Abstract
Enteroviruses, the most common cause of acute myocarditis, are also supposed aetiological agents of dilated cardiomyopathy. Autoantibodies (anti-M7; Klein & Berg, Clin Exp Immunol 1990; 58:283–92) directed against flavoproteins with covalently bound flavin (αFp-Ab; Otto et al., Clin Exp Immunol 1998; 111:541–2) are detected in up to 30% of sera of patients with myocarditis and idiopathic dilated cardiomyopathy (IDCM). Mice inoculated with a myocarditic variant of coxsackievirus B3 (CVB3) were employed to study the occurrence of serum αFp-Ab following viral infection. The presence of αFp-Ab was analysed by Western blotting with the flavoprotein antigens 6-hydroxy-d-nicotine oxidase (6HDNO) and sarcosine oxidase (SaO). Of 10 sera from CVB3-infected mice, five showed a strong reaction with both antigens. The sera were reactive also to the mitochondrial covalently flavinylated proteins dimethylglycine dehydrogenase and sarcosine dehydrogenase. Sera of non-infected mice did not react with these antigens. A 6HDNO mutant protein with non-covalently bound FAD no longer reacted on Western blots with sera of CVB3-infected mice. Preincubation with FAD abolished or reduced the reaction of the sera with the 6HDNO antigen. At 2 weeks p.i. the αFp-Ab were of the IgM and IgG isotypes, at 7 and 9 weeks p.i. of the IgG isotype. The sera of CVB3-infected mice reproduced closely the antigenic specificity of the anti-M7 sera of patients, lending further support to the role of coxsackieviruses in the pathogenesis of IDCM.
Keywords: anti-flavoprotein antibodies, Coxsackievirus B3, mitochondria, myocarditis, cardiomyopathy
INTRODUCTION
Some patients with myocarditis or dilated cardiomyopathy of unknown aetiology (IDCM) were shown to present serum autoantibodies to a heart and liver mitochondrial membrane protein of approximately 64 kD, a liver mitochondrial matrix protein of approximately 90 kD and antibodies to bacterial sarcosine oxidase (anti-M7) [1,2]. The mitochondrial membrane antigen was identified as the 68 180-D flavoprotein subunit [3] of the succinate dehydrogenase complex [4]. The liver mitochondrial matrix antigen was identified as the 91 391-D flavoprotein dimethylglycine dehydrogenase (DMGDH) and in addition as the closely related 101 000-D flavoprotein sarcosine dehydrogenase (SaDH). Besides these mitochondrial proteins, the sera also reacted with bacterial flavoproteins as antigen, such as sarcosine oxidase (SaO) and 6-hydroxy-d-nicotine oxidase (6HDNO) [4]. The common motif of all these flavoenzymes is the covalent attachment of the flavin cofactor to a histidine residue of the polypeptide chain [5]. The main common epitope recognized by the anti-flavoprotein antibodies (αFp-Ab) was shown by epitope mapping to be a peptide containing the covalently attached flavin moiety [6]. A mutant 6HDNO unable to bind FAD covalently was no longer recognized by the αFp-Abs [4].
Viral infections have been reported to be associated with dilated cardiomyopathy [7]. One potential cause of IDCM appears to be an enteroviral infection, especially with coxsackie B viruses [8]. In a mouse model, infection with cardiotropic coxsackievirus B3 (CVB3) leads to an acute myocarditis which may progress to a dilated cardiomyopathy [9]. In this study we tested in the mouse model the assumption of a possible correlation between a viral infection of the myocardium and the presence of αFp-Ab in the sera of CVB3-infected animals.
MATERIALS AND METHODS
Chemicals and immunochemicals
All chemicals and biochemicals were of highest purity available. Goat anti-mouse IgG, goat anti-mouse IgM, anti-human IgG and anti-rabbit IgG conjugated with alkaline phosphatase were from Sigma (Deisenhofen, Germany). Endoprotease Lys-C was from Sigma, and trypsin at highest purity available was from Promega (Mannheim, Germany). Antisera against 6HDNO, SaO, rat liver DMGDH and SaDH were raised in rabbits according to standard protocols [10].
