Abstract
This study was designed to compare the degree of lymphocyte apoptosis and Fas–Fas ligand (FasL) expression in AIDS patients and long-term non-progressors (LTNPs) and correlate these parameters with apoptosis-associated perturbations in lymphocyte function. LTNPs had a lower frequency of apoptotic CD4+ and CD8+ T cells compared with subjects with AIDS. This correlated with a lower frequency of cells expressing Fas and FasL. The frequency of selected lymphocyte populations exhibiting a disrupted mitochondrial transmembrane potential (ΔΨm) and increased superoxide generation was lower in LTNPs than in patients with AIDS; these abnormalities were associated with lower levels of caspase-1 activation in LTNPs. The results indicate a significantly reduced level of apoptosis and apoptosis-associated parameters in LTNPs than in patients developing AIDS. Based on these findings, a crucial role for mitochondria can be predicted in the process of lymphocyte apoptosis during the evolution of AIDS.
Keywords: long-term non-progressors, lymphocyte apoptosis, mitochondria
INTRODUCTION
An enhanced apoptotic turnover contributes to the progressive decline in CD4+ T lymphocyte numbers characteristic of AIDS [1–3]. A small subset of untreated HIV-infected subjects, who are referred to as long-term non-progressors (LTNPs), show little or no net T cell loss and remain asymptomatic for periods longer than at least 7 years after seroconversion with a relatively low viral burden in the peripheral blood and lymphoid organs [4,5]. The reasons for such an extraordinarily benign disease course of an otherwise rapidly evolving infection are not completely understood, but there is some evidence suggesting that peripheral lymphocytes from LNTPs have a relatively low level of apoptosis compared with lymphocytes from subjects progressing to AIDS [6–8].
Several different factors account for the increased lymphocyte apoptosis seen in HIV-infected progressors. These factors include increased transduction of the apoptotic signal of the Fas–Fas ligand (FasL) pathway [9–12] through the endogenous mediators ceramide [13–15] and IL-1β-converting enzyme (ICE, caspase-1) [16–18], as well as the generation of oxidant stress [19–21].
Since the mechanisms responsible for the slow rate of lymphocyte apoptosis in LTNPs are largely unknown, this study was designed to compare the degree of lymphocyte apoptosis and Fas–FasL expression and correlate them with apoptosis-associated perturbations in lymphocyte function in AIDS patients and in a group of LTNPs who remained clinically asymptomatic with stable CD4+ T counts for at least 7 years after seroconversion. Our results demonstrate that LTNPs have lower frequencies, compared with AIDS patients, of (i) apoptotic CD4+ and CD8+ T cells; (ii) lymphocytes expressing Fas and FasL; and (iii) selected lymphocyte populations exhibiting a disrupted mitochondrial transmembrane potential (ΔΨm) and increased superoxide generation.
MATERIALS AND METHODS
Patients and lymphocyte isolation
Peripheral blood mononuclear cells (PBMC) were obtained from 12 male HIV+ LTNPs and from 12 consecutively recruited male patients with clinical or laboratory parameters of AIDS (Centers for Disease Control and Prevention 1993, C3) living in the Community of San Patrignano (Rimini, Italy). All patients with AIDS were receiving zidovudine (600 mg daily) and trimethoprim-sulfamethoxazole (oral 15 mg/kg daily of the trimethoprim component) for prophylaxis of Pneumocystis carinii pneumonia. None of the patients had an opportunistic infection within 1 month of the tests being done. The demographic and clinical characteristics of LTNPs and AIDS patients who entered this study are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of long-term non-progressors (LTNPs) compared with AIDS patients (CDC stage 3)
| Characteristic | LTNPs | AIDS | P |
|---|---|---|---|
| No | 12 | 12 | NS |
| M/F sex ratio | 9/3 | 10/2 | NS |
| Age, years | |||
| Median ±s.d. | 36 ± 5 | 33 ± 4 | NS |
| Range | 30–44 | 24–42 | |
| Years since seroconversion | |||
| Median ±s.d. | 10 ± 2 | 3 ± 1 | < 0·000 01 |
| Range | 8–12 | 1–5 | |
| Viraemia, particles/μl | |||
| Median ±s.d. | 2100 + 1451 | 53 800 + 27 432 | 0·0004 |
| Range | 900–8300 | 3200–104 500 | |
| CD4+ T cells, no./μl | |||
| Median ±s.d. | 804 ± 83 | 35 ± 16 | < 0·0001 |
| Range | 680–911 | 21–67 | |
| CD8+ T cells, no./μl | |||
| Median ±s.d. | 1351 ± 142 | 280 ± 82 | < 0·000 01 |
| Range | 1287–1789 | 173–460 | |
| Total lymphocytes, no./μl | |||
| Median ±s.d. | 2630 ± 172 | 411 ± 114 | < 0·000 01 |
| Range | 2383–2885 | 269–633 | |
NS, Not significant.
