Abstract
IL-10 displays modulatory properties on the synthesis of platelet-activating factor (PAF), a potent inflammatory mediator of vascular injury. Despite the fact that IL-10 is considered to be an anti-inflammatory cytokine, IL-10 levels correlate with disease activity in SLE. Moreover, in SLE IL-10 is unable to exert its immunosuppressive and anti-inflammatory effects. We have investigated the ability of IL-10 to stimulate PAF production from monocytes of SLE patients. Spontaneous and IL-10-stimulated PAF production by peripheral blood monocytes was measured in active (n = 13) and inactive (n = 14) SLE patients and in 15 normal control subjects. We observed that monocytes derived from patients with active SLE, but not from controls or inactive SLE, spontaneously produced significant amounts of PAF. Moreover, IL-10 enhanced the synthesis of PAF from monocytes of active SLE patients only. IL-10-induced PAF production correlated with the severity of the disease and with the extent of proteinuria. These results indicate that IL-10 only stimulates the synthesis of PAF from monocytes of SLE patients when immunologically active, suggesting that IL-10 may possess a paradoxical proinflammatory effect in SLE by promoting the production of PAF, a secondary mediator of inflammation.
Keywords: IL-10, systemic lupus erythematosus, platelet-activating factor, monocytes, proteinuria
INTRODUCTION
SLE is an autoimmune disease characterized by polyclonal B cell activation with overproduction of pathogenic antibodies, and decreased in vitro and in vivo cellular responses [1,2]. The role of IL-10 in the immunological abnormalities present in SLE has been extensively studied. IL-10 is over-expressed in lupus patients [3], correlates with disease activity [4,5] and mediates, at least in part, B cell differentiation and excessive immunoglobulin production [6].
It is well established that IL-10 exerts immunosuppressive and anti-inflammatory effects in a variety of ways, including suppression of cytokine production [7], down-regulation of the antigen-presenting and co-stimulatory function of monocytes [8,9], as well as inhibition of T cell proliferation [10]. These latter effects could explain the dysfunction of both T helper lymphocytes and antigen-presenting cells (APC) observed in SLE patients [11].
Although IL-10 is commonly considered a suppressive cytokine on monocytes, recent papers have proposed that IL-10 may also display activating functions. Indeed, it has been demonstrated that IL-10 primes the production of cytokines [12] and platelet-activating factor (PAF) [13] by human monocytes stimulated with bacterial lipopolysaccharide (LPS). Moreover, IL-10 up-regulates PAF and formyl peptide receptor expression on the monocyte cell surface [14].
PAF (1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphocholine) is a phospholipid mediator of inflammation with diverse and potent biological actions [15,16], which is produced by a large range of cells, including circulating monocytes [17,18]. PAF acts through a specific receptor [19] and is considered to be a mediator of cell-to-cell communication on account of its role as an intercellular or intracellular messenger [15,20,21]. Increased levels of both circulating and urinary PAF have been observed in experimental and human lupus nephritis and correlated with the severity of proteinuria [22,23]. Moreover, a causative role for PAF in lupus glomerular injury has been suggested by the reduction of renal damage induced by PAF receptor blockade in murine SLE [24,25].
The aim of the present study was to evaluate whether monocytes of lupus patients produced increased levels of PAF and whether IL-10 was involved in this increase. The results demonstrate that IL-10 directly induced PAF synthesis from monocytes of active SLE patients, but not from those of inactive patients or normal subjects. Moreover, PAF levels correlated with the severity of the disease and with the extent of proteinuria.
PATIENTS AND METHODS
Human subjects
Study subjects included patients with SLE (n = 27) and healthy control subjects (n = 15). All patients met the American College of Rheumatology diagnostic criteria for SLE. SLE activity was assessed by the disease activity index for lupus patients (SLEDAI) described by Bombardier et al. [26]. Thirteen patients had active disease (> 6 at the SLEDAI score), while 14 had inactive lupus (Table 1). Fifty-eight percent of patients were female, and their mean age was 37 years. Fifteen healthy controls (64% female, mean age 35 years) were studied.
Table 1.
