases may be viewed as an attempt to restore IFN-γ production that is rendered unsuccessful, probably due to defective expression of the IL-12 receptor. Although expression of the IL-12 receptor has been found to be increased in bronchoalveolar lavage fluid (BALF) from patients with active TB [21], Zhang et al. [22] demonstrated that reduced IFN-γ production by peripheral blood T cells of TB patients correlated with poor expression of IL-12Rβ1 and IL-12Rβ2, and that TGF-β neutralization led to recovered IL-12R expression and IFN-γ synthesis. Accounts from the same group documented that decreased IFN-γ production by M. tuberculosis-stimulated PBMC in adults or children with TB was not linked to inadequate IL-12 production [23,24]. Furthermore, studies evaluating the number of peripheral IL-12-producing cells, as well as cytokine expression in lymph nodes or BAL, showed elevated IL-12 production in TB patients [25–27].
In partial agreement with these reports, our approach of studying TB patients with different pulmonary involvement revealed that IL-12 synthesis was only augmented in advanced TB cases, who also displayed lower IFN-γ production. In addition, these patients exhibited a trend to present increased levels of IL-4, which has been recognized to decrease the expression of IL-12Rβ2, thereby preventing the action of IL-12 on T-helper cell differentiation [20,28].
Despite being inefficient at promoting a better IFN-γ response, the question arises whether too much IL-12 may promote some detrimental effects. Studies measuring IL-12 concentrations from BAL of TB patients showed higher IL-12 levels in those lungs with greater disease manifestations [29]. Furthermore, work in IFN-γ gene knockout mice infected with Leishmania donovani suggests that IL-12 may induce a TNF-α response independent of IFN-γ [30]. This lends some support to our finding of increased TNF-α amounts in TB cases with severe disease, although advanced patients also produced increased levels of TGF-β, a cytokine that is known to counteract TNF-α production by monocytes [31]. Taken together, these findings lead us to envisage a more complex interactive scenario, in which influences operating on cytokine responsiveness and cytokine expression may also depend on the context being examined.
Our data regarding IFN-γ measurements contrast with other studies in which the IFN-γ synthesis of healthy tuberculin reactors appeared to be increased by comparison with TB patients [23,32]. In fact, despite developing an adequate lymphoproliferative response, cytokine production by PBMC from our controls was lower than that seen in patients. Antigen-specific proliferation tests in these controls may be an indicator of prior mycobacterial exposure but one not strong enough to elicit a substantial cytokine response. One reason for such a reduced in vitro response might be a distinct level of sensitization to mycobacterial antigens, i.e. our controls had no antecedent of direct contact with TB. Additional factors accounting for differences between the present study and the above cited studies [23,32] may be the variations in experimental culture conditions, such as cell concentrations and the antigen characteristic or dose with which cells were stimulated. That proliferative T cell responses do not necessarily correlate with cytokine production has also been demonstrated by Surcel et al. [33]. Compared with healthy PPD responders, TB patients in their studies showed a slightly decreased thymidine incorporation following stimulation with mycobacterial antigens in presence of an elevated number of IFN-γ- and IL-4-secreting cells, especially the latter.
Even though the TGF-β-favoured intracellular replication of M. tuberculosis within human macrophages [8] has not been corroborated by other studies [34], the presence of TGF-β in TB is also thought to participate in the suppressed T cell responses accompanying this disease [15]. At a general level, TGF-β was found to suppress both IFN-γ expression [35] and the development of Th1 cells from helper T cells in vitro [36] favouring a preferential expansion of Th2-type cells [37]. Regarding TB, synthesis of TGF-β appeared to be increased in both blood monocytes and lung granuloma macrophages in TB patients [38]. Further studies, in which PBMC from TB patients were stimulated with M. tuberculosis antigens, documented augmented TGF-β production compared with values yielded by PBMC from healthy PPD-positive individuals [39]. Such increased TGF-β synthesis was accompanied by decreased amounts of IFN-γ, with the abrogation of TGF-β (by adding anti-TGF-β antibodies or natural TGF-β inhibitors to PBMC cultures) resulting in increased IFN-γ production by cells from diseased individuals [39,40]. Concerning other reports, studies with murine T cells also showed that TGF-β down-regulated IL-12Rβ2 expression, leading to decreased IFN-γ production in response to IL-12 [41]. This was not the case when using human monocytes, as no TGF-β-related alteration in IL-12 production in response to in vitro M. tuberculosis infection was detected [42]. In light of all these findings, the increased TGF-β levels in advanced TB patients in this study may account for their lower IFN-γ values, the trend to show augmented IL-4 concentrations, and the unimpaired IL-12 production.