PBMC were separated from heparinized peripheral blood by Lymphoprep gradient centrifugation (Nycomed, Oslo, Norway), washed twice with PBS and resuspended in RPMI 1640 (Life Technologies, Inc., Paisley, UK) medium supplemented with 10% heat-inactivated fetal calf serum (FCS; Life Technologies), 10 U/ml penicillin/streptomycin (Life Technologies), 10 mm HEPES (Sigma Chemical Co., St Louis, MO), and 1 mm l-glutamine (Life Technologies) (complete medium). In the apoptosis assay, PBMC (5 × 105/ml) were cultured in complete medium for 12 h at 37°C in a 5% CO2-humidified atmosphere. In addition, for the analysis of mitochondrial functions, aliquots of cells were isolated and maintained in complete culture medium at 4°C until labelling.
Expression of surface and intracellular antigens
The absolute counts of cells bearing either the CD4, CD8 or the Fas/FasL phenotype were determined by flow cytometry. PBMC were stained with the following antibodies: PE-labelled anti-hCD4 or anti-hCD8 (Becton Dickinson Immunocytometry Systems, San Josè, CA), anti-hCD95/Fas/APO1 (Upstate Biotechnology Inc., New York, NY) and FITC-labelled anti-mouse IgM (Sigma), anti-hCD95L/FasL (PharMingen, San Diego, CA) and anti-mouse IgG FITC conjugate (Sigma). For staining of surface antigens 5 × 105 PBMC were washed in PBS containing 1% bovine serum albumin (BSA; Sigma) and 0·1% sodium azide (PBS–BSA–NaN3) followed by incubation for 20 min at 4°C with the MoAbs previously described. For determination of background staining, cells were incubated with 20 μl each of mouse IgG1 FITC and mouse IgG1 PE (Becton Dickinson Immunocytometry Systems). Then, after washing twice with PBS–BSA–NaN3 containing 2% FCS, the labelled cells were analysed by flow cytometry using a FACScan flow cytometer (Becton Dickinson). For each sample 10 000 viable lymphocytes were gated, according to size (forward scatter, FSC) and granularity (side scatter, SSC) parameters.
Assessment of cells undergoing apoptosis
Staining of apoptotic nuclei with propidium iodide
Lymphocyte apoptosis was quantified as the percentage of cells with hypodiploid DNA using the technique of Nicoletti et al. [22–24]. Briefly, following a short-term culture, cell suspensions were centrifuged at 200 g for 10 min. For staining of surface antigens, aliquots of 1 × 106 cells were incubated with FITC-conjugated MoAbs as previously described and, after washing, the pellet was gently resuspended in 1 ml of hypotonic fluorochrome solution (50 μg/ml propidium iodide (PI) in 0·1% sodium citrate plus 0·1% Triton X-100, 0·05 mg/ml RNase A; Sigma). Cells were kept overnight at 4°C, then analysed in their staining solution using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems) equipped with a 15-mW air-cooled 488-nm argon–ion laser. Apoptotic nuclei appeared as a broad hypodiploid DNA peak which was easily distinguished from the narrow peak of nuclei with normal (diploid) DNA content in the red fluorescence channel. Orange PI fluorescence was collected after a 585/42 nm band pass (BP) filter and was displayed on a four-decade log scale. Acquisition on the flow cytometer was done in the low sample flow rate setting (12 μl/min) to improve the coefficient of variation on the DNA histograms. Lymphocytes, including live, early apoptotic and late apoptotic cells, were gated on the basis of their FSC and SSC parameters, and fluorescence data were gated on FSC versus PI fluorescence dual-parameter contour plots for exclusion of monocytes, debris and clumps. This method of gating allowed ready discrimination of debris (very low FSC and decreased PI fluorescence) from dead cells (low FSC and high PI fluorescence). A minimum of 10 000 events was collected on each sample.
Phenotypic analysis of apoptotic T cells
Quantification and phenotypic analysis of apoptotic cells from the short-term cultured lymphocytes were performed by staining apoptotic cells with 7-amino-actinomycin D (7-AAD; Sigma) as reported by Schmid et al. [25]. This method was shown to discriminate between early and late apoptotic cells due to the increased membrane permeability of the latter group. Cultured lymphocytes were first incubated with FITC-conjugated MoAbs against surface antigens as described above, and washed cells were then incubated with 20 μg/ml of 7-AAD for 20 min at 4°C protected from light. Stained cells were further fixed with 1% paraformaldehyde in PBS in the presence of 20 μg/ml of non-fluorescent actinomycin D (Sigma) to block 7-AAD staining within apoptotic cells and avoid non-specific labelling of living cells. Finally, the double-stained cells were incubated overnight at 4°C in the dark and were then analysed in their staining solution by a FACScan flow cytometer (Becton Dickinson). The green fluorescence was collected after a 530/30 BP nm filter, the red fluorescence from 7-AAD was filtered through a 650 long pass filter. Scattergrams were generated by combining FSC with 7-AAD fluorescence, and regions were drawn around clear-cut populations having either negative (live cells), dim (early apoptotic cells), or bright fluorescence (late apoptotic cells). A minimum of 10 000 events was collected on each sample.