Clinical and laboratory characteristics of SLE patients
| Patient number | Glomerulonephritis class | Proteinuria (g/day) | SLEDAI score | Basal PAF (pg/106 cells) | IL-10-stimulated PAF (pg/106 cells) |
|---|---|---|---|---|---|
| 1 | III | 2·9 | 14 | 500 | 750 |
| 2 | III + V | 3 | 15 | 450 | 780 |
| 3 | NB | 0·2 | 12 | 50 | 120 |
| 4 | IV | 2·4 | 4 | 35 | 45 |
| 5 | NB | 0·2 | 8 | 30 | 110 |
| 6 | V | 1·7 | 16 | 65 | 180 |
| 7 | IV | 0·5 | 4 | 85 | 90 |
| 8 | NB | 0·2 | 3 | 45 | 45 |
| 9 | III | 0·61 | 2 | 25 | 25 |
| 10 | III + V | 0·4 | 2 | 58 | 58 |
| 11 | NB | 0·18 | 4 | 48 | 65 |
| 12 | IV + V | 0·3 | 4 | 20 | 20 |
| 13 | III | 0·3 | 2 | 20 | 35 |
| 14 | III + V | 0·08 | 6 | 45 | 50 |
| 15 | NB | 0·3 | 4 | 50 | 110 |
| 16 | III + V | 0·3 | 2 | 88 | 86 |
| 17 | V | 2·3 | 6 | 35 | 44 |
| 18 | III | 1·4 | 4 | 28 | 42 |
| 19 | III | 2·1 | 22 | 56 | 120 |
| 20 | III | 1·7 | 4 | 46 | 45 |
| 21 | NB | 3 | 12 | ND | 225 |
| 22 | NB | 1 | 24 | 56 | 104 |
| 23 | V | 3 | 18 | 150 | 200 |
| 24 | III + V | 1·3 | 14 | 60 | 100 |
| 25 | V | 3 | 10 | 270 | 320 |
| 26 | III + V | 1·7 | 12 | 60 | 65 |
| 27 | IV | 7·3 | 22 | 90 | 365 |
Disease activity was indicated using the SLEDAI score. PAF production by monocytes was valuated in basal conditions and after IL‐10 (20 ng/ml) stimulation, as described in PATIENTS and METHODS. NB, Not biopsy.
Cell preparation
After obtaining informed consent, heparinized blood was obtained from both patients and healthy subjects and peripheral blood mononuclear cells (PBMC) were immediately isolated by Ficoll–Hypaque gradient centrifugation, washed three times with PBS and suspended in RPMI 1640 (Gibco, Paisley, UK) containing 0·25% bovine serum albumin (BSA).
Monocytes present in PBMC were detected by non-specific esterase staining and immunofluorescence positivity with the anti-CD14 Leu-M3 MoAb (Becton Dickinson, Milano, Italy) and resuspended at 1 × 106/ml density. Monocytes were isolated from PBMC by adhesion to plastic dishes, as described by Valone & Epstein [18]. Non-adherent cells were removed by vigorous washing. Adherent cells were >90% monocytes as detected by non-specific esterase staining, and less than one platelet/10 monocytes was detected at the microscopic observation after staining with May–Grunwald–Giemsa. Monocyte viability was >90%, as assessed by the trypan blue dye exclusion test.
IL-10 measurement
To detect IL-10 production from PBMC, cells (1 × 106) were incubated in RPMI plus 0·25% BSA at 37°C, 5% CO2 for 24 h. IL-10 released into culture supernatants was determined by a sandwich ELISA (Genzyme Corp., Cambridge, MA), according to the instructions provided by the manufacturer.
Purification and quantification of PAF
The production of PAF from monocytes stimulated with IL-10 (Genzyme) was studied. After removal of non-adherent cells, monocytes were equilibrated for 15 min in Tris-buffered Tyrode containing 0·25% delipidized BSA (fraction V, tested for <1 ng endotoxin per mg, obtained from Sigma Chemical Co., St Louis, MO), as previously described [17]. The cells were subsequently incubated at 37°C for 15 min with either vehicle alone or with vehicle plus IL-10 (20 ng/ml). To assess the specificity of the IL-10-induced effect, the cytokine was inactivated by boiling for 10 min or was preabsorbed with 10 μg/ml anti-IL-10 rabbit polyclonal antibody (Sigma), as previously described [13]. Moreover, to exclude LPS contamination, selected experiments were conducted in the presence of 5 μg/ml Polymyxin B pre‐incubated for 30 min at 37°C. The supernatants and the cell pellets were extracted according to a modification of the Bligh & Dyer procedure [27], with formic acid added to lower the pH of the aqueous phase to 3·0. Each individual experiment was performed in duplicate.