Experimental animals
Four to 8-week-old male SJL/OlaHSD (Harlem Sprague Dawley Inc., USA) mice were infected in the hind leg with 2 × 103 plaque-forming units (PFU) of a myocarditic Woodruff variant of coxsackievirus B3. A first lot of 10 mice were killed 3, 9 and 13 weeks post-infection (p.i.) and blood samples were collected. The sera were stored at −20°C until use. Sera from six uninfected mice were taken as controls. The hearts of the mice were fixed in buffered 10% formalin and dissected in 0·5 cm thick horizontal slices. The tissue was paraffin-embedded using standard techniques, and 5 μm thick sections were made from each slice. They were stained using haematoxylin–eosin and Elastica van Gieson. Microscopic pictures were taken from representative myocardial lesions. Sera of a second lot of 30 CVB3-infected mice were taken from 10 animals each at 2, 7 and 9 weeks p.i. and the sera of five additional uninfected mice were taken as controls.
Isolation of mitochondrial proteins
Rat liver mitochondria were prepared as described in [11]. Separation of the soluble matrix fraction was performed by four times freeze–thawing of the washed mitochondria in liquid nitrogen followed by centrifugation at 100 000 g for 30 min at 2°C.
Purification of bacterial antigens
6HDNO wild-type and the 6HDNO.Cys mutant proteins were isolated as 6His-tagged proteins by affinity chromatography on Ni2+-chelating Sepharose as described in Berthold et al. [12].
Western blotting
Mitochondrial matrix protein (50 μg) and 1 μg of the purified proteins were resolved by SDS–PAGE and transferred onto nitrocellulose (Optipran BA-S 85; Schleicher & Schuell, Dassel, Germany) using a semidry horizontal apparatus (BioRad, München, Germany). Individual lanes of the membrane were incubated with mouse sera at a dilution of 1:200, with human αFp-Ab serum at a dilution of 1:500 and with rabbit sera at a dilution of 1:2000. Second antibodies conjugated to alkaline phosphatase were used at a dilution of 1:30 000 for the goat anti-mouse IgG, goat anti-mouse IgM and goat anti-human IgG and of 1:8000 for the goat anti-rabbit IgG. 5-bromo-4-chloro-3-indolyl-phosphate/nitrotetrazolium blue was used as substrate.
Immunoinhibition
The reaction of the CVB3-infected mice with the 6HDNO and SaO antigens was competed by incubating the sera overnight with 10 μm FAD. Following incubation with FAD, the sera were used to decorate Western blots of 6HDNO and SaO.
Protease digest of 6HDNO
6HDNO (1 mg) was digested at 37°C for 4 h with either 20 μg trypsin or for 9 h with 5 μg endoprotease Lys-C. The tryptic and endoprotease Lys-C digest was separated by SDS–PAGE on 17·5% and 12% polyacrylamide gels, respectively. The FAD-containing peptides on the gel were identified by their UV light fluorescence in 10% acidic acid.
