Abstract
The autoimmune nature of primary biliary cirrhosis (PBC) is well established. We tested the hypothesis that fetal microchimerism indicated by the persistence of circulating fetal cells in women years after pregnancy might contribute to the aetiopathogenesis of PBC through a graft-versus-host-like response. We extracted DNA from the peripheral blood cells of 36 women carefully selected from 173 consecutive PBC patients, who were matched with 36 healthy women by age, age of last son, and number of children. Both patients and controls had to have male offspring, and no history of miscarriages or blood transfusions; they could not be twins. We tested all of the samples for the presence of two specific Y-chromosome sequences (SY154 and SRY) by amplifying DNA in a nested polymerase chain reaction. Y-chromosome-specific DNA was detected in the peripheral blood cell DNA of 13 (36%) of the 36 women with PBC and in 11 (31%) of the 36 healthy controls. The two groups of PBC patients with and without male DNA sequences were similar in terms of their clinical, biochemical, and serological features. Y-chromosome sequences were found in three of the four PBC women with associated systemic sclerosis. All of the 24 Y-positive samples contained SY154 sequences, but only three PBC patients and six controls showed the presence of both SY154 and SRY sequences. This discrepancy may suggest that not only fetal cells but also fragments of fetal DNA are present in maternal circulation. Overall, our data do not support the hypothesis that fetal microchimerism plays a significant role in the onset or progression of PBC.
Keywords: autoimmunity, liver disease, chronic cholestasis, Y-chromosome
INTRODUCTION
Primary biliary cirrhosis (PBC) is a chronic progressive liver disease of autoimmune origin, which shares important clinical and histological features of autoimmunity with chronic graft-versus-host disease (GVHD) [1,2]. Both conditions can be associated with autoimmune diseases such as Sjögren's syndrome (SS) or systemic sclerosis (SSc), both can have serum autoantibodies and, more importantly, both have similar histological patterns of mononuclear cell liver infiltrates and bile duct damage [1,2].
It has long been known that chronic GVHD is a chimeric disease that occurs in recipients of allogeneic stem cell transplants [3]. In view of the recent observation that allogeneic fetal cells can persist in healthy women for decades after pregnancy [4], Nelson has suggested that fetal microchimerism might be involved in the aetiopathogenesis of some autoimmune diseases, such as SSc (which more often affects middle-aged women and frequently in the years after childbearing), probably by initiating a graft-versus-host-like response [5]. In accordance with this hypothesis, non-self nucleated fetal cells have been found in the peripheral blood of women with SSc more frequently and in larger amounts than in healthy women [6–8]; interestingly, fetal cells have also been found in the skin lesions of SSc patients [7].
In line with the original theory, it has been more recently speculated that fetal microchimerism may also play a role in the aetiopathogenesis of PBC [9]. A high proportion of Y-chromosome sequences has been found in liver specimens taken from patients with PBC or other liver diseases [10]. No data are yet available concerning the presence of circulating fetal cells in women with PBC.
In the present study, we estimated the presence of Y-chromosome sequences (i.e. the prevalence of peripheral blood Y-microchimerism) in the maternal circulation of carefully selected women with PBC; for purposes of comparison, a group of healthy pair-matched women was also investigated. Particular care was taken to avoid any known confounding factor and sources of non-fetal allogeneic circulating cells, such as blood transfusions, abortions and twinship. We also investigated whether the presence of specific male sequences in PBC is associated with particular disease characteristics.
PATIENTS and METHODS
Study population and design
The PBC population consisted of 36 patients from whom whole blood was drawn in order to extract the total DNA to be tested for highly specific Y-chromosome sequences. These patients were identified on the basis of strict inclusion and exclusion criteria from a larger series of 173 PBC patients who consecutively attended our centre between November 1998 and August 1999. The diagnosis of PBC was based on internationally accepted criteria [11]. Six of the 36 patients who were anti-mitochondrial antibody (AMA)-negative but otherwise met the diagnostic criteria for the disease were considered as having AMA-negative PBC, as previously reported [12,13]. All of the patients had to be female and negative for hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (HCV) according to the study protocol. The date of birth of the patients' first child had to precede the date of the earliest suspected evidence of liver disease. They had to have at least one son, no history of miscarriages or blood transfusions, and could not be twins. The diagnosis of associated SSc was defined using the criteria of LeRoy et al. [14]. Ursodeoxycholic acid was being administered to 25 (69%) of the patients as the only treatment for liver disease at the time of blood sampling.