PAF was quantified after extraction and purification by thin layer chromatography (TLC; silica gel plates 60 F254; Merck, Darmstardt, Germany) and high-pressure liquid chromatography (HPLC; μPorasil column; Millipore Chromatographic Division, Waters, Milford, MA) following aggregation of washed rabbit platelets, as previously reported [13,17]. The detection limit of PAF on washed rabbit platelets in our system was 2 pg/ml of synthetic PAF (1-O-octadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine, Bachem Feinchemikalien, Bubendorf, Switzerland).
The biologically active material extracted from cells and supernatants in different experiments was characterized by comparison with synthetic PAF, according to the following criteria [16]: (i) induction of platelet aggregation by a pathway independent of both ADP and arachidonic acid/thromboxane A2-mediated pathways; (ii) specificity of platelet aggregation as inferred from the inhibitory effect of 5 μm WEB 2170 (Boehringer, Ingelheim, Germany) or CV 3988 (Takeda Chemical Industries, Kyoto, Japan), two different PAF receptor antagonists; (iii) TLC and HPLC behaviour and physico-chemical characteristics, such as inactivation by strong bases and by phospholipase A2 treatment, but resistance to phospholipase A1, acids, weak bases and 5 min heating in boiling water.
Statistical analysis
Statistically significant differences were determined using anova with Newman Keuls multicomparison test. Correlation coefficients were determined using multiple linear regression analysis.
RESULTS
PAF production from monocytes of inactive and active lupus patients
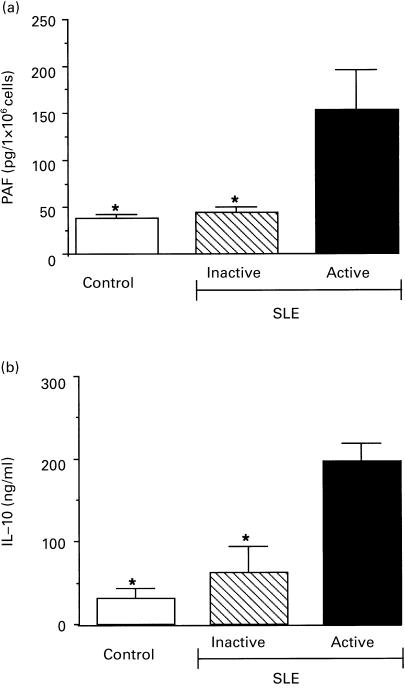
The spontaneous production of PAF from monocytes of active SLE patients (n = 13) and inactive SLE patients (n = 14) was evaluated. Monocytes derived from active SLE patients produced significantly higher levels of PAF compared with inactive patients. In contrast, PAF levels from the monocytes of inactive patients did not differ significantly from those of healthy controls (Fig. 1a). At the time studied (15 min incubation), PAF was mainly detected in association with the cell fraction (82 + 7%), and was released into the supernatant after further incubation (2 h) (data not shown).
Fig. 1.
Spontaneous production of platelet-activating factor (PAF) or IL-10 from cells of 15 normal subjects (Control) or from 13 active and 14 inactive SLE patients. (a) Cell-associated PAF spontaneously synthesized by 1 × 106 monocytes after 15 min incubation at 37°C. (b) Spontaneously released IL-10 by 1 × 106 peripheral blood mononuclear cells after 24 h incubation at 37°C. anova with Newman Keuls multicomparison test was performed among active SLE patients versus inactive SLE and control subjects (*P < 0·05) and inactive SLE versus control subjects (no statistical difference).