RESULTS
Histological examination of the hearts of CVB3-infected mice
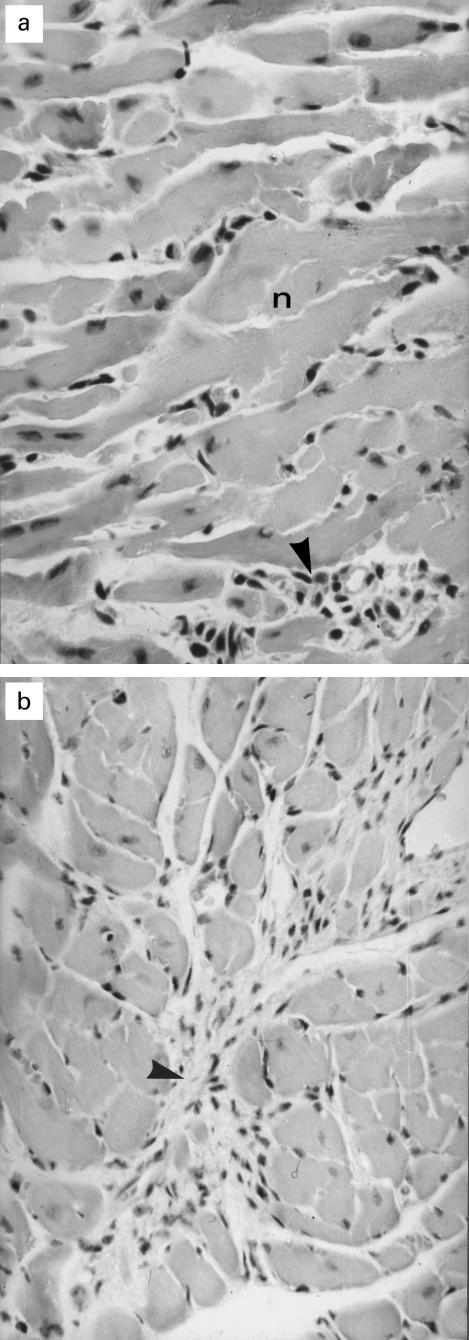
Histological examination of the hearts 3 weeks p.i. (two mice) revealed a focally accentuated lymphohistiocytic and necrotizing myocarditis (Fig. 1a). At 9 weeks and at 13 weeks p.i. in seven hearts minimal and microfocal lymphohistiocytic infiltrations of the myocardial interstitium were found. One heart of this experimental group showed a diffuse interstitial fibrosis, which included sparse mesenchymal cells (Fig. 1b).
Fig. 1.
Histopathology of hearts of coxsackievirus B3 (CVB3)-infected mice. (a) Myocarditis due to CVB3 infection showing myocytic necrosis (n) and lymphohistiocytic infiltration (arrowhead); mice, 2 weeks p.i., H–E staining; primary mag. × 200. (b) Marked diffuse interstitial fibrosis including mesenchymal proliferation (arrowhead) in the heard of a mouse 13 weeks p.i.; H–E staining; primary mag. × 100.
Sera of CVB3-infected mice contain αFp-Ab
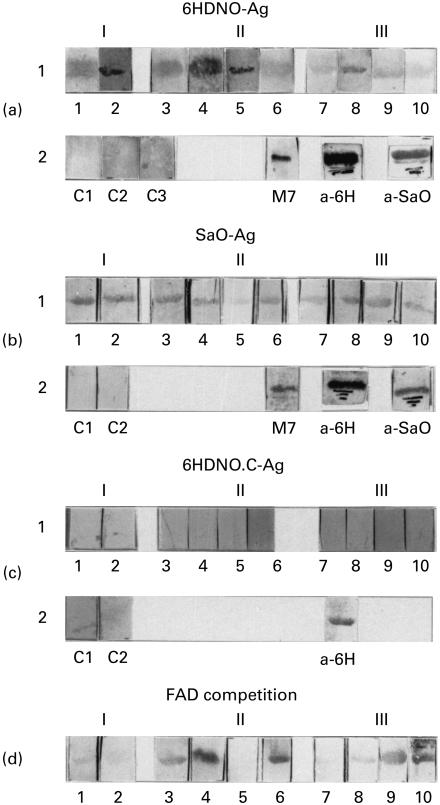
It has been shown previously [4,6] that sera of patients with myocarditis and IDC contain αFp-Ab. These antibodies recognize as antigens, besides mitochondrial flavoproteins, the bacterial enzymes 6HDNO of Arthrobacter nicotinovorans, an enzyme of 48·9 kD with FAD bound to His-71 and the flavoprotein subunit of SaO of Corynebacterium sp., a tetrameric enzyme with FMN covalently attached to the 45-kD subunit. These flavoproteins were employed as standard bacterial antigens. The 6HDNO mutant with His-71 replaced by a Cys residue no longer binds FAD covalently. This mutant protein served as a FAD-less control antigen. From 10 CVB3-infected mice, two were killed 3 weeks (I), four 9 weeks (II) and four 13 weeks (III) p.i. The sera from infected mice reacted with the 6HDNO antigen (Fig. 2a1), with different intensity. A strong reaction was observed with sera 1, 2, 3, 4, 5, and 8. None of the control sera reacted with the 6HDNO antigen (Fig. 2a2). Additional controls were performed with human αFp-Ab serum (Fig. 2a2, M7), with rabbit anti-6HDNO serum (Fig. 2a2, a-6H) and with rabbit anti-SaO serum (Fig. 2a2, a-SaO). Immunization of rabbits with 6HDNO or SaO induces αFp-Ab which cross-react with these antigens [4]. The sera from CVB3-infected mice also reacted with the SaO antigen, the strongest reaction giving sera 1, 2, 3, 4, 8 and 9 (Fig. 2b1). Thus, five of the sera from CVB3-infected mice reacted strongly with both flavoproteins. Figure 2b2 shows the results with two control mice sera out of six, which were all negative, the reaction