Abstract
In the present study, the concentration of TGF-β1 secreted by adherent cells isolated from human peripheral blood mononuclear cells (PBMC) and either stimulated with PGL-1 or lipopolysaccharide (LPS) or left unstimulated was determined by ELISA. The cells were isolated from untreated patients with different clinical forms of leprosy and healthy individuals. The adherent cells exhibited spontaneous release of TGF-β1 in all clinical forms of leprosy and in healthy individuals; however, lepromatous leprosy/borderline leprosy (LL/BL) patients presenting erythema nodosum leprosum (ENL) displayed significantly higher concentrations of TGF-β1 than either the other patients studied or the controls. These high TGF-β1 levels were consistently observed when LL/BL ENL cells were stimulated with phenolic glycolipid (PGL-1) or LPS, and even in the absence of a stimulus (P < 0·01). The most significant differences in TGF-β1 levels were observed when comparing the results in the presence of PGL-1 from ENL with, in order of significance: tuberculoid leprosy (TT) patients (P < 0·001), LL/BL patients without ENL (P < 0·01), healthy individuals (P < 0·01) and borderline-borderline/borderline-tuberculoid (BB/BT) patients with reversal reaction (RR) (P < 0·01). The BB/BT patients produced equivalent levels of TGF-β1 compared with LL/BL patients without ENL, for all types of stimuli (P > 0·05). In contrast, TT patients produced the lowest levels of TGF-β1 among all the subjects studied (both patients and healthy controls), especially following PGL-1 stimulation (P < 0·001, and P < 0·05, respectively). In conjunction with our previous data regarding TGF-β1 expression in dermal lesions, it appears that TGF-β1 probably plays different roles in leprosy: (i) to mediate a suppressive action locally, associated with the presence of PGL-1, and (ii) to induce proinflammatory effects when secreted systemically by monocytes, thereby acting as a modulatory cytokine in the acute inflammatory reactions of ENL and associated with the Th2 immune response in multibacillary forms of leprosy.
Keywords: leprosy, PGL-1, transforming growth factor-beta 1, ELISA, peripheral blood mononuclear cells
INTRODUCTION
Leprosy, caused by the intracellular pathogen Mycobacterium leprae, provides an extraordinary opportunity to investigate the human immune regulation of infection, because it presents a spectrum of clinical manifestations that correlate with the immune response of the host against the pathogen [1]. At one end of the clinical spectrum is tuberculoid leprosy (TT) that typifies the resistant response characterized by restricted growth of the pathogen. The number of lesions are few but tissue and nerve damage is frequent. At the opposite end is lepromatous leprosy (LL), in which the patients are unable to contain the infection and their lesions are diffusely distributed in the skin resulting in many viable M. leprae (up to 1010/g of tissue). These clinical presentations are correlated with the level of cell-mediated immunity (CMI), which is high in TT patients and in healthy exposed individuals but is strikingly absent in LL patients, and is associated with an inverse relationship with the humoral response. There is a potent antibody response in LL, but not TT, and this response is therefore not thought to play a role in protection. It has been demonstrated that there is a clear correlation between the clinical forms of leprosy and the state of mononuclear phagocyte activation in the lesions. In TT, the lesions are characterized by a predominance of CD4+ T cells and type-1 cytokines including IL-2 [2–4], interferon-gamma (IFN-γ) [4,5], IL-1β [5], tumour necrosis factor-alpha (TNF-α) [5] and IL-12 [6]. By contrast, in LL the skin lesions are characterized by a predominance of CD8+ T cells and type-2 cytokines including IL-4, IL-5 and IL-10 [4]. Moreover, reactional episodes may occur during the natural course of the disease, during treatment and even after treatment. The reversal reaction (RR) seems to be associated with a sudden increase in CMI against M. leprae antigens and is characterized by a predominantly type-1 cytokine profile (IL-1β, TNF-α, IL-2 and IFN-γ) in the lesions of the borderline patients. The erythema nodosum leprosum (ENL) type of reaction, which occurs in multibacillary leprosy patients, is a more systemic reaction than the RR and is immunopathologically more complex as well [7]. In this reaction, it has been shown that there is a selective increase in IL-6, IL-8 and IL-10 levels, whereas the levels of IL-4 and IL-5 remain unchanged [8].