IL-10 stimulates PAF production from monocytes of active lupus patients
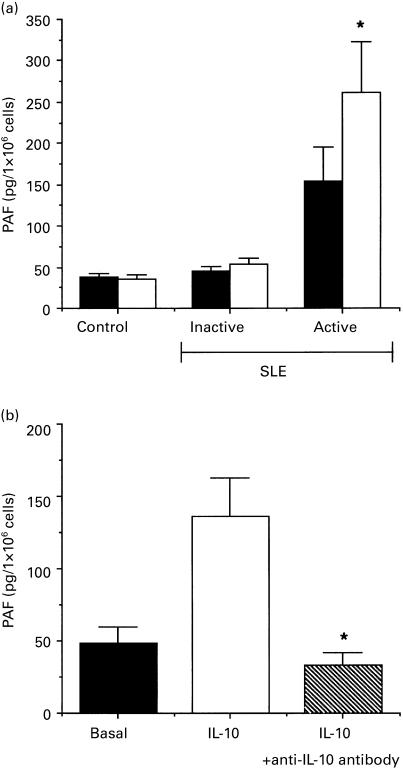
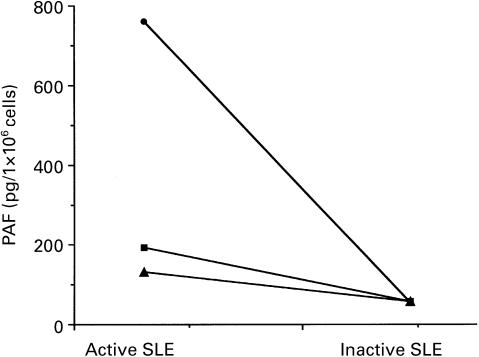
We found that PBMC from active lupus patients released significantly higher levels of IL-10 than from inactive patients or normal healthy controls (Fig. 1b), as has previously been reported [3]. To evaluate a possible role for IL-10 in the production of PAF, monocytes were stimulated for 15 min with 20 ng/ml IL-10. We previously demonstrated that this dose of IL-10 was unable to induce PAF production by monocytes from healthy subjects, but was able to prime PAF synthesis after stimulation with LPS [13]. As for normal subjects, monocytes from inactive patients did not produce PAF after stimulation with IL-10. In contrast, monocytes obtained from active patients significantly increased PAF synthesis after stimulation with this cytokine (Fig. 2a). The specificity of the observed stimulation was demonstrated by the abrogation of PAF enhancement when IL-10 was immuno-absorbed (Fig. 2b). Similar results were obtained with heat inactivation of IL-10 (data not shown). Moreover, preincubation of IL-10 with Polymyxin B, that binds and inactivates LPS, did not affect the IL-10-induced stimulation, indicating that this effect was not dependent on LPS contamination (data not shown). In three active SLE patients, IL-10-induced PAF synthesis was significantly reduced after the remission of the disease (Fig. 3).
Fig. 2.
IL-10-induced platelet-activating factor (PAF) synthesis from active SLE monocytes. (a) PAF synthesis from monocytes unstimulated (▪) or stimulated with 20 ng/ml IL-10 (□). PAF was detected as being cell-associated. A statistical difference between the basal and stimulated PAF levels was observed in the active SLE patient group (n = 14, *P < 0·05) but not in the inactive (n = 14) or control (n = 15) groups. (b) PAF produced by monocytes (1 × 106) derived from three active SLE patients stimulated with 20 ng/ml IL-10 (□) or with 20 ng/ml IL-10 preabsorbed with the anti-IL-10 antibody (hatched column). Inhibition of IL-10-induced PAF was observed after immuno-absorbtion of IL-10. *P < 0·05.
Fig. 3.
Total amount of platelet-activating factor (PAF) synthesized by monocytes derived from three SLE patients during an active and subsequent inactive phase of the disease, as evaluated by SLE Disease Activity Index (SLEDAI) score. Cells (1 × 106) were stimulated with IL-10 (20 ng/ml) for 15 min at 37°C.
Correlation between PAF levels and clinical or biological markers of disease activity
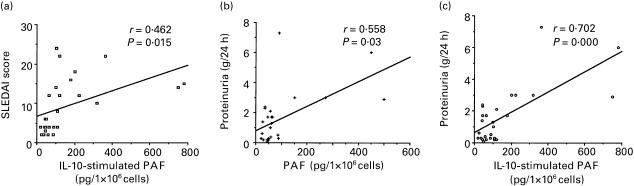
A significant correlation was observed between disease activity, evaluated using the SLEDAI score [26], and levels of PAF after stimulation with IL-10 (P = 0·015, Fig. 4a). No such correlation was observed under basal (i.e. absence of IL-10 stimulation) conditions. Moreover, the amount of proteinuria positively correlated with the amount of PAF produced by monocytes, both unstimulated (P = 0·030; Fig. 4b) and stimulated with IL-10 (P = 0·00008, Fig. 4c). No significant correlation was observed between PAF levels and other laboratory parameters such as platelet count, haemoglobin concentration, serum creatinine, complement and anti-DNA antibody levels. No significant correlation was observed with patient age or with type, dose or duration of therapy.
