Abstract
Tumour necrosis factor-alpha (TNF-α), IL-1α and IL-6 production by human monocytes in response to a clinical strain of the Gram-negative encapsulated bacteria Neisseria meningitidis and an isogenic lpxA− strain deficient in LPS was investigated. Wild-type N. meningitidis at concentrations between 105 and 108 organisms/ml and purified LPS induced proinflammatory cytokine production. High levels of these cytokines were also produced in response to the lpxA− strain at 107 and 108 organisms/ml. The specific LPS antagonist bactericidal/permeability-increasing protein (rBPI21) inhibited cytokine production induced by LPS and wild-type bacteria at 105 organisms/ml but not at higher concentrations, and not by LPS-deficient bacteria at any concentration. These data show that proinflammatory cytokine production by monocytes in response to N. meningitidis does not require the presence of LPS. Therapeutic strategies designed to block LPS alone may not therefore be sufficient for interrupting the inflammatory response in severe meningococcal disease.
Keywords: lipopolysaccharide, monocytes, proinflammatory cytokines, Neisseria meningitidis
INTRODUCTION
Infections caused by Neisseria meningitidis are an important cause of mortality and morbidity world wide [1]. Despite recent advances in intensive care, the mortality for patients presenting with severe meningococcal sepsis remains between 20% and 50%. Those that survive may have extensive tissue injury sometimes requiring amputation and/or skin grafting [2].
The host inflammatory response in meningococcal sepsis is generally believed to be induced by LPS [3,4]. LPS complexed to CD14 and LPS binding protein signals through Toll-like receptors (TLR) to activate NFκB and induce production of proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), IL-1 and IL-6 [5,6]. Novel therapeutic interventions in Gram-negative septicaemia have been directed at modulating the effects of LPS [7] but with limited success [8–11]. The reason why LPS antagonists have not been effective is presently unknown. One possibility is that LPS may not be the only or even the main bacterial component responsible for inducing host inflammatory responses.
In the present study, we examined monocyte proinflammatory cytokine production induced by a clinical isolate of serogroup B N. meningitidis and an isogenic strain lpxA− that is completely deficient in LPS [12]. Our results show that LPS is not required for cytokine production in response to N. meningitidis and that strategies designed to block LPS alone may not be sufficient for interrupting the proinflammatory cytokine response.
MATERIALS AND METHODS
Bacteria and LPS
Neisseria meningitidis serogroup B (strain H44/76) is a clinical isolate from a case of fatal septicaemia [13]. A viable, LPS-deficient isogenic strain lpxA− derived from H44/76 was constructed by insertional inactivation of the lpxA gene required for lipid A biosynthesis [12,14]. Absence of LPS in the strain was confirmed by Limulus amoebocyte assay, MoAb binding, and gas chromatography/mass spectrometry. Bacteria were grown on gonococcal agar (Difco, West Mosley, UK) supplemented with Vitox (Oxoid, Basingstoke, UK). Purity of the lpxA− strain was maintained by culturing with 100 μg/ml of kanamycin (Sigma, Poole, UK) [12]. Bacteria were used in stationary phase after culture for 18 h. Suspensions were prepared in RPMI 1640 without phenol red (Gibco, Paisley, UK) and the optical density (OD) measured at 540 nm. Viability was determined with a modified Miles and Misra technique [15]. An OD of 1 was shown to be equivalent to 109 viable organisms per ml. Bacteria were fixed in 0·5% paraformaldehyde in PBS for 15 min and washed thoroughly in RPMI. Meningococcal LPS from N. meningitidis (H44/76) was prepared as previously described [16,17]. The final product contained <0·3% protein and was without detectable nucleic acids.
Stimulation of monocytes
Monocytes were stimulated with N. meningitidis H44/76, the lpxA− LPS-deficient strain or purified meningococcal LPS at the indicated concentrations in a modified whole blood culture technique described previously [18]. Brefeldin A (Sigma) was added at 10 μg/ml to prevent cytokine secretion. Cultures were incubated on a rocking platform for 4 h at 37°C before analysis for intracellular cytokine production. Where indicated, 10 μg/ml of the LPS antagonist rBPI21 (recombinant bactericidal/permeability-increasing protein; a gift from Xoma, Berkeley, CA) were also added.