Host resistance against mycobacterial organisms is principally dependent on the bactericidal and/or bacteriostatic functions of macrophages activated by cytokines such as IFN-γ and TNF-α. The latter is mainly produced by activated macrophages and up-regulates the microbicidal activity of human monocytes or monocyte-derived macrophages against mycobacteria [8,43,44], although high local and systemic levels of TNF-α may promote a severe inflammatory response [10]. This contention may apply to our results, since the greater the amount of lung involvement the higher the TNF-α production. Similar findings were recently reported by analysing TNF-α concentrations in BALF [29]. Several pieces of evidence concur, pointing to a harmful effect of TNF-α in TB. Thus, Law et al. [11] showed that bronchoalveolar cells obtained from an affected lung produced more TNF-α than cells recovered from the uninvolved side. Other authors showed that monocyte TNF production was higher in patients with fever and cachexia [45], whereas our own findings in TB patients receiving immunotherapy with M. vaccae indicated that improved weight gain and time to become apyrexial was related to a greater fall in TNF-α serum levels, compared with patients receiving chemotherapy alone [46]. Similarly, reduced TNF-α production in TB patients undergoing thalidomide treatment was accompanied by accelerated weight gain [47].
Concerning the role of NO, even though this molecule exerts a cytocidal or cytostatic activity against a variety of pathogens, including mycobacteria [12,13,48], it is becoming increasingly evident that NO can be regarded as a double-edged sword, with excessive or long-lasting production being likely to result in damaging effects. It follows, that the ability of the host to synthesize NO does not necessarily lead to a favourable infection outcome, as suggested by our results in which higher levels of nitrite accumulation were more likely to prevail in cases with advanced disease. Following the demonstration that inducible NO synthase (iNOS), and mRNA regulating its synthesis, are present in human alveolar macrophages from TB patients [49], increased levels of NO were also detected in the exhaled air of patients with active TB, as was spontaneous nitrite production from cultured alveolar macrophages [50]. This report also documents that cases with more severe disease showed a reduced ability to generate NO [50]. At first sight this result may seem at variance with the present findings. Nevertheless, it should be noted that our measurements were carried out in cultured PBMC and may be more indicative of the accompanying systemic repercussions in cases with severe disease. Regarding its synthesis, NO is known to be induced and modulated by different compounds, particularly the up-regulatory IFN-γ and TNF-α cytokines [51]. Some evidence in the human system suggests that iNOS expression in monocytes/macrophages does not seem to be up-regulated by TNF-α and IFN-γ [52], while other investigations have demonstrated the generation of nitrite in response to TNF-α [53]. Recent studies employing human alveolar macrophages reported an inverse correlation between NO production and TGF-β synthesis [15]. Our strategy of using PBMC however, showed that higher levels of nitrite accumulation in culture supernatants from TB patients were accompanied by increased TNF-α production, in the presence of significant amounts of TGF-β; a finding that illustrates the intricate network of regulatory influences surrounding the production of biologic response modifiers.
To summarize, the present results may contribute to a clearer picture of the net effect of the interactions among the examined mediators involved in the immune response to M. tuberculosis. This is essential for a better comprehension of the immunopathological mechanisms underlying the distinct clinical forms of pulmonary TB.
Acknowledgments
This work was supported by grants from the Alberto J. Roemmers Foundation.
REFERENCES
- 1.Raviglione MC, Snider De, Jr, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Del Prete GF, De Carli M, Mastromauro C, Macchia D, Biagiotti R, Ricci M. Purified protein derivative of Mycobacterium tuberculosis and excretory/secretory antigen(s) of Toxocara canis expand in vitro human T cell with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991;88:346–50. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dlugovitzky D, Bay ML, Rateni L, et al. In vitro synthesis of interferon-γ, interleukin-4, transforming growth factor-β, and interleukin 1-β by peripheral blood mononuclear cells from tuberculosis patients. Relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;49:210–7. doi: 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptative immunity. Ann Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 7.Briscoe DM, Henault LE, Geehan C, Alexander SI, Lichtman AH. Human endothelial cell costimulation of T cell IFN-γ production. J Immunol. 1997;159:3247–56. [PubMed] [Google Scholar]
- 8.Hirsch CS, Yoneda T, Ellner JJ, Averill LE, Toossi Z. Enhancement of intracellular growth of M. tuberculosis in human monocytes by transforming growth factor beta. J Infect Dis. 1994;170:1229–37. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 9.Kindler V, Sapino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Ann Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 11.Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799–804. doi: 10.1164/ajrccm.153.2.8564135. [DOI] [PubMed] [Google Scholar]
- 12.Chan J, Xing Y, Magliozzom RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–6. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toosi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997;78:247–55. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 14.Tomioka H, Sato K, Maw WW, Saito H. The role of TNFα, IFNγ, TGFβ and NO in the expression of immunosuppressive functions of splenic macrophages induced by M. avium complex infection. J Leuk Biol. 1995;58:704–12. doi: 10.1002/jlb.58.6.704. [DOI] [PubMed] [Google Scholar]