Analysis of mitochondrial functions
For the simultaneous determination of surface markers and ΔΨm, cells were first stained with PE-labelled anti-hCD4 or anti-hCD8 (Becton Dickinson) and anti-Fas (PharMingen) antibodies (30 min on ice). Cells were washed (5 min; 600 g; 4°C) in ice-cold staining buffer (PBS, pH 7·2, supplemented with 2% BSA) (Sigma) followed by exposure for 15 min at 37°C to 40 nmol/l 3,3′-dihexyloxacarbo-cyanine iodide (3) (DiOC6; Molecular Probes, Eugene, OR) [26]. For the simultaneous assessment of surface markers and mitochondrial reactive oxygen species (ROS) generation, such as superoxide and hydroxyperoxide, cells were first stained with PE-labelled anti-hCD4 or anti-hCD8 antibodies and then exposed for 15 min at 37°C to 2 mmol/l hydroethidine (HE; Molecular Probes) [27] or for 1 h at 37°C to 5 mm 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes) [27,28], respectively. In control experiments, cells were labelled after preincubation with the uncoupling agent carbonyl cyanide m-chlorophenyl-hydrazone (mClCCP; 50 mmol/l, 37°C, 30 min; Sigma), or the ROS-generating agent menadione (1 mmol/l, 37°C, 1 h; Sigma). For DCFH-DA, a positive control (cells kept for 2 min in 15 mm H2O2 and washed three times) was inserted. Analysis were performed using a FACScan cytofluorometer (Becton Dickinson). FSC and SSC parameters were gated on the major population of normal-sized lymphoid cells. After suitable compensation, fluorescence was recorded at different wavelengths: FITC, DiOC6(3) and DCFH-DA at 525 nm (FL-1), PE at 575 nm (FL-2) and HE at 600 nm (FL-3).
Statistical analysis
The primary purpose of this study was to compare several parameters, both actual values and percentages, between the patients with AIDS and LTNPs. Individual P values for each separate parameter comparison were obtained using the Wilcoxon's rank sum test, and the unadjusted P values are reported. Multiple P values were adjusted following the method developed by Hochberg [29], and a non-parametric procedure for simultaneously evaluating multiple endpoints was used [30]. All P values are two-tailed.
RESULTS
Fas–FasL expression and lymphocyte apoptosis
Patients with AIDS had lower CD4+ and CD8+ T counts in comparison with LTNPs (P < 0·0001 for CD4 and <0·000 01 for CD8) (Table 1). Furthermore, expression of both Fas and FasL was found to be significantly increased among CD4+ and CD8+ T cell subsets from the AIDS subjects with respect to LTNPs (P = 0·0002 and <0·000 01 for Fas-positive CD4+ and Fas-positive CD8+ T cells, respectively; P < 0·000 01 and = 0·002 for FasL-positive CD4+ and FasL-positive CD8+ T cells, respectively) (Table 2).
Table 2.
Apoptotic CD4+ and CD8+ T lymphocytes and Fas–FasL expression (see Materials and methods for details)
| Parameter | LTNPs | AIDS | P |
|---|---|---|---|
| Apoptotic CD4 (%) | |||
| (stained with PI) | |||
| Median ±s.d. | 3·4 ± 0·7 | 31·8 ± 5 | < 0·000 01 |
| Range | 2·3–4·4 | 21·4–36·5 | |
| Apoptotic CD8 (%) | |||
| (stained with PI) | |||
| Median ±s.d. | 5·5 ± 1·1 | 27·7 ± 3 | < 0·000 01 |
| Range | 3·5–7·6 | 20·5–31·2 | |
| Apoptotic CD4 (%) | |||
| (stained with 7-AAD) | |||
| Median ±s.d. | 3·3 ± 0·8 | 31·7 ± 5 | < 0·000 01 |
| Range | 2·1–4·5 | 20·7–38·9 | |
| Apoptotic CD8 (%) | |||
| (stained with 7-AAD) | |||
| Median ±s.d. | 5·4 ± 1·1 | 26·9 ± 2·4 | < 0·000 01 |
| Range | 3·7–7·8 | 22·6–30·2 | |
| Fas CD4 (%) | |||
| Median ±s.d. | 38 ± 8·2 | 71 ± 18 | 0·0002 |
| Range | 16–51 | 31–85 | |
| Fas CD8 (%) | |||
| Median ±s.d. | 26·7 ± 6 | 52·7 ± 12 | < 0·000 01 |
| Range | 16–35 | 22–67 | |
| FasL CD4 (%) | |||
| Median ±s.d. | 29·3 ± 11 | 70 ± 17 | < 0·000 01 |
| Range | 1–46 | 33–98 | |
| FasL CD8 | |||
| Median ±s.d. | 39·7 ± 11 | 71 ± 2 | 0·002 |
| Range | 13–50 | 10–88 | |
PI, Propidium iodide; 7-AAD, 7-amino-actinomycin D; LTNP, long-term non-progressor.