Intracellular determination of monocyte cytokine production
After stimulation, 200-μl aliquots of the blood cultures were dispensed into 5-ml Falcon tubes (cat. no. 2054) and stained with CD14–FITC MoAb (TUK4) (Dako, Ely, UK). Erythrocytes were then lysed with FACS lysing buffer (Becton Dickinson, Oxford, UK), and the cells fixed in 250 μl of 4% paraformaldehyde for 15 min, and permeabilized with 50 μl of Leucoperm (Serotec Ltd, Oxford, UK) at room temperature. Monocytes were identified by gating on CD14. Granulocytes were excluded by forward and right angle scatter. Intracellular cytokines were detected with PE-conjugated MoAbs to TNF-α, IL-6 or IL-1α (Becton Dickinson) and analysed with a FACScalibur using Cellquest software (Becton Dickinson). Statistical comparisons were made by paired t-test using Sigmaplot.
RESULTS AND DISCUSSION
Monocyte proinflammatory cytokine production in response to wild-type N. meningitidis bacteria and the LPS-deficient lpxA− strain
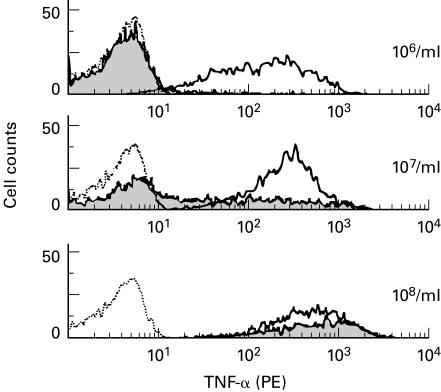
Intracellular TNF-α production by CD14+ monocytes was compared following activation with wild-type N. meningitidis and the isogenic lpxA− strain added at concentrations between 106 and 108 organisms/ml. The results of a typical experiment from more than six are given in Fig. 1. TNF-α was produced in response to wild-type bacteria added at concentrations between 106 and 108 organisms per ml in a dose-dependent manner. Equivalent high levels of TNF-α production were induced by the lpxA− strain at 108/ml. TNF-α was also induced but at lower levels with 107 and 106 lpxA− per ml. This result clearly shows that N. meningitidis can induce proinflammatory cytokine production by monocytes in the absence of LPS.
Fig. 1.
Tumour necrosis factor-alpha (TNF-α) production by monocytes in response to wild-type Neisseria meningitidis and the LPS-deficient lpxA− strain. Blood was cultured with the bacteria at a concentration of 106−108 organisms/ml for 4 h in the presence of Brefeldin A and the cells then surface stained for CD14, permeabilized and stained intracellularly for TNF-α. The results are expressed as FACS histograms using Cellquest software. Dotted line, unstimulated; solid line, wild-type N. meningitidis; grey fill, lpxA− LPS-deficient N. meningitidis.
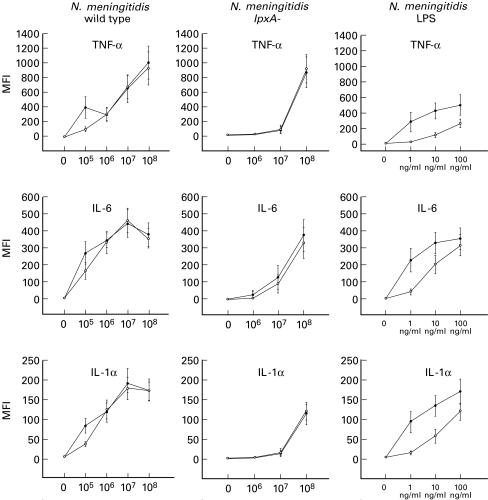
We next compared the ability of wild-type and lpxA− bacteria to induce IL-1α, IL-6 and TNF-α in the presence and absence of the LPS antagonist rBPI21(Fig. 2). At 108 organisms/ml, the wild-type and lpxA− bacteria induced equivalent high levels of all three cytokines. Significant cytokine production (P < 0·05) was also obtained with 107 organisms/ml, although the response to wild-type bacteria was higher than to the lpxA− strain. Below this concentration, the response to wild-type bacteria was still easily detectable but the response to the lpxA− strain was very low or absent. LPS at concentrations >1 ng/ml also induced cytokine production.