Fig. 4.
The correlation between platelet-activating factor (PAF) levels and disease activity score or proteinuria was determined for 27 SLE patients. (a) Correlation between activity score and IL-10-induced PAF synthesis. (b,c) Correlation between proteinuria (evaluated as g for 24 h) and spontaneous (b) or IL-10-induced (c) PAF production.
DISCUSSION
In the present study, we demonstrate that monocytes derived from patients with active SLE, but not from controls or inactive SLE, spontaneously produce significant amounts of PAF. Moreover, IL-10 enhanced the synthesis of PAF from monocytes of active SLE patients only. A positive correlation between PAF levels and the severity of the disease and the extent of proteinuria was found.
PAF is a proinflammatory mediator produced early in response to several immunological stimuli, including immunocomplexes and proinflammatory cytokines [17,18]. Moreover, PAF itself mediates some of the biological effects exerted by cytokines like tumour necrosis factor-alpha (TNF-α) and IL-8 [28]. However, not all the biological effects of PAF are proinflammatory. It has recently been shown that PAF is involved in the inhibition of proinflammatory cytokine production during macrophage phagocytosis of apoptotic cells, possibly through the synthesis of transforming growth factor-beta (TGF-β) [29].
Previous studies demonstrated increased intravascular levels of PAF in patients with SLE [22]. It has been suggested this may depend on both increased PAF synthesis by circulating leucocytes and reduction of acetyl hydrolase activity, the main catabolic enzyme of PAF [22]. In the present study, we demonstrate that monocytes purified from patients with active SLE spontaneously synthesize significant amounts of PAF, suggesting that these cells are primed in vivo to synthesize PAF and can therefore contribute to the increased levels of circulating PAF observed in SLE patients.
Monocyte activation may follow either phagocytosis of immunocomplexes or cytokine stimulation. Among the cytokines able to stimulate the synthesis of PAF, IL-10 is a potential candidate, since it has been shown that IL-10 production by PBMC is increased in SLE [3]. We therefore investigated whether the enhanced synthesis of PAF by monocytes was dependent upon a biological effect of IL-10. In a previous study we observed that IL-10 per se is not able to trigger PAF synthesis by monocytes of normal subjects [13]. However, it primes PAF synthesis induced by LPS [13]. We now report that IL-10 directly stimulated PAF synthesis in monocytes obtained from patients with active SLE, whereas it was ineffective on those from SLE patients with quiescent disease and from normal subjects. Moreover, the shift from active to inactive disease in three patients paralleled the reduction of IL-10-induced synthesis of PAF. These results indicate that monocytes obtained from SLE patients during the active phase of the disease are activated and primed in vivo to respond to IL-10.
IL-10 is considered an anti-inflammatory cytokine, which suppresses the synthesis and release of several proinflammatory cytokines, such as TNF-α, IL-1, IL-6 and IL-8, from LPS-activated cells (for review, see [7]). Moreover, IL-10 down-regulates the production of oxygen radicals [7]. However, the enhanced production of IL-10 is considered detrimental in SLE patients [30] and it has been suggested that it may contribute to the expansion of autoreactive B lymphocytes [6]. Indeed, increased production of IL-10 correlates with the activity of the disease [4,5]. The results of this study suggest that IL-10 may also contribute to the pathogenesis of SLE via a proinflammatory effect by inducing the synthesis of PAF, a mediator involved in glomerular and vascular injury.
Previous studies suggested that IL-10 could combine proinflammatory and immunosuppressive actions on target cells [12–14]. Moreover, it has been shown that IL-10 is unable to exert a suppressive effect on monocytes derived from SLE patients [31]. Similar abnormalities in the response to IL-10 were also observed in rheumatoid synovial dendritic cells and monocytes, that are resistant to the immunosuppressive effects of IL-10 [32–34]. We propose here that IL-10, by triggering the synthesis of PAF in active SLE patients, may switch from an anti-inflammatory to a proinflammatory cytokine and may therefore contribute to tissue injury. Consistent with this hypothesis is the observation that IL-10 mediates in vivo leucocyte extravasation into the pancreatic tissue of transgenic mice expressing IL-10 in the islets of Langerhans [35]. Altogether, these observations suggest a paradoxical proinflammatory effect of IL-10 in immunological diseases such as SLE and rheumatoid arthritis (RA). The mechanism of this modification of IL-10 cell responsiveness is unclear. A possible hypothesis involves the modulation of the cell surface IL-10 receptor 1, which has been observed for example in RA [32]. However, other mechanisms may also be involved, including synergy between the different mediators involved in the transduction of inflammatory signals.