For control purposes, we extracted DNA from the peripheral blood cells of 36 healthy women with at least one son and no known previous autoimmune disease, abortions or blood transfusions; they could not be twins. To rule out any suspicion of an underlying PBC, all of them were tested for AMA by means of indirect immunofluorescence and had to be negative. The healthy subjects were pair-matched with the PBC patients on the basis of two age classes (≤ 50 versus >50 years), four age classes of the last son (0–10 versus 10–20 versus 20–30 versus ≥ 30 years), and three classes by number of children (one or two versus three or four versus more than five children).
Serum liver function and lipids, immunoglobulins, HBsAg, antibody to hepatitis B core antigen, and antibody to HCV were all assessed by means of routine laboratory methods; anti-mitochondrial, anti-nuclear, anti-centromere and anti-smooth muscle antibodies were determined using indirect immunofluorescence (IIF). The presence of symptoms was defined as the occurrence of pruritus, jaundice or major complications of cirrhosis: i.e. hepatic encephalopathy, variceal bleeding, ascites requiring diuretic therapy, or hepatocellular carcinoma. Disease duration was calculated as the time between the date of the earliest suspected evidence of liver disease and the date of blood sampling. The patients with no fibrosis at liver biopsy (i.e. those at Ludwig's stage I and II [15]) were considered as having early stage disease; those with fibrosis or cirrhosis (i.e. stage III or IV) were considered as having advanced disease.
The study protocol respected the ethical guidelines of the 1975 Declaration of Helsinki and subsequent modifications, and all of the patients and healthy controls gave their consent in writing after being informed about the nature of the study.
Determination of Y-chromosome sequences in peripheral blood
All of the peripheral blood samples from the PBC and healthy subjects were obtained at the same clinical centre, where they were in-batch tested under blinded conditions. Genomic DNA was obtained from 5 ml of anti-coagulated whole blood using the standard phenol chloroform technique. All of the DNAs were tested by means of nested polymerase chain reaction (PCR) in order to investigate the presence of two Y-specific sequences: one located in Yp 11.3 and included in the SRY gene, and the other corresponding to a sequence-tagged site (STS) sequence (SY154) of the DAZ (deleted in azoospermia) region on Yq 11.23. One aliquot from each DNA sample was tested more than once for each Y sequence in order to verify the repeatability of the results. The SRY primers (1F, 1R and the nested primer 1FB) were taken from the SRY gene sequence (GenBank Accession no. X53772), and the SY154 primers (154A, 154B and the nested primer 154A1) from the clone NH0086G22 sequence (GenBank Accession no. AC006366). The primer and their relative positions in the sequences are listed in Table 1. The Y specificity of the sequences was confirmed by the absence of any amplified product when the DNA obtained from the peripheral blood of a woman with no history of pregnancy or abortion was used as a template in the amplification mixture. In order to evaluate the sensitivity of our technique, we mixed male and female DNA at various ratios in order to observe the Y-DNA signal after the nested PCR for both SY154 and SRY up to a dilution of 1:20 000 of male:female DNA. In the setting of this technique, we performed several experiments with different Y-primer sequences but could not reproduce the same Y-specific results reported by others [7,16], because the DNA from the healthy female subject with no history of pregnancy or abortion frequently showed the amplification signal. In particular, we tested primers described by Artlett et al.(Y1, Y2, Y3, Y4) [7] and primers described by Lo et al.(Y5, Y6, Y7, Y8) [16].
Table 1.