Fig. 2.
Cytokine production by monocytes activated with wild-type Neisseria meningitidis, lpxA− LPS-deficient strain and purified meningococcal LPS. Blood was cultured with the bacteria at a range of concentrations from 105 to 108 organisms/ml or with purified LPS at concentrations of 1, 10 and 100 ng/ml for 4 h in the presence of Brefeldin A. The cells were then surface stained for CD14, permeabilized and stained intracellularly for IL-1α, IL-6 and tumour necrosis factor-alpha (TNF-α). The results were determined as median fluorescence intensity (MFI) from histograms as shown in Fig. 1 and plotted as the mean ±s.e.m. from six separate experiments. •, Response without bactericidal/permeability-increasing protein (rBPI21); ○, response obtained in the presence of 10 μg/ml of rBPI21.
Addition of rBPI21 to the cultures inhibited cytokine production induced by LPS at concentrations up to 100 ng/ml (P < 0·05), confirming its activity as an LPS antagonist. Cytokine production induced with wild-type N. meningitidis bacteria at 105 organisms/ml was also significantly inhibited in all six experiments, typically to about 50% of control levels (P < 0·05). At higher concentrations of bacteria, rBPI21 did not inhibit cytokine production. Furthermore, it did not inhibit the response to the lpxA− strain at any concentration of bacteria tested. It is possible that the concentration of rBPI21 used in these experiments was not high enough to inhibit LPS present on the bacteria above 106/ml. Quantification of the LPS content in N. meningitidis based on spectrophotometric analysis of the LPS-specific sugar 2-keto-3-deoxyoctonic acid (KDO) showed that there are approximately 1·5 × 105 LPS molecules (mol. wt 4044) per N. meningitidis (H44/76) bacterium. LPS at 100 ng/ml is therefore equivalent to about 108 bacteria. As rBPI21 significantly inhibited responses to LPS at 100 ng/ml, the failure to inhibit cytokine production induced by 106−108 organisms/ml cannot be explained by the presence of high concentrations (> 100 ng/ml) of LPS. It is possible that rBPI21 does not completely inhibit cell-associated LPS, in which case the response with higher concentrations of bacteria (> 105/ml) may have been due in part to LPS together with other bacterial components. This may explain the inability of lpxA− bacteria to stimulate significantly at concentrations <107/ml. Nevertheless, our experiments show unequivocally that N. meningitidis can activate monocytes to produce proinflammatory cytokines in the absence of LPS. Various components of Gram-negative bacteria such as pili, outer membrane proteins, and opacity factors have been shown to activate NFκB and AP1/cjun in epithelial cells and may contribute to activation by the lpxA-strain [19–21]. Our results suggest that LPS may not have an exclusive role in monocyte activation by intact bacteria at concentrations >105/ml. The concentrations of bacteria present in septicaemic patients have been estimated at between 103 and >105/ml [22,23], but more recent estimates using quantitative polymerase chain reaction have indicated much higher concentrations of ≥107/ml (R. Borrow, personal communication). This bacterial load is significantly higher than previously estimated by quantitative blood cultures.
Our data indicate that bacteria at this concentration can induce an inflammatory response in the absence of LPS. Therapeutic measures aimed at blocking endotoxin may not be as effective as originally hoped [9,10]. Additive/synergistic effects of LPS and non-LPS pathways may explain our observation that wild-type bacteria frequently induce higher TNF-α levels than high concentrations of LPS and should be considered in meningococcal disease. Prompt administration of anti-microbial therapy in addition to anti-LPS therapy may be critical for avoiding catastrophic activation of host inflammatory processes induced by both LPS and non-LPS activated pathways. Future strategies for treating this disease should be directed against both LPS and non-LPS pathways.
Acknowledgments
We thank Dominic Jack and the staff of Clinical Microbiology Laboratory at Great Ormond Street Hospital. We also would like to thank XOMA for providing us with the rBPI21 and Russ Dedrick for his valuable comments on the manuscript. G.D. is funded by Royal College of Physicians.