of SaO antigen with human αFp-Ab serum, the reaction with rabbit anti-6HDNO serum, which cross-reacts on Western blots with SaO because of its αFp-Ab, and the reaction of rabbit anti-SaO serum with SaO. None of the sera from CVB3-infected mice and none of the control sera reacted with the FAD-less 6HDNO.Cys mutant protein (Fig. 2c1,2). The 6HDNO.Cys protein however, was recognized as expected by the rabbit 6HDNO antiserum (Fig. 2c2, a-6H). These results indicate that the sera from CVB3-infected mice contain antibodies which, similar to the human anti-M7 sera and the rabbit sera induced with 6HDNO or SaO, were directed against flavoproteins with covalently attached FAD.
Fig. 2.
Sera of coxsackievirus B3 (CVB3)-infected mice contain antibodies directed against flavoproteins with covalently bound FAD. Western blots with 6-hydroxy-d-nicotine oxidase (6HDNO), sarcosine oxidase (SaO) and the 6HDNO His-71 to Cys mutant protein (6HDNO-Ag, SaO-Ag and 6HDNO.C-Ag, respectively) were prepared as outlined in Materials and Methods. Sera were collected from 10 CVB3-infected mice 3 weeks (I, mice 1, 2), 9 weeks (II, mice 3, 4, 5, 6) and 13 weeks (III, mice 7, 8, 9, 10) p.i. and used to decorate the Western blots in a1, b1 and c. (a2) Western blots with 6HDNO as antigen decorated with sera of non-infected mice (C1, C2, C3) as negative controls, and with human anti-flavoprotein antibody (αFp-Ab) serum (M7), rabbit anti-6HDNO serum (a-6H), rabbit anti-sarcosine oxidase serum (a-SaO), respectively, as positive controls. (b1) As in (a1), but with SaO as antigen. (b2) Two negative controls with sera of non-infected animals are shown (C1, C2), and the positive controls as in (a2). (c1) As in (a1, b1) but with the 6HDNO cysteine mutant devoid of FAD as antigen. (c2) C1 and C2 represent Western blots with mutant 6HDNO.Cys as antigen and decorated with sera from non-infected mice, and a-6H shows a positive control with 6HDNO.Cys as antigen and decorated with rabbit anti-6HDNO serum. (d) Western blots with 6HDNO as antigen, decorated with the sera from CVB3-infected mice following preincubation of the sera with 10 μm FAD.
Neutralization of αFp-Ab in sera of CVB3-infected mice by incubation with FAD
Incubation of the sera of CVB3-infected mice with FAD resulted in an inhibition of the immunoreaction with 6HDNO (Fig. 2d, sera 1, 2, 5 and 8). In these sera, the bound FAD must represent a main epitope recognized by the αFp-Ab. The αFp-Ab in the other mice sera may bind to the flavin only in the context of a larger conformational epitope of the flavoproteins [6].