PCR primers used for the Y assay of primary biliary cirrhosis and control cases
| Primer | Position | Sequence 5′ → 3′ |
|---|---|---|
| SY154A (sense) | 94527–94546 | Tttgcaccaggattaagtga |
| SY154A1 (sense) | 94591–94610 | Gtaaccattactgaaaccag |
| SY154B (antisense) | 94748–94771 | Ttttttcagataaactttcagtgg |
| 1F (sense) | 543–566 | Cagtgtgaaacgggagaaaacagt |
| 1FB (sense) | 631–543 | Agaggcgcaagatggctctagag |
| 1R (antisense) | 798–812 | Cttccgacgaggtcgatacttata |
The SRY PCR was performed in a 50-μl final volume containing 50 mm KCl, 10 mm Tris–HCl, 1·5 mm MgCl2, 0·1% Triton X-100, 0·4 μm of each primer, a deoxyribonucleoside triphosphate mixture containing AUCG at a concentration of 200 μm each. Taq DNA Polymerase was purchased from Promega (Catalogue no. M1661; Promega Corp., Madison, WI). The use of dUTP instead of dTTP allowed us to destroy all of the amplified products in the case of contamination by means of the application of Uracil N-glycosylase (Perkin Elmer, Norwalk, CT), an enzyme capable of cutting the DNA sequences containing dUTP. The SY154 PCR was performed in a 10-μl final volume containing the same components as that used for the SRY PCR except for MgCl2 (1·75 mm). As template for the first PCR, we used 100 ng of genomic DNA for the SY154 amplification and 300 ng for the SRY amplification; the template of the nested PCR was, respectively, 1 μl or 3 μl of the first PCR for the SY154 or SRY amplification. As there is a high risk of contamination when using nested PCR, we adopted all of our previously described precautions in order to avoid it [17]. In brief, the PCR mixtures of the first and nested PCRs were prepared in a laminar flow cabinet, the genomic DNA was added in a different room, and the template of the nested PCR (an amplified product) was added to the mixture in a specific PCR room; the amplified products and the instruments used for their processing were never used outside the PCR room. Following these precautions, we never found any contamination in the negative controls used for each PCR (one tube without DNA and two tubes with DNA obtained from the peripheral blood of two women who had never undergone pregnancy or abortion).
PCR conditions were: SRY: 30 cycles of 95°C for 60 s, 60°C for 60 s and 72°C for 60 s, with a final extension of 5 min at 72°C; Y154: 29 cycles of 94°C for 60 s, 55°C for 120 s and 72°C for 120 s, with a final extension of 4 min at 72°C. After the nested PCR, the amplified products (10 μl) were loaded together with a DNA marker on a 2% agarose gel containing ethidium bromide, electrophoresed in 0·5% TBE at 100 V, and then visualized by means of UV.
Statistical analysis
To compare the groups of patients defined on the basis of their fetal microchimerism status, the χ2 or Fisher's exact test were used to analyse the categorical variables. In the case of the continuous variables, the Mann–Whitney test was used to compare two groups, and the Kruskal–Wallis non-parametric one-way analysis of variance test was used to compare more than two groups. The statistical comparisons were made using Stata Statistical Software (Stata Corp., College Station, TX). All of the analyses were two-sided, and P <0·05 was considered statistically significant.
RESULTS
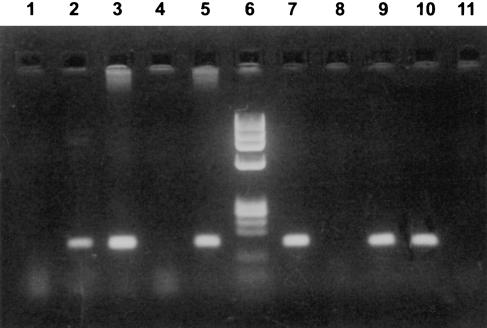
The main characteristics of the PBC patients and control subjects at time of blood sampling are shown in Table 2. Y-chromosome-specific sequences were found in 13 of the 36 blood samples from PBC patients (36%) and in 11/36 control samples (31%). Three of the 13 Y-positive PBC samples and six of the 11 Y-positive controls contained both SRY and SY154 sequences. The remaining 15 Y-positive samples (10 PBC and five control samples) were positive only for the SY154 amplification. Peripheral blood Y-chromosome sequences persisted for years after pregnancy (in one patient and in her control for 60 and 61 years). The results of a representative DNA amplification experiment are shown in Fig. 1.
Table 2.
Characteristics of primary biliary cirrhosis (PBC) and healthy women at time of blood sampling
| PBC patients (n = 36) | Healthy subjects (n = 36) | |
|---|---|---|
| Age, years | 60 ± 12 (34–87) | 59 ± 13 (34–86) |
| Interval between blood sampling | 30 ± 12 (6–60) | 30 ± 14 (3–61) |
| and most recent son, years | ||
| Pregnancies | ||
| Male (n = 101) | 1·4 ± 0·7 (1–4) | 1·4 ± 0·6 (1–3) |
| Female (n = 47) | 0·6 ± 0·8 (0–3) | 0·8 ± 0·7 (0–2) |
| All children (n = 148) | 2·0 ± 1·2 (1–6) | 2·1 ± 0·9 (1–5) |
Mean values ± s.d. (range).
Fig. 1.