- 15.Toossi Z, Ellner JJ. The role of TGF-β in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol. 1998;87:107–14. doi: 10.1006/clin.1998.4528. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme I. Immunity to mycobacteria. Curr Opin Immunol. 1993;5:497–502. doi: 10.1016/0952-7915(93)90029-r. [DOI] [PubMed] [Google Scholar]
- 19.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet. 1997;349:1513–5. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 20.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taha RA, Minshall EM, Olivenstein R, et al. Increased expression of IL-12 receptor mRNA in active pulmonary tuberculosis and sarcoidosis. Am J Respir Crit Care Med. 1999;160:1119–23. doi: 10.1164/ajrccm.160.4.9807120. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Gong J, Presky D, Xue W, Barnes PF. Expression of the IL-12 receptor β1 and β2 subunits in human tuberculosis. J Immunol. 1999;162:2441–7. [PubMed] [Google Scholar]
- 23.Zhang M, Lin Y, Iyer D, Gong J, Abrams JS, Barnes PF. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–4. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan S, Gong J, Zhang M, Samten B, Hanna LE, Narayanan PR, Barnes PF. Cytokine production in children with tuberculosis infection and disease. Clin Infect Dis. 1999;28:1290–3. doi: 10.1086/514794. [DOI] [PubMed] [Google Scholar]
- 25.Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. IFN-γ and IL-12 are increased in active compared with inactive tuberculosis. Am J Resp Crit Care Med. 1997;155:1135–9. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Zhang M, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–6. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munk ME, Mayer P, Anding P, Feldmann K, Kaufmann SHE. Increased numbers of interleukin-12-producing cells in human tuberculosis. Infect Immun. 1996;64:1078–80. doi: 10.1128/iai.64.3.1078-1080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casarini M, Ameglio F, Alemanno L, et al. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am J Respir Crit Care Med. 1999;159:143–8. doi: 10.1164/ajrccm.159.1.9803066. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AP, Murray HW. Intracellular antimicrobial activity in the absence of IFN gamma: effect of IL-12 in experimental visceral Leishmaniasis in IFN-gamma gene-disrupted mice. J Exp Med. 1997;185:1231–9. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor β and interleukin-10. J Biol Chem. 1992;267:23301–6. [PubMed] [Google Scholar]
- 32.Sánchez FO, Rodríguez JI, Agudelo G, García LF. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–8. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surcel HM, Troye-Blomberg M, Paulie S, Anderson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Warwick Davies J, Lowrie DB, Cole PJ. Selective deactivation of human monocyte functions by TGF-beta. J Immunol. 1995;155:3186–93. [PubMed] [Google Scholar]
- 35.Durham DM, Arkins S, Edward CK, Dantzer R-, III, Kelley KW. Role of interferon-γ in counteracting the suppressive effects of transforming growth factor-β2 and glucocorticoids on the production of tumor necrosis factor-α. J Leuk Biol. 1990;48:473–81. doi: 10.1002/jlb.48.6.473. [DOI] [PubMed] [Google Scholar]
- 36.Swain SL, Huston G, Tonkonogy S, Weinberg A. Transforming growth factor β and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 37.Takeuchi M, Kosiewicz MM, Alard P, Streilein JW. On the mechanism by which transforming growth factor-β2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997;27:1648–56. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- 38.Toosi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 39.Hirsch CS, Hussain R, Toosi Z, Dawood G, Shadid F, Ellner JJ. Cross modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch CS, Ellner JJ, Blinkhorn R, Tossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor β. Proc Natl Acad Sci USA. 1997;94:3926–31. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorham JD, Guler ML, Fenoglio U, Gubler U, Murphy KM. Low dose TGF-β attenuates IL-12 responsiveness in murine T cells. J Immunol. 1998;161:1664–70. [PubMed] [Google Scholar]
- 42.Fulton SA, Cross JV, Toossi ZT, Boom WH. Regulation of interleukin-12 by interleukin-10, transforming growth factor-β, tumor necrosis factor-α, and interferon-γ in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–14. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 43.Denis M, Gregg EO. Recombinant tumor necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990;71:139–41. [PMC free article] [PubMed] [Google Scholar]
- 44.Bermudez LE, Patrofsky MW, Young LS. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992;60:4245–52. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadranel J, Philippe C, Perez J, Milleron B, Akoun G, Ardaillou R, Baud L. In vitro production of tumor necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990;81:319–24. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dlugovitzky D, Bottasso O, Dominino JC, Valentini E, Hartopp R, Singh M, Stanford C, Stanford J. Clinical and serological studies of tuberculosis patients receiving immunotherapy with Mycobacterium vaccae. Respiratory Med. 1999;93:557–62. doi: 10.1016/s0954-6111(99)90155-5. [DOI] [PubMed] [Google Scholar]
- 47.Tramontana JM, Utaipat U, Molloy A, et al. Thalidomide treatment reduces tumor necrosis factor α production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med. 1995;1:384–97. [PMC free article] [PubMed] [Google Scholar]
- 48.De Groote MA, Fang FC. NO inhibitions: anti-microbial properties of nitric oxide. Clin Infect Dis. 1995;21(Suppl. 2):162–5. doi: 10.1093/clinids/21.supplement_2.s162. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson S, Bonecini-Almeida MDG, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11:809–15. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 51.Drapier JC, Wietzerbin J, Hibbs Jb., Jr IFN-γ and TNF-α induce the l-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–92. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 52.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–63. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- 53.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leuk Biol. 1991;49:380–2. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]