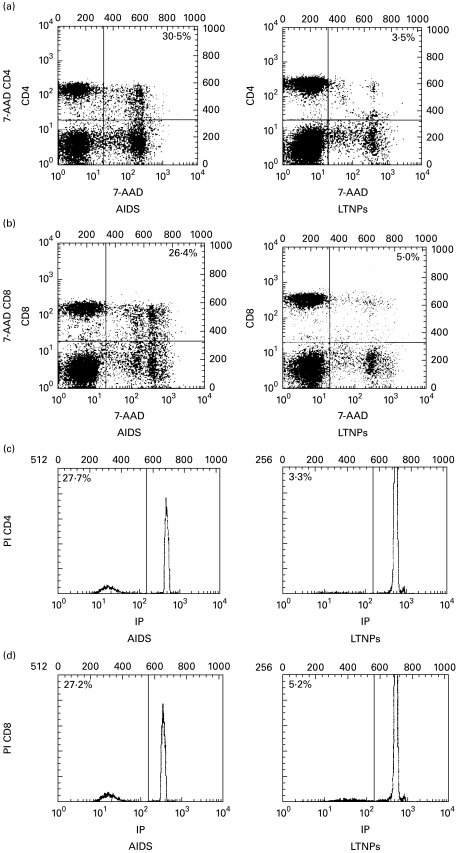
These findings were associated with an increased susceptibility of lymphocytes to apoptosis. In fact, an increased number of lymphocytes were undergoing apoptosis in AIDS patients compared with LTNPs. This was established by staining apoptotic nuclei with PI [22], which detects late events of apoptosis such as chromatin condensation and DNA fragmentation [24]. Following 12 h of incubation in complete medium, the percentage of spontaneous apoptosis was significantly increased in CD4+ and CD8+ T lymphocytes from patients at the AIDS stage compared with the rate in LTNPs (31·8 ± 5·3 and 27·7 ± 2·4 in AIDS patients, 3·4 ± 0·7 and 5·5 ± 1·1 in LTNPs, respectively, for CD4+ and CD8+ T cells; P < 0·000 01 for both parameters), whereas the level of apoptosis was only 1% in lymphocytes from control donors (Table 2). We confirmed these results by measuring apoptosis also with 7-AAD, a fluorescent DNA-intercalating agent which only penetrates the membrane of cells undergoing apoptosis and thus exhibiting a shrunken phenotype (reduced FSC) (P < 0·000 01 for both parameters) (Table 2). Furthermore, this staining discriminates between early and late apoptotic cells [25], and Fig. 1 shows that both methods produced comparable results when measuring the extent of lymphocyte apoptosis in lymphocytes from AIDS patients and LTNP individuals. The FSC and SSC characteristics of cells stained with PI and 7-AAD confirmed that cells were indeed undergoing apoptosis (data not shown).
Fig. 1.
Assessment of apoptotic peripheral blood T lymphocytes from AIDS patients and long-term non-progressors (LTNPs). Two-colour staining was performed for the simultaneous determination of apoptosis and CD4/CD8 surface phenotype. Quantification of apoptosis was performed by staining either apoptotic cells with 7-amino-actinomycin D (7-AAD) (a,b) or apoptotic nuclei with propidium iodide (PI) (c,d), as described in Materials and methods. Numbers refer to the percentage of apoptotic cells among the CD4+ (a,c) and CD8+ (b,d) T lymphocytes. Results are representative of two independent experiments each performed on two different subjects.
Generation of ROS
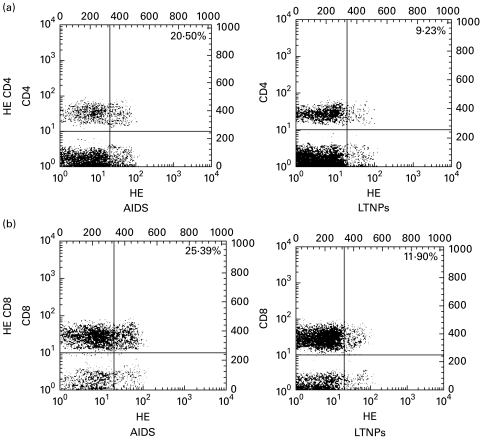
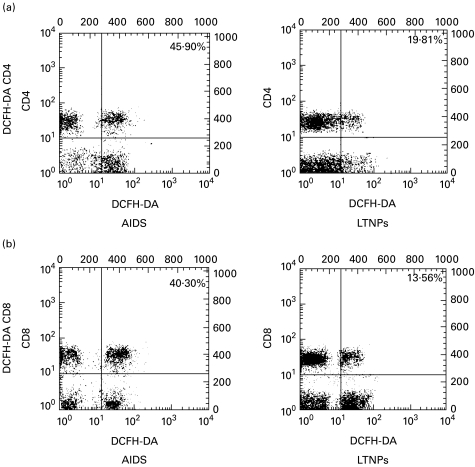
As shown in Table 3, circulating lymphocytes from HIV+ donors contained a fraction of cells which are able to oxidize the non-fluorescent lipophilic (i.e. membrane-permeable) dye HE into the hydrophilic fluorescent product Eth. Since HE is particularly sensitive to the superoxide anion, this change is thought to reflect the generation of this anion [27]. Moreover, lymphocytes were labelled using DCFH-DA, a fluorochrome that detects hydroperoxide generation [27,28]. We found that the percentage of cells bearing an Ethhigh and DCFH-DA+ phenotype was elevated in AIDS patients compared with LTNPs. Statistical analysis revealed a highly significant difference between the two groups with respect to CD4+ and CD8+ T cells stained with DCFH-DA (P < 0·000 01 for both parameters) (Table 3). A significant increase in the Ethhigh CD4+ T cell subset was also found in the AIDS group compared with LTNPs (P < 0·000 01) (Table 3). There was a similar trend for the Ethhigh CD8+ T cell subset, although the difference was only marginally significant (P = 0·03) (Table 3).