In the present study, a positive correlation was observed between the IL-10-enhanced synthesis of PAF from monocytes and the activity score of the disease. Moreover, both spontaneous and IL-10-induced PAF levels correlated with the extent of proteinuria. PAF has been implicated in several experimental models of renal injury, including lupus nephritis [28]. PAF affects glomerular filtration, participates in renal immune injury by favouring immune complex deposition, increasing glomerular permeability to proteins [36]. Indeed, experimental studies using PAF receptor antagonists demonstrated an amelioration of lupus nephritis in MRL/mpJ lpr/lpr mice [24] and New Zealand Black × New Zealand White mice [25]. Furthermore, preliminary studies in humans suggest that blockade of the PAF receptor may improve renal injury and vascular inflammatory lesions in SLE patients [37]. During the active phase of the disease, the enhanced IL-10-induced PAF synthesis together with PAF-reduced catabolism [22] may favour increased intravascular concentrations of this potent autacoid which may contribute to the vascular and renal injury seen in SLE.
In conclusion, these results indicate a further role for IL-10 in the pathogenesis of SLE. IL-10 may display a paradoxical proinflammatory effect in the acute phase of the disease through the activation of monocytes and by promoting the production of secondary mediators involved in vascular and renal injury.
Acknowledgments
This work was supported by the by the National Research Council (CNR), Targeted Project Biotechnology, and by MURST 60%. We thank Dr Justin Mason for the helpful suggestions.
REFERENCES
- 1.Handwergwe BS. Lymphocyte biology in lupus. Curr Opin Rheumatol. 1990;2:749–63. doi: 10.1097/00002281-199002050-00011. [DOI] [PubMed] [Google Scholar]
- 2.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunol Rev. 1995;144:269–390. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 3.Llorente L, Richaud-Patin Y, Fior L, Alcocer-Varela J, Wijdenes J, Morel-Fourrier B, Galanaud P, Emilie D. In vivo production of interleukin-10 by non T cells in rheumatoid arthritis, Sjogren syndrome, and systemic lupus erythematosus: a potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara E, Gourley MF, Lee S, Klinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: interferon-γ-secreting cells in the peripheral blood. Arthritis Rheum. 1996;3:379–85. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 5.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–95. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KW, O'garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down-regulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–9. [PubMed] [Google Scholar]
- 10.de Waal Malefyt R, Yssel H, De Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 11.Via CS, Tsokos GS, Bermas B, Clerici M, Shearer GM. T cell–antigen-presenting cell interactions in human systemic lupus erythematosus. Evidence for heterogeneous expression of multiple defects. J Immunol. 1993;151:3914–22. [PubMed] [Google Scholar]
- 12.Adib-Conquy M, Petit AF, Marie C, Fitting C, Cavaillon JM. Paradoxical priming effects of IL-10 on cytokine production. Int Immunol. 1999;11:689–98. doi: 10.1093/intimm/11.5.689. [DOI] [PubMed] [Google Scholar]
- 13.Bussolati B, Mariano F, Montrucchio G, Piccoli G, Camussi G. Modulatory effect of interleukin-10 on the production of platelet-activating factor and superoxide anions by human leukocytes. Immunology. 1997;90:440–4. doi: 10.1111/j.1365-2567.1997.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Modulation of formyl peptide receptor expression by IL-10 in human monocytes and neutrophils. J Immunol. 1999;162:3590–5. [PubMed] [Google Scholar]
- 15.Venable ME, Zimmerman GA, McIntyre TM, Prescott SM. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res. 1993;34:691–702. [PubMed] [Google Scholar]
- 16.McManus L, Woodard DS, Deavers SI, Pinckard RN. Biology of disease: PAF molecular heterogeneity, pathobiological implications. Lab Invest. 1993;69:639–50. [PubMed] [Google Scholar]