The presence of large amounts of bacilli in the lesions of LL demonstrates the inability of macrophages to process these microorganisms. This may be explained, at least in part, by the presence of a cytokine that inhibits the microbicidal activity of macrophages. A cytokine with macrophage-suppressing activity, such as TGF-β1 [9,10], has been demonstrated in diseases caused by intracellular parasites [11–14] and in dermal lesions of patients with borderline leprosy (BL) and LL [15]. TGF-β1 is a product of activated monocytes [16], among other inflammatory cells, and is one of the most fascinating cytokines because it has a plethora of immunoregulatory effects which are described as bifunctional [17]. This cytokine is a potent proinflammatory and immunosuppressive molecule, in addition to its effects on cellular growth and differentiation [18]. TGF-β1 plays roles in the suppression of T cell responses, inhibiting both IFN-γ [19] and IL-2 expression [20], and has the ability to inhibit the lytic activity of macrophages by suppressing the production of intermediate oxygen-reactive and nitrogen-reactive factors [9,10], leading to the progression of infection. Recently, we have shown that TGF-β1 is produced by macrophages in LL and BL skin lesions, probably as part of the M. leprae evasion mechanism [15]. The role of circulating monocytes directed against M. leprae and its products, and their relationship with the production of TGF-β1 by macrophages resident in this microenvironment is however, unknown.
To investigate the immunoregulatory network at a systemic level, we determined the concentrations of TGF-β1 secreted by blood monocytes from patients with different clinical forms of leprosy and healthy individuals, when these cells were stimulated in vitro with either phenolic glycolipid (PGL-1) antigen from M. leprae or bacterial lipopolysaccharide (LPS).
PATIENTS and METHODS
Patients and healthy individuals
Nineteen untreated patients with the following forms of leprosy were studied: LL (n = 6); BL (n = 4); borderline-borderline (BB, n = 2), borderline-tuberculoid (BT, n = 4), and TT (n = 3). Among the multibacillary patients (LL/BL, n = 10), five presented ENL (n = 5) or Jopling type-2 leprosy reaction. Among the patients from the borderline group (BB/BT, n = 6), three presented RR (n = 3) of Jopling type-1 leprosy reaction. A group of healthy individuals (n = 9) was studied as a control group. The patients were clinically classified by the bacilloscopic index (BI) (mean counts of bacilli on heat-fixed dermal smears obtained from skin lesions and from usually involved areas, such as ear lobes and elbows, and stained by the method of Ziehl–Neelsen) [21], by the Mitsuda reaction (intradermal reaction with a suspension of heat-killed M. leprae containing 6 × 107 bacilli/ml) and by histopathological evaluation, according to Ridley & Jopling criteria [1].
Monocyte cell culture and stimuli
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque and platelets were removed from the monocytes by washing in Versene buffer and differential centrifugation as previously described [22,23]. Monocytes were cultured in RPMI 1640 in the presence of 2·5% heat-inactivated human AB serum at 37°C for 1 h. After removing the non-adherent cells, the adherent monolayers were then cultured in the presence of different stimuli, such as PGL-1 (1 μg/ml; Laboratory of Mycobacterial Research, National Institute for Medical Research, London, UK) and LPS (10 μg/ml; Sigma Chemical Co., St Louis, MO), or in serum-free RPMI 1640 (Gibco, Life Technologies, Paisley, UK) only as a control. The supernatants were harvested after 48 h culture.