Table 3.
Generation of reactive oxygen species by CD4+ and CD8+ T lymphocytes as assessed with staining with the fluorochromes hydroethidine (HE) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (see Materials and methods for details)
| Parameter | LTNPs | AIDS | P |
|---|---|---|---|
| HE CD4 (%) | |||
| Median ±s.d. | 13·1 ± 3 | 22·1 ± 2·6 | < 0·000 01 |
| Range | 9–18·8 | 18–25 | |
| HE CD8 (%) | |||
| Median ±s.d. | 14·7 ± 2·3 | 17 ± 6·2 | 0·03 |
| Range | 12–19 | 13–34 | |
| DCFH-DA CD4 (%) | |||
| Median ±s.d. | 26·3 ± 6·5 | 41·4 ± 4·2 | < 0·000 01 |
| Range | 10–30 | 31–46 | |
| DCFH-DA CD8 (%) | |||
| Median ±s.d. | 13·4 ± 3·6 | 31·1 ± 4·2 | < 0·000 01 |
| Range | 4–21 | 29–40 | |
LTNPs, Long-term non-progressors.
Typical cytofluorographic plots using staining with Eth and DCFH-DA are shown in Figs 2 and 3.
Fig. 2.
Assessment of superoxide anion generation in peripheral blood T lymphocytes from AIDS patients and long-term non-progressors (LTNPs). Two-colour staining was performed for the simultaneous determination of CD4/CD8 surface phenotype and superoxide anion generation. Cells from representative AIDS patients and LTNPs were labelled, as described in Materials and methods, using markers for superoxide anion generation (hydroxyethidine (HE)) and MoAbs to CD4/CD8 antigens (a/b, respectively). Numbers refer to the percentage of cells bearing an HE→Ethhigh phenotype among the CD4+ and CD8+ T lymphocytes. In the FACScan profiles, apoptotic cells were identified either by forward light scatter (FSC)/side scatter (SSC) criteria or by positive staining with 7-amino-actinomycin D (7-AAD), as described in Materials and methods. The cursors were set according to the background staining defined with mouse IgG isotypic controls. Results are representative of two independent experiments each performed on two different subjects.
Fig. 3.
Assessment of hydroxyperoxide generation in peripheral blood T lymphocytes from AIDS patients and long-term non-progressors (LTNPs). Two-colour staining was performed for the simultaneous determination of CD4/CD8 surface phenotype and hydroxyperoxide generation. Cells from representative AIDS patients and LTNPs were labelled, as described in Materials and methods, using markers for hydroxyperoxide generation (2′,7′-dichlorofluorescein diacetate (DCFH-DA)) and MoAbs to CD4/CD8 antigens (a/b, respectively). Numbers refer to the percentage of DCFH-DA-positive cells among the CD4+ and CD8+ T lymphocytes. Results are representative of two independent experiments each performed on two different subjects.
Mitochondrial activity
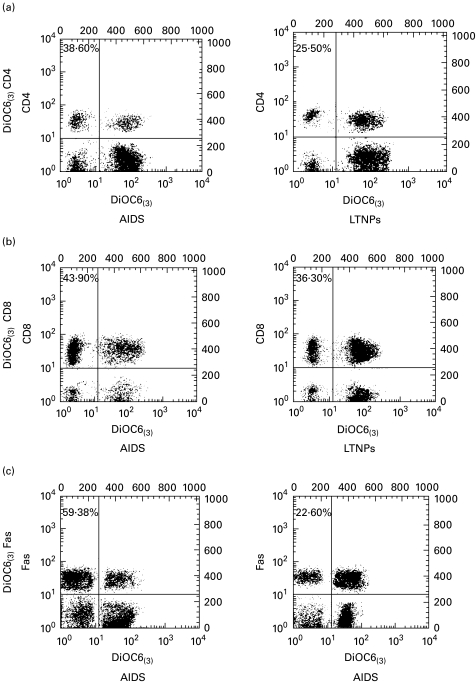
The low incorporation of DiOC6(3), a cationic lipophilic fluorochrome that allows for the assessment of mitochondrial ΔΨm, is thought to reflect the dissipation of mitochondrial ΔΨm [26]. This change constitutes an early and irreversible step in the effector phase of apoptosis [31–34]. A relatively high percentage of peripheral blood T lymphocytes from HIV+ donors incorporated low levels of DiOC6(3). However, there was no correlation between the clinical status of HIV carriers and the level of DiOC6(3) incorporation when we investigated the CD4+ and CD8+ T populations. Indeed, the differences observed between AIDS subjects and LTNPs were significant with respect to the DiOC6(3)low CD4+ T cells (P = 0·008) but not to CD8+ T cells (P = 0·24) (Table 4). By contrast, we observed that patients with AIDS had a higher percentage of DiOC6(3)low lymphocytes expressing the Fas antigen compared with such lymphocytes from LTNPs (P = 0·002) (Table 4).
Table 4.