Isotypes and persistence of αFp-Ab in sera of CVB3-infected mice
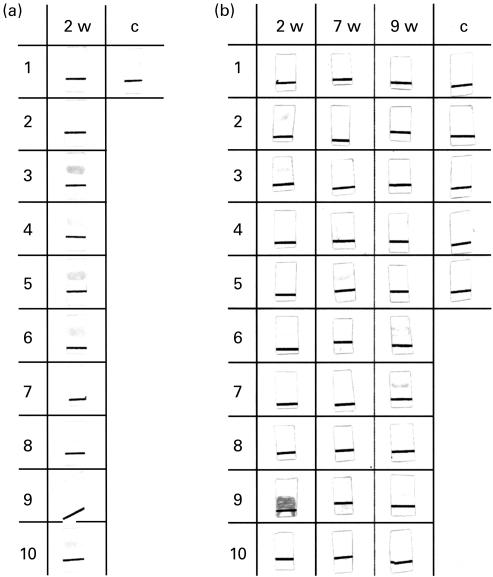
An additional lot of five sera of control mice and 30 sera of CVB3-infected mice was tested, 10 sera each, at 2, 7 and 9 weeks p.i. for the presence of αFp-Ab on Western blots with 6HDNO as antigen (Fig. 3). Six of the mice sera collected 2 weeks p.i. contained α6HDNO-Ab of the IgM isotype (Fig. 3a, nos 3, 4, 5, 6, 9, 10) and four contained α6HDNO-Ab of the IgG isotype (Fig. 3b, 2 weeks, nos 2, 3, 6 and 9). Sera 3, 6 and 9 contained both antibody isotypes. α6HDNO-Ab persisted in sera of some CVB3-infected mice 7 weeks p.i. (Fig. 3b, no. 5) and 9 weeks p.i. (Fig. 3b, nos 6, 7, 9). At this time point the α6HDNO-Ab were all of the IgG isotype. None of the control sera from uninfected mice showed α6HDNO-Ab of the IgM or IgG isotypes (Fig. 3a, c and b, c).
Fig. 3.
Antibody idiotypes of anti-flavoprotein antibody (αFp-Ab) in the sera of coxsackievirus B3 (CVB3)-infected mice. Thirty mice were infected with CVB3. Sera of 10 mice each were collected 2, 7 and 9 weeks p.i. and tested on Western blots with 6-hydroxy-d-nicotine oxidase (6HDNO) as flavoprotein antigen with goat anti-mouse IgM-specific and goat anti-mouse IgG-specific second antibody. Sera of mice 2 weeks p.i. showed α6HDNO-Ab of the IgM isotype (a, 3, 4, 5, 6, 9, 10) and the IgG isotype (b, 2, 3, 6, 9). The sera of mice 7 weeks (b, 5) and 9 weeks p.i. (b, 6, 7, 9) were of the IgG isotype only. The black line on the Western blots served as orientation.
Sera from CBV3-infected mice contain antibodies directed against mitochondrial flavoproteins
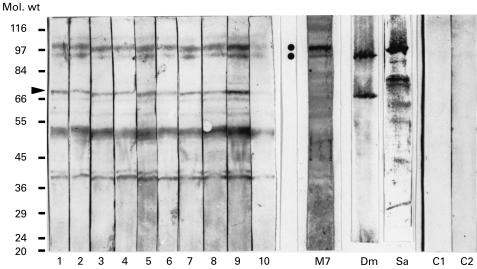
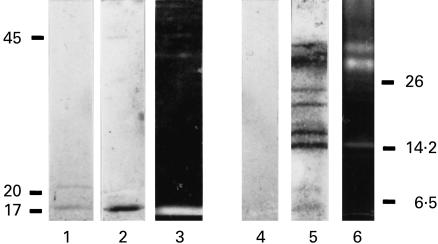
Western blots of rat liver mitochondrial proteins were tested with the sera from CBV3-infected mice (Fig. 4). Two reacting proteins were in the molecular weight range of 90 kD to 100 kD and corresponded to two proteins which reacted with human αFp-Ab (Fig. 4, M7). They were previously identified as dimethylglycine dehydrogenase and sarcosine dehydrogenase [4] and reacted with the respective specific antisera (Fig. 4, Sa and Dm). A third mitochondrial protein recognized by the mice sera (Fig. 4, arrowhead) could represent the flavoprotein subunit of the succinate dehydrogenase complex, a protein of 68 kD. This protein of the inner mitochondrial membrane may be released into the soluble protein fraction during the preparation of mitochondrial matrix. The nature of the additional reacting protein antigens is unknown. However, it is known that anti-mitochondrial autoantibodies (AMA) against various mitochondrial proteins are found in myocarditis and IDCM [13,14], and can be induced in mice by CVB3 infection [15,16].