- 17.Camussi G, Aglietta M, Coda R, Bussolino F, Piacibello W, Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF. monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981;42:191–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Valone FH, Epstein LB. Biphasic platelet-activating factor synthesis by human monocytes stimulated with IL-1, tumor necrosis factor or IFN-γ. J Immunol. 1988;141:3945–50. [PubMed] [Google Scholar]
- 19.Chao W, Olson MS. Platelet activating factor receptors and signal transduction. J Biochem. 1993;292:617–29. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JM, Henson PM. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986;137:2653–61. [PubMed] [Google Scholar]
- 21.Pinckard RN, Woodard DS, Showell HJ, Conklyn MJ, Novak MJ, McManus L. Structural and (patho) physiological diversity of PAF. Clin Rew Allergy. 1994;12:329–59. doi: 10.1007/BF02802299. [DOI] [PubMed] [Google Scholar]
- 22.Tetta C, Bussolino F, Modena V, Montrucchio G, Segoloni G, Pescarmona G, Camussi G. Release of platelet-activating factor in systemic lupus erythematosus. Int Arch Allergy Appl Immunol. 1990;91:244–56. doi: 10.1159/000235124. [DOI] [PubMed] [Google Scholar]
- 23.Macconi D, Noris M, Benfenati E, Quaglia R, Pagliarino G, Remuzzi G. Increased urinary excretion of platelet activating factor in mice with lupus nephritis. Life Sci. 1991;48:1429–37. doi: 10.1016/0024-3205(91)90179-f. [DOI] [PubMed] [Google Scholar]
- 24.Baldi E, Emancipator SN, Hassan MO, Dunn MJ. Platelet activating factor receptor blockade ameliorates murine systemic lupus erythematosus. Kidney Int. 1990;38:1030–8. doi: 10.1038/ki.1990.309. [DOI] [PubMed] [Google Scholar]
- 25.Morigi M, Macconi D, Riccardi E, Boccardo P, Zilio P, Bertani T, Remuzzi G. Platelet-activating factor receptor blocking reduces proteinuria and improves survival in lupus autoimmune mice. J Pharmacol Exp Ther. 1991;258:601–6. [PubMed] [Google Scholar]
- 26.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 27.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 28.Camussi G. Interactive effects of tumor necrosis factor and platelet activating factor in the pathogenesis of glomerular injury. Lab Invest. 1994;70:4351–60. [PubMed] [Google Scholar]
- 29.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcocer-Varela J, Llorente L, Alarcon-Segovia D. Immunoregulatory circuits and potential treatment of connective tissue diseases. Int Arch Allergy Immunol. 1996;111:348–541. doi: 10.1159/000237391. [DOI] [PubMed] [Google Scholar]
- 31.Mongan AE, Ramdahin S, Warrington RJ. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997;46:406–12. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald KP, Pettit AR, Quinn C, Thomas GJ, Thomas R. Resistance of rheumatoid synovial dendritic cells to the immunosuppressive effects of IL-10. J Immunol. 1999;163:5599–607. [PubMed] [Google Scholar]
- 33.Hart PH, Ahern MJ, Jones CA, Jones KL, Smith MD, Finlay Jone JJ. Synovial fluid macrophages and blood monocytes differ in their response to IL-4. J Immunol. 1993;151:3370–80. [PubMed] [Google Scholar]
- 34.Chomarat P, Banchereau J, Miossec P. Differential effects of interleukins 10 and 4 on the production of interleukin-6 by blood and synovium monocytes in rheumatoid arthritis. Arthritis Rheum. 1995;38:1046–54. doi: 10.1002/art.1780380805. [DOI] [PubMed] [Google Scholar]
- 35.Wogensen L, Huang X, Sarvetnick N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin 10 in the islets of Langerhans. J Exp Med. 1993;178:175–85. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camussi G, Tetta C, Coda R, Benveniste J. Release of platelet-activating factor in human pathology. I. Evidence for the occurrence of basophil degranulation and release of platelet-activating factor in systemic lupus erythematosus. Lab Invest. 1981;44:241–51. [PubMed] [Google Scholar]
- 37.Clark WF, Parbtani A, Huff MW, Spanner E, de Salis H, Chin-Yee I, Philbrick DJ, Holub BJ. Flaxseed: a potential treatment for lupus nephritis. Kidney Int. 1995;48:475–80. doi: 10.1038/ki.1995.316. [DOI] [PubMed] [Google Scholar]