TGF-β1 detection
TGF-β1 concentrations were determined in cell-free supernatants. TGF-β1 detection was done by enzyme immunoassay (Promega, Madison, WI) designed for the sensitive and specific detection of the biologically active molecule in an antibody sandwich format. Briefly, flat-bottomed 96-well plates were coated with MoAb to TGF-β1 (at 1:1000 dilution) which binds the soluble molecule from solution. The captured TGF-β1 was bound by a second specific polyclonal antibody (at 1:1000 dilution) and, after washing, the amount of specifically bound secondary antibody was detected using a species-specific antibody conjugated to peroxidase as a tertiary reactant. This immunoassay is able to detect a minimum of 25 pg/ml of TGF-β1 and has ≤5% cross-reactivity with TGF-β2 or TGF-β3 at 10 ng/ml. The Kruskal–Wallis test for unpaired samples was used for statistical analysis.
RESULTS
Table 1 summarizes the data obtained from patients in terms of clinical and laboratory information. Initial time-course studies revealed that optimal TGF-β1 release into the supernatant occurred after 48 h of culture. The results demonstrate that the adherent cells exhibited spontaneous release of TGF-β1 in all clinical forms of leprosy and in healthy individuals. High TGF-β1 levels were detected for the majority of the leprosy patients in comparison with healthy individuals, except for the TT patients (Table 2). Generally, these findings occurred independently of stimulus type in the established conditions for cell culture (Figs 1, 2 and 3).
Table 1.
Characteristics of the patients infected by Mycobacterium leprae, according to their clinical forms and laboratory data
| Patients | Sex | Year/ origin | Age (years) | Clinical form | Leprosy reaction | BI | Mitsuda test (mm) | Histopathology |
|---|---|---|---|---|---|---|---|---|
| P1 | F | 96, UDI | 45 | BB | Type 1 (RR) | 0 | 0 | BB Granuloma, AARB 2+ |
| P2 | M | 96, UDI | 45 | LL | Type 2 (ENL) | 4·5 | 0 | LL Infiltrate, AARB 5+ |
| P3 | M | 97, UDI | 35 | LL | – | 5·25 | 0 | LL Infiltrate, AARB 5+ |
| P4 | F | 97, UDI | 44 | LL | – | 2·0 | 0 | LL Infiltrate, AARB 4+ |
| P5 | F | 97, UDI | 35 | LL | – | 3·0 | 0 | LL Infiltrate, AARB 4+ |
| P6 | F | 97, UDI | 61 | BB | Type 1 (RR) | 0 | 2 | BB Granuloma, AARB 1+ |
| P7 | F | 97, UDI | 25 | LL | – | 5·5 | 0 | LL Infiltrate, AARB 6+ |
| P8 | M | 97, UDI | 67 | BT | – | 0 | 6 | BT Granuloma, AARB− |
| P9 | F | 97, RP | 46 | BL | Type 2 (ENL) | 3·0 | 0 | BL Infiltrate, AARB 4+ |
| P10 | M | 97, RP | 68 | TT | – | 0 | 5 | TT Granuloma, AARB− |
| P11 | M | 97, RP | 46 | BT | – | 0 | 1 | BT Granuloma, AARB 1+ |
| P12 | M | 97, RP | 54 | LL | Type 2 (ENL) | 3·75 | 0 | LL Infiltrate, AARB 5+ |
| P13 | M | 97, RP | 25 | TT | – | 0 | 7 | TT Granuloma, AARB− |
| P14 | F | 97, RP | 45 | BL | Type 2 (ENL) | 3·0 | 0 | LL Infiltrate, AARB 3+ |
| P15 | M | 97, RP | 22 | BL | Type 2 (ENL) | 3·0 | 0 | BL Infiltrate, AARB 3+ |
| P16 | M | 98, RP | 54 | TT | – | 0 | 8 | TT Granuloma, AARB− |
| P17 | F | 98, RP | 57 | BL | – | 1·5 | 0 | BL Infiltrate, AARB 4+ |
| P18 | M | 98, RP | 41 | BT | Type 1 (RR) | 0 | 5 | BT Granuloma, AARB− |
| P19 | M | 99, RP | 36 | BT | – | 0 | 5 | BT Granuloma, AARB 1+ |
RR, Reversal reaction; ENL, erythema nodosum leprosum; BI, bacilloscopic index; AARB, alcohol-acid-resistant bacilli; UDI, Uberlândia – MG; RP, Ribeirão Preto – SP, Brazil.