Fig. 4.
Sera of coxsackievirus B3 (CVB3)-infected mice react with mitochondrial antigens. The lanes of a Western blot of soluble rat mitochondrial proteins were cut into strips and the individual strips were decorated with sera of CVB3-infected mice (1–10), with human anti-flavoprotein antibody (αFp-Ab) serum (M7), with rabbit anti-dimethylglycine dehydrogenase serum (Dm), with rabbit anti-sarcosine dehydrogenase serum (Sa), and with sera of non-infected mice (C1, C2). The two dots indicate the position on the Western blot of the flavoenzymes sarcosine dehydrogenase and dimethylglycine dehydrogenase, respectively. Arrowhead indicates the position of the assumed flavoprotein subunit of succinate dehydrogenase. Molecular weight markers are indicated in kD.
Sera of CVB3-infected mice immunoreact with the endoprotease Lys-C-generated FAD peptide but not with tryptic FAD peptides of 6HDNO
αFp-Ab isolated from human sera recognized as epitope the FAD bound to His-71 and a stretch of amino acids on both sites of the histidyl-FAD [6]. Trypsin digestion, which cuts three amino acid residues in front and 17 residues behind the histidyl-FAD, resulted in FAD peptides which were no longer recognized by the αFp-Ab. When the serum of a CVB3-infected mouse was tested with the tryptic and endoprotease Lys-C digest of 6HDNO the mouse serum recognized the endoprotease Lys-C-generated peptide, but not the tryptic digest of 6HDNO (Fig. 5). This finding agrees with results obtained with αFp-Ab from human serum [6].
Fig. 5.
Sera of coxsackievirus B3 (CVB3)-infected mice recognize the endoprotease Lys-C generated 6-hydroxy-d-nicotine oxidase (6HDNO) FAD peptide but not the tryptic FAD peptides. 6HDNO was digested with endoprotease Lys-C (lanes 1 and 3) or trypsin (lanes 2 and 4), the peptides were separated by SDS–PAGE on a 12% or a 17·5% polyacrylamide gel, respectively, and blotted to nitrocellulose. The Western blots were decorated with the serum of the CVB3-infected mouse no. 8 (lanes 1 and 4) or with rabbit anti-6HDNO serum as control (lanes 2 and 5). Lanes 3 and 6 show the UV light fluorescence in 10% acidic acid of small molecular weight endoprotease Lys-C and tryptic peptides. The mouse serum reacted only with the endoprotease Lys-C-generated FAD peptide (lane 1) but not with the trypsin-generated FAD peptides (lane 4). The sizes in kD of molecular weight markers are indicated on the left and right sides of the panel.
DISCUSSION
Coxsackie B viruses, enteroviruses of the Picornavirus family, are the most common cause of myocarditis [17], which may develop to DCM [18]. The occurrence of serum autoantibodies to various myocardial antigens in patients with myocarditis and IDCM has been reported [13,14,19–21]. Recently a new type of anti-mitochondrial autoantibody (anti-M7) was described in 25–30% of sera from patients with myocarditis and IDCM [1,2]. An analysis of the antigenic specificity of these sera revealed the presence of antibodies directed against mitochondrial and bacterial flavoenzymes with covalently bound flavin cofactor [4,6]. If the occurrence of these αFp-AB in the sera of patients were the result of a viral infection of the myocardium, one may predict that antibodies with similar specificity may be found in the mouse model of CVB3-induced myocarditis.