CD4+ and CD8+ T lymphocytes and Fas-positive lymphocytes with disrupted mitochondrial transmembrane potential as shown by the incorporation of the (3,3′-dihexyloxacarbo-cyanine iodide (3) (DiOC6(3)) fluorochrome (see Materials and methods for details)
| Parameter | LTNPs | AIDS | P |
|---|---|---|---|
| DiOC6(3)low CD4 (%) | |||
| Median ±s.d. | 27·6 ± 8 | 28·3 ± 2·5 | 0·008 |
| Range | 23–47 | 34–42 | |
| DiOC6(3)low CD8 (%) | |||
| Median ±s.d. | 33·5 ± 10·3 | 41·5 ± 5 | 0·24 |
| Range | 24–62 | 28–45 | |
| DiOC6(3) Fas (%) | |||
| Median ±s.d. | 27·8 ± 13·2 | 44·8 ± 5·9 | 0·002 |
| Range | 5–57 | 36–59 | |
LTNPs, Long-term non-progressors.
Typical cytofluorographic plots using staining with DiOC6(3) are shown in Fig. 4.
Fig. 4.
Assessment of mitochondrial transmembrane potential (ΔΨm) in peripheral blood T lymphocytes from AIDS patients and long-term non-progressors (LTNPs). Two-colour staining for the simultaneous determination of surface phenotype and ΔΨm. Cells from representative AIDS patients and LTNPs were labelled, as described in Materials and methods, using markers for ΔΨm (3,3′-dihexyloxacarbo-cyanine iodide (3) (DiOC6(3))) and for CD4, CD8 and Fas antigens (a,b,c, respectively). Numbers refer to the percentage of lymphocytes with disrupted mitochondrial transmembrane potential (DiOC6(3)low) among the CD4, CD8 and Fas-positive cells. Results are representative of three independent experiments each performed on two different subjects.
DISCUSSION
We report here that LTNPs had a lower degree of lymphocyte apoptosis, with regard to either CD4+ or CD8+ T cells, compared with subjects progressing to AIDS. Previous studies have suggested that apoptosis is a critical contributory component to HIV disease progression, and data provided so far are consistent with the view that the persistent viraemia or the chronic state of immune activation that characterize HIV infection might be the primary mechanism responsible for the accelerated rate of apoptotic lymphocyte death in AIDS [1–3]. A strong correlation appears indeed to link the intensity of lymphocyte apoptosis and the degree of cell activation [6,35].
The reduced rate of apoptosis that we measured in lymphocytes from LTNPs in comparison with AIDS patients was associated with lower expression of Fas, which is both an activation marker and a death factor, and FasL. These results correlate well with previous evidence of significantly lower levels of lymphocyte-associated ceramide, an endogenous mediator of apoptosis which has been shown to play a crucial role in Fas-induced apoptotic signalling [13,14], in LTNPs in comparison with AIDS patients [7,36]. Other studies have recently suggested an involvement of the Fas–FasL system in the pathophysiology of the HIV-related CD4+ T lymphocyte decline, and up-regulation of Fas expression on both CD4+ and CD8+ T cells, as well as increased sensitivity of lymphocytes to Fas-induced apoptosis [9–12], have been reported in patients with HIV infection.
The increased level of lymphocyte apoptosis that we found in subjects with AIDS was associated with altered mitochondrial function. We measured a higher proportion of cells incorporating low levels of the DiOC6(3) fluorochrome among peripheral blood lymphocytes from AIDS patients than from LTNPs; in particular, DiOC6(3)low cells with decreased ΔΨm were significantly more frequent among the Fas+ subset irrespective of a CD4+ or CD8+ phenotype. In our opinion, these data further highlight, although indirectly, the relationship linking the expression of Fas with the increased rate of lymphocyte apoptosis in HIV progressors.
It is known that lymphocytes undergoing apoptosis suffer a sequential dysregulation of mitochondrial function early during the apoptotic process, and this implicates some functional influence of mitochondria on the regulation of apoptotic cell death [31–34]. In all experimental models of apoptosis, including those associated with Fas–FasL activation and those induced by oxidant stress, the alterations in nuclear morphology and degradation of chromosomal DNA, the hallmarks of the apoptotic process are invariably preceded by a step-wise dysregulation of mitochondrial function [31–34]. Lymphocytes exhibit first a reduction in mitochondrial ΔΨm, as quantifiable by means of suitable fluorochromes such as DiOC6(3), and then an additional decrease in ΔΨm as evidenced by increased superoxide anion-mediated oxidation of HE into the fluorescent product Eth [31–34]. The collapse in ΔΨm constitutes an early and irreversible step in the apoptotic effector phase and allows us to identify an additional pool of lymphocytes that are irreversibly committed to undergo apoptosis despite still lacking the characteristic morphological nuclear changes and the degradation of internucleosomal DNA associated with apoptosis [31–34].
The altered mitochondrial function, as shown by the disruption of ΔΨm that we determined in patients with AIDS rather than in LTNPs, appeared to be associated with oxidant stress and the increased generation of ROS. In fact, we found a greater frequency of (HE→Eth)high or DCFH-DAhigh cells among CD4+ and CD8+ T lymphocytes from patients with AIDS compared with LTNPs. Thus, based on our findings, the pool of lymphocytes with abnormal mitochondrial function and enhanced generation of ROS appears to be significantly greater in subjects progressing to AIDS than in LTNPs. Taking into account all our findings, a crucial role for mitochondria in the process of lymphocyte apoptosis during the evolution of the disease towards AIDS can be predicted.