Representative gel electrophoresis of polymerase chain reaction (PCR) products after amplification of Y-specific sequences of primary biliary cirrhosis (PBC) and control case DNAs. The nested PCR-amplified products for SY154 and SRY were, respectively, 181 bp and 182 bp. Lanes 1–5, PCR analyses with SY154 primers; lane 6, DNA size marker φx 174 digested with HAEIII; lanes 7–11, PCR analysis with SRY primers. Lanes 1 and 11 contain no DNA (blank); lanes 2 and 10 contain DNA from control case no. 8; lanes 3 and 9 contain DNA from PBC case no. 31 (PBC case no. 31 and control case no. 8 were positive for both SRY and SY154); lanes 4 and 8 contain DNA from a healthy female subject with no history of pregnancy or abortion; lanes 5 and 7 contain DNA from a healthy male subject.
The main clinical and laboratory characteristics of the PBC patients were compared on the basis of the presence of circulating male sequences. No significant difference was found between the patients with and without Y-chromosome sequences in terms of age, the age of the last son, parity, the presence of asymptomatic disease or advanced disease at histology, the presence of major complications of liver cirrhosis, or the duration of the disease (Table 3). Y sequences were detected in the blood of three (75%) of the four PBC patients with associated SSc (Table 3). No differences were found in the studied laboratory variables (Table 4). Positivity for non-organ-specific autoantibodies such as AMA, anti-nuclear and anti-smooth-muscle antibodies was equally distributed among the male-sequence-positive and -negative patients (Table 4); there was also no difference in the prevalence of specific anti-nuclear immunoreactivities against the centromere between the two groups of patients (Table 4).
Table 3.
Characterization of primary biliary cirrhosis (PBC) patients at time of blood sampling by the presence of specific male sequences
| With male sequences (n = 13) | Without male sequences (n = 23) | |
|---|---|---|
| Age (years) | 60 ± 12 | 60 ± 13 |
| Duration of disease (years) | 11 ± 5 | 10 ± 5 |
| Interval between first pregnancy and | 22 ± 11 (0·2–48) | 24 ± 12 (0·2–49) |
| earliest evidence of PBC (years) | ||
| Age of last son (years) | 30 ± 12 (6–59) | 30 ± 12 (11–60) |
| Pregnancies Male (n = 52) | 1·5 ± 0·7 (1–3) | 1·4 ± 0·8 (1–4) |
| Female (n = 20) | 0·2 ± 0·4 (0–1) | 0·7 ± 0·9 (0–3) |
| All children (n = 72) | 1·8 ± 0·7 (1–3) | 2·1 ± 1·4 (1–6) |
| No. (%) | No. (%) | |
| Asymptomatic | 7 (54) | 11 (48) |
| With histological stage III–IV | 8 (62) | 11 (48) |
| With complications of cirrhosis | 2 (15) | 2 (9) |
| With Sjögren's syndrome | 3 (23) | 3 (13) |
| With systemic sclerosis | 3 (23) | 1 (4) |
Mean values ± s.d. unless otherwise stated.
Table 4.
Biochemical and serological features of primary biliary cirrhosis patients at time of blood sampling by the presence of specific male sequences
| With male sequences (n = 13) | Without male sequences (n = 23) | |
|---|---|---|
| Alkaline phosphatase (U/l) | 459 ± 195 | 609 ± 471 |
| (n.v. < 279) | ||
| Aspartate aminotransferase (U/l) | 54 ± 20 | 65 ± 49 |
| (n.v. < 50) | ||
| Total bilirubin (mg/dl) | 1·0 ± 1·3 | 1·0 ± 1·5 |
| (n.v. < 1·0) | ||
| Albumin (g/dl) | 4·5 ± 0·7 | 4·6 ± 0·4 |
| (n.v. > 3·5) | ||
| Total gamma-globulins (g/dl) | 1·9 ± 1·0 | 1·8 ± 1·0 |
| (n.v. < 3·0) | ||
| IgG (mg/dl) | 1734 ± 783 | 1549 ± 770 |
| (n.v. < 1700) | ||
| IgA (mg/dl) | 402 ± 294 | 307 ± 240 |
| (n.v. < 450) | ||
| IgM (mg/dl) | 332 ± 168 | 395 ± 251 |
| (n.v. < 280) | ||
| No.(%) | No. (%) | |
| Positive for AMA | 10 (77) | 20 (87) |
| Positive for ANA | 7 (54) | 9 (39) |
| Positive for ACA | 3 (23) | 4 (17) |
| Positive for SMA | 2 (15) | 2 (9) |
Mean values ± s.d. unless otherwise stated.