The results presented in this study support this prediction. Several of the mouse sera showed on Western blots a strong reaction with bacterial flavoenzymes with covalently bound FAD as antigen. The sera reacted with mitochondrial antigens, two of which (dimethylglycine dehydrogenase and sarcosine dehydrogenase) were identical to the mitochondrial antigens recognized by human αFp-Ab. Thus the antigenic reactivity of the mouse sera resembled that of human anti-M7 sera. The common structural feature of these bacterial and mammalian enzymes is the histidyl(N3)-(8α)FAD linkage [6]. If correlated with the histological findings, which showed lymphocytic infiltration and necrosis of myocytes only at early stages p.i., the number of reacting sera decreased with time following the acute phase of myocarditis (of 12 sera 2 and 3 weeks p.i. eight were α6HDNO-Ab-postive, that is 66·6%, compared with six positive out of 28 sera taken at 7, 9 and 13 weeks p.i., that is 21·4%; similar results were obtained with SaO). Apparently an inflammatory process was required to maintain the production of these antibodies. αFp-Ab at 2 weeks p.i. were of the IgG and IgM isotypes. At later time points p.i. analysed in this work they were all of the IgG isotype.
The antibodies reacting with bacterial flavoenzyme antigens in CVB3-infected mice sera represent αFp-Ab. They did not react with flavin-free 6HDNO and the anti-flavoprotein activity of several sera could be neutralized in vitro by incubation with FAD. However, besides the flavin, additional features of the FAD peptide, such as secondary structural elements, may be important for antibody recognition and binding. Only the endoprotease Lys-C-generated 6HDNO peptide reacted with mouse serum, but not the trypsin-generated FAD peptides. This observation agrees with results obtained with the αFp-Ab of patients [6].
Antibodies to subunits of the mitochondrial respiratory chain complexes NADH dehydrogenase and cytochrom c oxidase [14], the mitochondrial α-ketoglutarate dehydrogenase complex [14] and the mitochondrial adenosine carrier [13], which are found in sera of patients with myocarditis and dilated cardiomyopathy, can be induced in the mouse model by CVB3 infection [15]. The presence of these antibodies may be part of the complex immune reactions associated with the establishment of a dilated cardiomyopathy in genetically predisposed mice and humans [7,8]. It has been demonstrated that antibodies to the adenosine carrier impair the function of cardiomyocytes [16]. Antibodies directed against epitopes of components of the mitochondrial respiratory chain and the citric acid cycle (α-ketoglutarate dehydrogenase) may interfere with the energy generation by this organelle [14]. The same may apply to αFp-Ab reacting with the flavoprotein subunit of succinate dehydrogenase, which represents complex two of the respiratory chain.
Flavoproteins with covalently bound FAD are present in all bacteria. Therefore a latent pool of T cells with affinity to the FAD peptide may be present in humans at any time. Presentation of FAD peptides on MHC II complexes misexpressed on cardiomyocytes following a viral infection, or presentation of FAD peptides by professional antigen-presenting cells following phagocytosis of cardiomyocyte debris, as a result of cellular destruction by the viral infection, may activate these T cells.
In conclusion, we show that in the murine model, infection with CVB3 can lead to the occurrence of αFp-Ab. Whether the presence of αFp-Ab in patients with myocarditis and IDCM results from a viral infection has yet to be proved. Our results however, support this possibility.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to R.B. We wish to thank Dr P. Bergeron (Montpellier, France) for the kind gift of sarcosine dehydrogenase antiserum and Dr J. Ilonen for critically reading the manuscript.