Different mechanisms may contribute to the mitochondrial alterations associated with HIV infection. HIV-specific gene products could directly affect mitochondrial function [19–21, 37–40]; however, it is more likely that mitochondrial function is predominantly altered indirectly by HIV infection. The relatively small proportion of lymphocytes actually infected with HIV in vivo and the observation that apoptosis occurs in both CD4+ and CD8+ T lymphocytes support this view. An additional mechanism of mitochondrial damage could be the oxidation of mitochondrial DNA caused by the use of anti-retroviral drugs, in particular zidovudine [41].
One point to be noted about this study is that the AIDS patients we investigated were fast progressors with a median time from seroconversion to the development of AIDS significantly shorter than that usually seen in developed countries [42]. We do not know whether this discrepancy has influenced the differences in the degree of apoptosis between the LTNPs and AIDS patients determined in this study. On the basis of our findings, the only conclusion we can draw is that once HIV infection has progressed to AIDS patients have a greater degree of lymphocyte apoptosis compared with asymptomatic LTNPs. Whether this is also influenced by the rate of disease progression remains to be established. Our work was not designed to test this hypothesis, and longitudinal investigations will be helpful to clarify this problem.
An additional point is that all the patients with AIDS in this study were on zidovudine therapy at the time of cell sampling for apoptosis analysis. The relative contribution of zidovudine to the increased lymphocyte apoptosis is unclear. Zidovudine and other nucleoside analogues have been shown to induce apoptosis in several in vitro systems [43–46]. In clinical studies of anti-retroviral therapy however, the suppression of viral replication is not clearly associated with a decline in lymphocyte apoptosis [47–49]. Rather, the marginal improvement in indices of apoptosis afforded by anti-retroviral therapy appears to be in part independent of the suppression of viral replication. Even though these data are somewhat conflicting, in our opinion the direct pro-apoptotic action of nucleoside analogues is probably marginal in vivo compared with the pro-apoptotic potential either directly or indirectly exploited by the virus. It is also likely that even low levels of HIV replication are sufficient to induce immune activation and lymphocyte apoptosis.
In conclusion, our data indicate a significantly reduced level of apoptosis and apoptosis-associated parameters in LTNPs compared with patients developing AIDS. Even though a number of questions remain, it appears plausible that a lower degree of Fas/FasL expression and/or enhanced apoptosis resistance associated with a lower degree of mitochondrial superoxide anion generation may contribute to delayed AIDS progression and improving the long-term outcome of HIV infection.
Acknowledgments
Informed consent was obtained from all patients, and human experimentation guidelines of the Ethical Committee of the Community of San Patrignano were followed in the conduct of clinical research.
REFERENCES
- 1.Gougeon ML, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–70. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen JC, Estaquier J, Idziorek T, De Bels F. The relevance of apoptosis to AIDS pathogenesis. Trends Cell Biol. 1995;5:27–40. doi: 10.1016/s0962-8924(00)88933-3. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Fauci AS. Apoptosis in HIV infection. Nature Med. 1995;1:111–20. doi: 10.1038/nm0295-118. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 5.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 6.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: the increased susceptibility to apoptosis of CD4+ and CD8+ T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 7.De Simone C, Cifone MG, Di Marzio L, et al. Cell-associated ceramide in HIV-1-infected subjects. AIDS. 1996;10:675–6. doi: 10.1097/00002030-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Liegler TJ, Yonemoto W, Elbeik T, Vittinghoff E, Buchbinder S, Greene WC. Diminished spontaneous apoptosis in lymphocytes from human immunodeficiency virus-infected long-term nonprogressors. J Infect Dis. 1998;178:669–79. doi: 10.1086/515378. [DOI] [PubMed] [Google Scholar]
- 9.Katsikis PD, Wunderlich ES, Craig CA, Herzenberg LA. Fas antigen stimulation induced marked apoptosis of T lymphocytes in HIV virus-infected individuals. J Exp Med. 1995;181:2029–36. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloand EM, Young NS, Kumar P, Weichold FF, Sato T, Maciejewski JP. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood. 1997;89:1357–63. [PubMed] [Google Scholar]
- 11.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–66. [PubMed] [Google Scholar]
- 12.Gehri R, Hahn S, Rothen M, Steuerwald M, Nuesch R, Erb P. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS. 1996;10:9–16. [PubMed] [Google Scholar]