AMA, Anti-mitochondrial antibodies; ANA, anti-nuclear antibodies; ACA, anti-centromere antibodies; SMA, anti-smooth-muscle antibodies.
n.v., normal values.
No difference was observed between the PBC women positive for both Y-specific sequences and those positive to only one.
The same prevalence of Y-chromosome sequences was found in the 25 patients receiving ursodeoxycholic acid at the time of blood sampling and in the untreated patients (36%).
DISCUSSION
PBC is an autoimmune disease that shares many clinical features with SSc. It has long been recognized that patients with these disorders develop tissue lesions similar to those found in chronic GVHD [1,2]. The very high frequency of fetal cells found in the blood of women with SSc decades after pregnancy has led to the hypothesis that they may play an aetiopathogenic role in the disease, probably by initiating a graft-versus-host-like response [6–8]. A similar hypothesis has more recently been proposed in PBC [9].
In the present study, we found specific male sequences in the blood of 13/36 PBC women (36%) with at least one son. However, our data do not support the hypothesis that maternal-fetus microchimerism plays a relevant role in the onset or progression of PBC, because the peripheral blood of the PBC women did not contain a higher frequency of male DNA than that of the healthy controls, and the presence of male DNA in the PBC patients was not associated with any particular characteristics of the disease.
To rule out any potential source of false-positive results, strict selection criteria were applied and only 36 of the 173 consecutively evaluated PBC patients were finally enrolled. None of them could be twins or have a history of miscarriages or blood transfusions because engraftments between twins and the engraftment of donor stem cells from blood transfusions have been described [18,19]. Abortion may represent another confounding factor because it is not known if fetal cells can persist in such cases and, more importantly, because the gender of the fetus is usually unknown. The same strict criteria were also applied to the selection of the 36 control subjects, in whom the presence of any known autoimmune disease was also excluded. The PBC and control groups were well matched in terms of age, the age of the last male child, and total parity. Although it is not known whether the total number of children is associated with the onset or outcome of PBC, we could not reasonably exclude the possibility that multiparity may affect fetal microchimerism. The study design enabled us to obtain a reasonable estimate of the persistence of fetal-derived DNA years after pregnancy in the PBC and healthy women, as well as to limit selection biases and known confounding factors.
It is interesting to note that we detected Y-chromosome-specific sequences in the blood of three of our four PBC patients with associated SSc, an observation that confirms the recently found association between circulating fetal microchimerism and SSc [6–8]. Unfortunately, we could not study a larger number of patients with both PBC and SSc because of the relatively low prevalence of SSc among PBC patients and our stringent selection criteria. The increased prevalence of circulating fetal microchimerism in PBC patients with SSc warrants further evaluation.
In order to ensure a high degree of sensitivity and specificity in detecting Y genes in female peripheral circulation, and rule out the presence of artefacts, we devised a nested PCR technique with two primers mapping to the long and short arms of the Y-chromosome. This procedure allowed us to find that, although all of the 24 Y-positive DNA samples contained the SY154 sequence, only nine were also positive for the SRY sequence. We cannot exclude the possibility that this finding may have been due to a difference in the sensitivity of the annealing of the primers of the two sequences to the target-specific DNA; in the other published studies, PCR amplification was carried out on only one sequence [6–8,10] and so no comparative data are available. Together with our difficulties in reproducing the PCR results obtained by others even when their primers were used [7,16], our observation may depend on the technical limitations of the currently available tools. We suggest that more than one DNA sequence is amplified if PCR is used to seek blood allogeneic fetal DNA in patients with autoimmune diseases. Another possible explanation for the discrepancy is that several Y-positive blood samples from our patient or control subjects did not contain entire fetal cells but only fragments of fetal-derived DNA. Indeed, fetal DNA has been recently identified in maternal plasma and serum samples [20,21]. The presence of circulating fetal cells might be limited to subjects showing both PCR signals.
We found a lower proportion of circulating fetal cells in women with PBC compared with the proportion of Y-chromosome sequences recently found in liver specimens taken from patients with PBC (36% versus 70%) [10]. Liver tissue data might be affected by the major flaw that the presence of fetal-specific DNA sequences in the liver tissues of women with hepatic injury and regeneration may be influenced by the amount of primary intrahepatic bone marrow-derived stem cells. A number of recent studies have demonstrated that a high proportion of transplanted allogeneic stem cells (such as CD34+ cells) can enter the liver through the circulation, localize in the intrahepatic stem cell compartment, and, depending on the extent of hepatocyte injury and regenerative process, differentiate into hepatocyte-like or cholangiocyte-like cells [22,23]. Accordingly, the fetal circulating CD34+ cells found in the peripheral blood of women who have given birth to at least one child [4,6–8] may undergo the same tissue localization and cellular differentiation. In view of the unknown and variable amount of fetal cells differentiating into hepatocytes, data from peripheral blood may better reflect the extent of maternal-fetus microchimerism in women with PBC.