Table 2.
Levels of TGF-β1 (mean ± s.d.) in supernatants of adherent cells from peripheral blood mononuclear cells (PBMC) from patients with different clinical forms of leprosy (LL/BL with ENL, LL/BL without ENL, BB/BT without RR, BB/BT with RR, TT) and healthy individuals (HI) measured by ELISA
| Clinical forms and healthy individuals | Type of stimulus | Concentration of TGF-β1 (pg/ml), 48 h, mean ± s.d. |
|---|---|---|
| LL/BL with ENL | RPMI | 6034·92 ± 3807·58* |
| (n = 5) | LPS | 6334·80 ± 3578·11* |
| PGL-1 | 6325·97 ± 3894·93* | |
| LL/BL without ENL | RPMI | 960·23 ± 411·86 |
| (n = 5) | LPS | 907·71 ± 344·30 |
| PGL-1 | 1065·86 ± 474·54 | |
| BB/BT without RR | RPMI | 1394·93 ± 171·00 |
| (n = 3) | LPS | 1740·80 ± 328·60 |
| PGL-1 | 1770·60 ± 287·80 | |
| BB/BT with RR | RPMI | 982·60 ± 139·20 |
| (n = 3) | LPS | 795·60 ± 331·50 |
| PGL-1 | 1516·98 ± 527·90 | |
| TT | RPMI | 204·98 ± 194·22** |
| (n = 3) | LPS | 208·43 ± 211·32** |
| PGL-1 | 105·42 ± 138·71**† | |
| HI | RPMI | 565·11 ± 315·23 |
| (n = 9) | LPS | 657·05 ± 358·04 |
| PGL-1 | 638·76 ± 285·01 |
Statistically significant compared with other clinical forms of leprosy or healthy individuals (LL/BL with ENL versus LL/BL without ENL, P < 0·01; LL/BL with ENL versus BB/BT with RR, P < 0·01; LL/BL with ENL versus TT, P < 0·001; LL/BL with ENL versus HI, P < 0·001).
Statistically significant compared with other clinical forms of leprosy (P < 0·001).
Statistically significant compared with healthy individuals under PGL-1 stimulation only (P < 0·05).
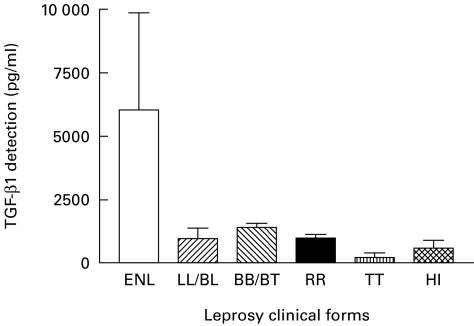
Fig. 1.
Detection of TGF-β1 in the supernatant of unstimulated monocytes. HI, Healthy individuals.
Fig. 2.
Detection of TGF-β1 in the supernatant of monocytes stimulated with lipopolysaccharide. HI, Healthy individuals.
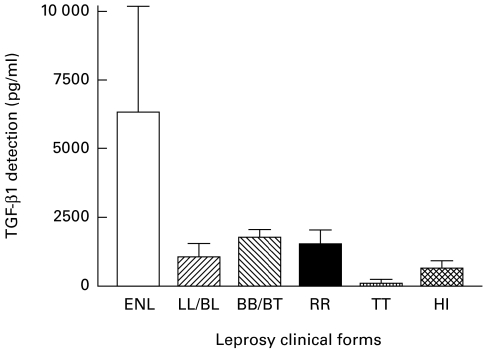
Fig. 3.
Detection of TGF-β1 in the supernatant of monocytes stimulated with phenolic glycolipid (PGL-1). HI, Healthy individuals.