- 13.Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activated an acidic sphingomyelinase. J Exp Med. 1994;180:1547–52. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995;14:5859–68. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulbins E, Bissonette R, Mahboubi A, et al. Fas-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2:341–51. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 16.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 17.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–6. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 18.Los M, Van de Craen M, Penning LC, et al. Requirement of an ICE/CED-3 protease for FAS/APO-1 mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 19.Westendorp MO, Shatrov VA, Schulze-Osthoff K, et al. HIV-1 tat potentiates TNF-induced NF-κB activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–53. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreck R, Rieber P, Bauerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–52. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenspan HC, Aruoma OI. Oxidative stress and apoptosis in HIV infection: a role for plant-derived metabolites with synergistic antioxidant activity. Immunol Today. 1994;15:209–13. doi: 10.1016/0167-5699(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti I, Migliorati G, Pagliacci C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1968;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 23.Dive C, Gregory CD, Phipps DJ, Evans DL, Milner AE, Wyllie AH. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992;1133:275–84. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 24.Wyllie AN, Morris RG, Smith AL, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1991;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- 25.Schmid I, Uittenbogaart CH, Keld B, Giorgi JV. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–57. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 26.Petit PX, O'connor JE, Grunwald D, Brown SC. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990;194:389–95. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 27.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′, 7′-dichlorofluorescein. J Leuk Biol. 1990;47:440–6. [PubMed] [Google Scholar]
- 28.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- 30.O'brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 31.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–72. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamzami N, Marchetti P, Castedo M, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–77. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petit PX, LeCoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations of mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995;130:157–67. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 35.Michel P, Balde AT, Roussilhon C, Aribot G, Sarthou JL, Gougeon ML. Reduced immune activation and T cell apoptosis in human immunodeficiency virus type 2 compared with type 1: correlation of T cell apoptosis with β2 microglobulin concentration and disease evolution. J Infect Dis. 2000;181:64–75. doi: 10.1086/315170. [DOI] [PubMed] [Google Scholar]
- 36.Moretti S, Alesse E, Di Marzio L, et al. Reduction of HIV infection-associated apoptosis by l-carnitine: a pilot study. Blood. 1998;91:3817–24. [PubMed] [Google Scholar]
- 37.Somasundaran M, Zapp ML, Beattie LK, Pang L, Byron KS, Bassel GJ, Sullivan JL, Singer RH. Localization of HIV RNA in mitochondria of infected cells: potential role in cytopathogenicity. J Cell Biol. 1994;126:1353–60. doi: 10.1083/jcb.126.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macreadie IG, Thorburn DR, Kirby DM, Castelli LA, de Rozario NL, Azad AA. HIV-1 protein Vpr causes gross mitochondrial dysfunction in the yeast Saccharomyces cerevisiae. FEBS Letters. 1997;410:145–9. doi: 10.1016/s0014-5793(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 39.Jacotot E, Ravagnan L, Loeffler M, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandstrom PA, Tebbey PW, Vancleaven S, Buttke TM. Lipid hydroperoxides induce apoptosis in T-cells displaying a HIV-associated glutathione peroxidase deficiency. J Biol Chem. 1994;2:798–801. [PubMed] [Google Scholar]
- 41.De la Asuncion JG, Del Olmo ML, Sastre F, Pallardo VL, Vina J. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology. 1999;29:985–7. doi: 10.1002/hep.510290353. [DOI] [PubMed] [Google Scholar]
- 42.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;335:1131–7. [PubMed] [Google Scholar]
- 43.Sailaja G, Nayak R, Antony A. Azidothymidine induces apoptosis in mouse myeloma cell line Sp2/0. Biochem Pharmacol. 1996;52:857–62. doi: 10.1016/0006-2952(96)82183-6. [DOI] [PubMed] [Google Scholar]
- 44.Sundseth R, Joyner SS, Moore JT, Dorusife RE, Dev IK. The anti-human immunodeficiency virus agent 3′-fluorothymidine induces DNA damage and apoptosis in human lymphoblastoid cells. Antimicrob Agents Chemother. 1996;40:331–5. doi: 10.1128/aac.40.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viora M, Di Genova G, Rivabene R, Malorni W, Fattorossi A. Interference with cell cycle progression and induction of apoptosis by dideoxynucleoside analogs. Int J Immunopharmacol. 1997;19:311–21. doi: 10.1016/s0192-0561(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto KI, Tsunoda R, Okamoto M, Shigeta S, Baba M. Stavudine selectively induces apoptosis in HIV type 1-infected cells. AIDS Res Hum Retrovir. 1997;20:193–9. doi: 10.1089/aid.1997.13.193. [DOI] [PubMed] [Google Scholar]
- 47.Roger PM, Breittmayer JP, Arlotto C, Pugliese P, Pradier C, Bernard-Pomier G, Dellamonica P, Bernard A. Highly active anti-retroviral therapy (HAART) is associated with a lower level of CD4+ T cell apoptosis in HIV-infected patients. Clin Exp Immunol. 1999;118:412–6. doi: 10.1046/j.1365-2249.1999.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patki AH, Purvis SF, Valdez H, et al. HIV infection perturbs DNA content of lymphoid cells: partial correction after ‘suppression’ of virus replication. AIDS. 1999;13:1177–85. doi: 10.1097/00002030-199907090-00005. [DOI] [PubMed] [Google Scholar]
- 49.Kotler DP, Shimada T, Snow G, Winson G, Chen W, Zhao M, Inada Y, Clayton F. Effect of combination antiretroviral therapy upon rectal mucosa HIV RNA burden and mononuclear cell apoptosis. AIDS. 1998;12:597–604. doi: 10.1097/00002030-199806000-00008. [DOI] [PubMed] [Google Scholar]