Although our data show that PBC patients do not differ from healthy subjects in terms of the frequency of the detection of blood fetal microchimerism, its high prevalence in our patients prevents us from definitely ruling out the possibility that it may play a role in the aetiopathogenesis of the disease. A number of questions remain open. It seems to be important to discover whether there is a difference in the cell populations that can persist in the blood of women with and without PBC, because qualitative rather than quantitative differences may be responsible for inducing the graft-versus-host-like aggression of the disease. It is also important to discover whether genetic factors (such as similarity or homozygosity between mother and child) may affect microchimerism in PBC, as has been suggested in the case of SSc [6]. Finally, the intriguing hypothesis that not only fetal cells but also isolated fragments of fetal DNA are present in maternal circulation needs further investigation.
Acknowledgments
Study supported in part by Fondazione Anna Villa Rusconi, Varese, Italy (to C.D.A.) and by grant 9806210866 from M.U.R.S.T., Rome, Italy.
REFERENCES
- 1.Epstein O, Thomas HC, Sherlock S. Primary biliary cirrhosis is a dry gland syndrome with features of chronic graft-versus-host disease. Lancet. 1980;1:1166–8. doi: 10.1016/s0140-6736(80)91621-9. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ. Chronic graft-versus-host disease and primary biliary cirrhosis: sorting the puzzle pieces. Lab Invest. 1994;70:589–92. [PubMed] [Google Scholar]
- 3.Klingebiel T, Schlegel PG. GVHD: overview on pathophysiology, incidence, clinical and biological features. Bone Marrow Transplant. 1998;21:S45–49. [PubMed] [Google Scholar]
- 4.Bianchi DW, Zickwolf GK, Weil GJ, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson JL. Maternal-fetal immunology and autoimmune disease. Is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191–4. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–62. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 7.Artlett CM, Smith JB, Jimenez SA. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998;338:1186–91. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- 8.Evans PC, Lambert N, Maloney S, et al. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–7. [PubMed] [Google Scholar]
- 9.McDonnell WM. Is primary biliary cirrhosis a complication of pregnancy? Hepatology. 1998;28:593–4. doi: 10.1002/hep.510280243. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Lindor K, Gish R, et al. Fetal microchimerism alone does not contribute to the induction of primary biliary cirrhosis. Hepatology. 1999;30:833–8. doi: 10.1002/hep.510300410. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan MM. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–80. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 12.Invernizzi P, Crosignani A, Battezzati PM, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–5. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P, Battezzati PM, Crosignani A, et al. Antibody to carbonic anhydrase II is present in primary biliary cirrhosis (PBC) irrespective of antimitochondrial antibody status. Clin Exp Immunol. 1998;114:448–54. doi: 10.1046/j.1365-2249.1998.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets, and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 15.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchows Arch [A] 1978;379:103–12. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 16.Lo YMD, Patel P, Sampietro M, et al. Detection of single-copy fetal DNA sequence from maternal blood. Lancet. 1990;335:1463–4. doi: 10.1016/0140-6736(90)91491-r. [DOI] [PubMed] [Google Scholar]
- 17.De Andreis C, Simoni G, Rossella F, et al. HIV-1 proviral DNA polymerase chain reaction detection in chorionic villi after exclusion of maternal contamination by variable number of tandem repeats analysis. AIDS. 1996;10:711–5. doi: 10.1097/00002030-199606001-00004. [DOI] [PubMed] [Google Scholar]
- 18.De Moor G, De Bock G, Noens L, et al. A new case of human chimerism detected after pregnancy: 46,XY karyotype in the lymphocytes of a woman. Acta Clin Belg. 1988;43:231–5. doi: 10.1080/17843286.1988.11717936. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Paglieroni T, Ohto H, et al. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–39. [PubMed] [Google Scholar]
- 20.Lo YMD, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 21.Lo YMD, Hjelm NM, Fidler C, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med. 1998;339:1734–8. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 22.Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 23.Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–40. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]