Adherent cells from patients with LL/BL presenting ENL displayed significantly higher concentrations of TGF-β1 in the culture supernatants in the presence of both PGL-1 (Fig. 3) and LPS (Fig. 2), and without stimulation (Fig. 1) compared with patients with all other clinical forms of leprosy (P < 0·01) or healthy individuals (P < 0·001) (Table 2).
On the other hand, the TGF-β1 levels produced by the adherent cells from TT patients, in the presence of either PGL-1 or LPS or in unstimulated cultures, were lower than in all other clinical forms of leprosy (P < 0·001). In addition, these TT TGF-β1 values were equivalent to the results obtained with PBMC from healthy individuals either stimulated with LPS or left unstimulated (P > 0·05) (Table 2). When the cells were stimulated by PGL-1 however, the macrophages from TT patients produced TGF-β1 at levels significantly lower than adherent cells from healthy individuals (P < 0·05) (Fig. 3).
When TGF-β1 production by adherent cells was compared between BB/BT patients without reaction and BB/BT patients with RR, no significant differences were observed for all cell culture conditions (P > 0·05; Table 2; Figs 1, 2 and 3). Furthermore, macrophage cultures from patients with BB/BT with type 1 Jopling reaction (RR) produced significantly lower levels of TGF-β1 when compared with cells from LL/BL patients with type-2 Jopling reaction (ENL) (P < 0·01, P < 0·001, P < 0·05, for unstimulated, LPS- and PGL-1-stimulated cells, respectively).
DISCUSSION
In the present study it was shown that adherent cells from PBMC exhibited spontaneous release of TGF-β1 in all clinical forms of leprosy and in healthy individuals. The most significant result was that patients with ENL showed markedly higher TGF-β1 concentrations than all other patients and controls studied. Since TGF-β1 primes macrophages to express inflammatory gene products in response to particulate stimuli [24], these findings have important implications in ENL where the scavenging macrophage is most likely to encounter phagocytosable bacilli, so amplifying the inflammatory response.
After exposure to phagocytic stimuli, macrophages synthesize and release inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, which are greatly increased in ENL patients [8,25,26]. TGF-β1 regulates the production of IL-1, IL-6 and TNF-α [27–29]. These are important cytokines in the inflammatory response and may induce macrophage differentiation and acute-phase protein synthesis [30]. IL-6 is involved in up-regulation of TGF-β1 [31] and increased concentrations of IL-6 inhibit the T cell response via induction of TGF-β1 [32]. Continuous secretion of TGF-β1 by activated inflammatory cells might result in excessive attraction of these cells into the lesions. As seen in ENL, this event may lead to tissue injury mediated by leucocytes, in addition to the induction of prostaglandin E2 (PGE2) [33], with additional immunosuppressive effects on monocytes, lymphocytes and neutrophils [34].
Furthermore, it was observed in the present study that monocytes from LL/BL patients without ENL presented similar levels of TGF-β1 to those found in BB/BT patients with or without RR. These results indicate that the peripheral levels of this cytokine might not be associated with the situation in cutaneous lesions [15]. Probably, this apparent preference of M. leprae for dermal macrophages and Schwann cells promotes the basis for a locally induced immune response, leading to immunosuppression in response to M. leprae antigens. Evidence exists suggesting that macrophages in LL/BL patients may be efficient at phagocytosis, but less efficient at other functions, such as antigen presentation [35].
In BB/BT patients, an immunologically unstable group, the RR seems to be associated with a sudden increase in CMI, triggered by the burden of immunologically detected antigen at a particular site or sites [36]. This phagocytic stimulus may also lead to production of TGF-β1 in a more endocrine mode of action [37], as a mechanism of self-regulation of TGF-β1 production by macrophages [38].