REFERENCES
- 1.Jones DM. Epidemiology of meningococcal disease in Europe and the USA. In: Cartwright K, editor. Meningococcal disease. Chichester: John Wiley & Sons Ltd; 1995. [Google Scholar]
- 2.Kirsch EA, Barton RP, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996;15:967–78. doi: 10.1097/00006454-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Mercier JC, Beaufils F, Hartmann JF, Azema D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16:27–33. doi: 10.1097/00003246-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 4.van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144–66. doi: 10.1128/cmr.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright SD. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–9. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler EJ, Fisher Cj, Jr, Sprung CL, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324:429–36. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 8.Derkx B, Wittes J, McCloskey RV European Paediatric Meningococcal Septic Shock Trial Study Group. Randomised, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. Clin Infect Dis. 1998;28:770–7. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 9.The French National Registry of HA-A (Centoxin) in Septic Shock. A cohort study of 600 patients. The National Committee for the Evaluation of Centoxin. Arch Intern Med. 1994;154:2484–91. [PubMed] [Google Scholar]
- 10.McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med. 1994;121:1–5. doi: 10.7326/0003-4819-121-1-199407010-00001. [DOI] [PubMed] [Google Scholar]
- 11.J5 study group. Treatment of severe infectious purpura in children with human plasma from donors immunized with Escherichia coli J5: a prospective double-blind study. J Infect Dis. 1992;165:695–701. doi: 10.1093/infdis/165.4.695. [DOI] [PubMed] [Google Scholar]
- 12.Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–50. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 13.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–8. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MS, Raetz CR. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987;262:5159–69. [PubMed] [Google Scholar]
- 15.Miles AAMSS. The estimation of the bactericidal power of blood. J Hygiene Camb. 1932;38:732–9. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen SR, Bry K, Thorseng K, Jantzen E. Heterogeneity of lipopolysaccharides of Neisseria meningitidis revealed by thin-layer chromatography combined with monoclonal antibodies. J Microbiol Methods. 1996;25:187–94. [Google Scholar]
- 17.Tsai CM, Frasch CE, Rivera E, Holstein HD. Measurement of lipopolysaccharide (endotoxin) in meningococcal protein and polysaccharide preparations for vaccines usage. J Biol Stand. 1989;17:249–58. doi: 10.1016/0092-1157(89)90017-6. [DOI] [PubMed] [Google Scholar]
- 18.Ison CA, Heyderman RS, Klein NJ, Peakman M, Levin M. Whole blood model of meningococcal bacteraemia—a method for exploring host–bacterial interactions. Microb Pathog. 1995;18:97–107. doi: 10.1016/s0882-4010(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 19.Dixon GLJ, Heyderman R, Kotowicz K, Jack DL, Andersen SR, Vogel U, Frosch M, Klein NJ. Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability-increasing protein are influenced by the cCapsulation and lipooligosaccharide structure of Neisseria meningitidis. Infect Immun. 1999;67:5626–33. doi: 10.1128/iai.67.11.5626-5633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naumann M, Wessler S, Bartsch C, Wieland B, Meyer TF. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappaB and activator protein 1 and the induction of inflammatory cytokines. J Exp Med. 1997;186:247–58. doi: 10.1084/jem.186.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naumann M, Rudel T, Wieland B, Bartsch C, Meyer TF. Coordinate activation of activator protein 1 and inflammatory cytokines in response to Neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J Exp Med. 1998;188:1277–86. doi: 10.1084/jem.188.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan TD, LaScolea LJJ. Neisseria meningitidis bacteremia in children: quantitation of bacteremia and spontaneous clinical recovery without antibiotic therapy. Pediatrics. 1987;80:63–67. [PubMed] [Google Scholar]
- 23.Zwahlen A, Waldvogel FA. Magnitude of bacteremia and complement activation during Neisseria meningitidis infection: study of two co-primary cases with different clinical presentations. Eur J Clin Microbiol. 1984;3:439–41. doi: 10.1007/BF02017367. [DOI] [PubMed] [Google Scholar]