The results obtained in the present study also demonstrate that TGF-β1 was secreted by adherent cells from TT patients at lower levels than patients with all other clinical forms of leprosy. In addition, healthy individuals produced equivalent levels of TGF-β1 when compared with TT patients. Following PGL-1 stimulation however, TT patients produced lower levels of TGF-β1 than healthy individuals. These findings are in agreement with our previous results for TGF-β1 detection in skin lesions [15], where this cytokine was not found in TT patients, indicating that the absence of TGF-β1 is associated with granuloma formation followed by activation of a cascade of proinflammatory cytokines, similar to that described in experimental models using mice with a TGF-β1 gene deletion [39,40].
In conclusion, these data suggest that TGF-β1 appears to play different roles in leprosy: it presents a proinflammatory effect on the inflammatory reaction, especially in ENL, stimulating the Th2 response and an immunosuppressive effect in the presence of PGL-1 or other M. leprae antigens.
Acknowledgments
This work was financially supported by the Brazilian Research Council (CNPq), Department of Internal Medicine, School of Medicine of Ribeirão Preto, University of São Paulo, and Federal University of Uberlândia, Minas Gerais, Brazil. The technical assistance of Maria Aparecida N. Ferreira, Deise Aparecida O. Silva, and helpful discussions and supplies from Terezila M. Coimbra and Célio L. Silva are gratefully acknowledged.
REFERENCES
- 1.Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 2.Longley J, Haregewoin A, Yemaneberhan T, Van Diepen TW, Nsibami J, Knowles D, Smith KA, Godal T. In vivo responses to Mycobacterium leprae: antigen presentation, interleukin-2 production, and immune cell phenotypes in naturally occurring leprosy lesion. Int J Lepr. 1985;53:385–94. [PubMed] [Google Scholar]
- 3.Modlin RL, Hofman FM, Horwitz DA, Husman LA, Gillis S, Taylor CR, Rea TH. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J Immunol. 1984;132:3085–90. [PubMed] [Google Scholar]
- 4.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 5.Arnoldi J, Gerdes J, Flad H-D. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am J Pathol. 1990;137:749–53. [PMC free article] [PubMed] [Google Scholar]
- 6.Sieling PA, Wang X-H, Gately MK, et al. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–47. [PubMed] [Google Scholar]
- 7.Naafs B. Leprosy reactions: new knowledge. Trop Geogr Med. 1994;46:80–84. [PubMed] [Google Scholar]
- 8.Yamamura M, Wang X-H, Ohmen JD, Uyemura TH, Bloom BR, Modlin RL. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- 9.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 10.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivation factor and transforming growth factors -β1-β2, and -β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J Immunol. 1990;145:940–4. [PubMed] [Google Scholar]
- 11.Barral-Neto M, Barral A, Brownell CE, Skeiky YAW, Ellingsworth LR, Twardzik DR, Reed SG. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–8. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 12.Bermudez LE, Covaro G, Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor-β and downregulation of expression of tumor necrosis factor receptors. Infect Immun. 1993;61:4126–30. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor-β (TGF-β) J Exp Med. 1991;174:539–45. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotz M, Seth P. TGF-β and HIV infection. Ann NY Acad Sci. 1993;685:501–11. doi: 10.1111/j.1749-6632.1993.tb35912.x. [DOI] [PubMed] [Google Scholar]
- 15.Goulart IMB, Figueiredo F, Coimbra T, Foss NT. Detection of transforming growth factor-β1 in dermal lesions of different clinical forms of leprosy. Am J Pathol. 1996;148:911–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–4. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl SM, McCartney-Francis N, Mergenhagen SE. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989;10:258–61. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield LM, Thompson NL, Flanders KC, O'connor-McCourt MD, Sporn MB. Transforming growth factor-beta: multifunctional regulator of cell growth and phenotype. Ann NY Acad Sci. 1988;551:290–7. doi: 10.1111/j.1749-6632.1988.tb22355.x. [DOI] [PubMed] [Google Scholar]
- 19.Espevick T, Figari IS, Shalaby MR, Lackides GA, Lewis GD, Shepard HM, Palladino MA. Inhibition of cytokine productions by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166:571–6. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'angeac AD, Dornand J, Emonds-Alt X, Jullien P, Garcia-Sanz JA, Erard F. Transforming growth factor type beta 1 (TGF-beta 1) down-regulates interleukin-2 production and up-regulates interleukin-2 receptor expression in a thymoma cell line. J Cell Physiol. 1991;147:460–9. doi: 10.1002/jcp.1041470312. [DOI] [PubMed] [Google Scholar]
- 21.Jopling WH, McDougall AC. Manual de Hanseníase. 2. London: Medical Book Ltd, W. Heinemann; 1991. pp. 11–55. [Google Scholar]
- 22.Channon JY, Kasper LH. Toxoplasma gondii induced immune suppression by human peripheral blood monocytes: role of gamma interferon. Infect Immun. 1996;64:1181–9. doi: 10.1128/iai.64.4.1181-1189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesion. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 24.Noble PW, Henson PM, Lucas C, Mora-Worms M, Carré PC, Riches DWH. Transforming growth factor-β primes macrophages to express inflammatory gene products in response to particulate stimuli by an autocrine/paracrine mechanism. J Immunol. 1993;151:979–89. [PubMed] [Google Scholar]
- 25.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–80. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parida SK, Grau GE, Zaheer AS, Murkherjee R. Serum tumor necrosis factor and interleukin-1 in leprosy and during lepra reactions. Clin Immunol Immunopathol. 1992;63:23–27. doi: 10.1016/0090-1229(92)90088-6. [DOI] [PubMed] [Google Scholar]
- 27.Chantry D, Turner M, Abney E, Feldman M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989;142:4295–300. [PubMed] [Google Scholar]
- 28.Turner M, Chantry D, Feldmann M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2:211–6. doi: 10.1016/1043-4666(90)90018-o. [DOI] [PubMed] [Google Scholar]
- 29.Musso T, Espinoza-Delgado I, Pulkki K, Gusella GL, Longo DL, Varesio L. Transforming growth factor beta downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood. 1990;76:2466–9. [PubMed] [Google Scholar]
- 30.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF) FASEB J. 1990;4:2860–7. [PubMed] [Google Scholar]
- 31.Gibellini D, Zauli G, Re MC, Milani D, Furlini G, Caramelli E, Capitani S, La Placa M. Recombinant human immunodeficiency virus type-1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF-β1 mRNA expression and protein synthesis in peripheral blood monocytes. Br J Haematol. 1994;88:261–7. doi: 10.1111/j.1365-2141.1994.tb05016.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou D, Munster A, Winchurch RA. Pathologic concentrations of interleukin 6 inhibit T cell responses via induction of activation of TGF-β. FASEB J. 1991;5:2582–5. doi: 10.1096/fasebj.5.11.1868982. [DOI] [PubMed] [Google Scholar]
- 33.Misra N, Selvakumar M, Sing S, Bharadwaj M, Ramesh V, Misra RS, Nath I. Monocyte derived IL-10 and PGE2 are associated with the absence of Th1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol Letters. 1995;48:123–8. doi: 10.1016/0165-2478(95)02455-7. [DOI] [PubMed] [Google Scholar]
- 34.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 35.Krahenbuhl J, Adams B. The role of the macrophage in resistance to the leprosy bacillus. Immunol Ser. 1994;60:281–302. [PubMed] [Google Scholar]
- 36.Ridley DS. Documenta Geigy. 3. Basle: Ciba-Geigy Ltd; 1990. Biopsy in leprosy; p. 63. [Google Scholar]
- 37.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor β1 has a longer plasma half-life in rats than active transforming growth factor β1, and a different tissue distribution. J Clin Invest. 1990;86:1976–84. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCartney-Francis N, Mizel D, Wong H, Wahl L, Wahl S. TGF-β regulates production of growth factors and TGF-β by human peripheral blood monocytes. Growth Factors. 1990;4:27–35. doi: 10.3109/08977199009011007. [DOI] [PubMed] [Google Scholar]
- 39.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulkarni AB, Huh C-G, Becker D, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]