REFERENCES
- 1.Klein R, Maisch B, Kochsiek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1984;58:283–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Berg PA. Anti-mitochondrial antibodies (anti-M7) in heart diseases recognize epitopes on bacterial and mammalian sarcosine dehydrogenase. Clin Exp Immunol. 1990;82:289–93. doi: 10.1111/j.1365-2249.1990.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch-Machin MA, Farnsworth L, Ackrell AC, Cochran B, Jackson S, Bindoff LA, Diamond AG, Turnbull DM. The sequence of the flavoprotein subunit of bovine heart succinate dehydrogenase. J Biol Chem. 1992;267:11553–8. [PubMed] [Google Scholar]
- 4.Otto A, Stähle I, Klein R, Berg PA, Pankuweit S, Brandsch R. Antimitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol. 1998;111:541–7. doi: 10.1046/j.1365-2249.1998.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker K, Brandsch R. Flavoproteins with a covalent histidyl (N3)-8α-riboflavin linkage. Biofactors. 1991;3:69–89. [PubMed] [Google Scholar]
- 6.Stähle I, Brizio C, Barile M, Brandsch R. Anti-mitochondrial flavoprotein autoantibodies of patients with myocarditis and dilated cardiomyopathy (anti-M7): interaction with flavin-carrying proteins, effect of vitamin B2 and epitope mapping. Clin Exp Immunol. 1999;115:404–8. doi: 10.1046/j.1365-2249.1999.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumori A. Molecular and immune mechanisms in the pathogenesis of cardiomyopathy. Jpn Circ J. 1997;61:275–91. doi: 10.1253/jcj.61.275. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Martino T, Opavski MA, Penninger J. Viral myocarditis: balance between viral infection and immune response. Can J Cardiol. 1996;12:935–43. [PubMed] [Google Scholar]
- 9.Hober D, Andreoletti L, Shen L, Copin MC, Desmidt A, Wattre P. Coxsackievirus B3-induced chronic myocarditis in mouse: use of whole blood culture to study the activation of TNFα-producing cells. Microbiol Immunol. 1966;40:837–45. doi: 10.1111/j.1348-0421.1996.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. pp. 92–135. [Google Scholar]
- 11.Otto A, Stoltz M, Sailer H-P, Brandsch R. Biogenesis of the covalently flavinylated mitochondrial enzyme dimethylglycine dehydrogenase. J Biol Chem. 1996;271:9823–9. doi: 10.1074/jbc.271.16.9823. [DOI] [PubMed] [Google Scholar]
- 12.Berthold H, Scanarini M, Abney CC, Frorath B, Northemann W. Purification of recombinant antigenic epitopes of human 68-kDa (U1) ribonucleoprotein antigen using the expression system pH6EX3 followed by metal chelating affinity chromatography. Prot Exp Purif. 1992;3:50–56. doi: 10.1016/1046-5928(92)90055-2. [DOI] [PubMed] [Google Scholar]
- 13.Schulze K, Becker BF, Schultheiss HP. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ Res. 1989;64:179–92. doi: 10.1161/01.res.64.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Pohlner K, Portig I, Pankuweit S, Lottspeich F, Maisch B. Identification of mitochondrial antigens recognized by antibodies in sera of patients with idiopathic dilated cardiomyopathy by two-dimensional gel electrophoresis and protein sequencing. Am J Cardiol. 1997;80:1040–5. doi: 10.1016/s0002-9149(97)00600-0. [DOI] [PubMed] [Google Scholar]
- 15.Neumann D, Rose NR, Ansari AA, Herskowitz A. Induction of multiple heart autoantibodies in mice with Coxsackievirus B3 and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–50. [PubMed] [Google Scholar]
- 16.Schulze K, Witzenbichler B, Christmann C, Schultheiss HP. Disturbance of myocardial energy metabolism in experimental myocarditis by antibodies against the adenine nucleotide translocator. Cardiovasc Res. 1999;44:91–100. doi: 10.1016/s0008-6363(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff JF. Viral myocarditis. Am J Pathol. 1980;101:427–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhard JR, Perry CM, Harkins S, Lane T, Mena I, Asensio VC, Cambell IL, Whitton JL. Coxsackievirus B3-induced myocarditis. Am J Pathol. 1998;153:417–28. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson CM, O'donoghue HL, Reed WD. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992;75:513–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against β-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Wolff PG, Kühl U, Schultheiss HP. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J. 1989;117:1303–9. doi: 10.1016/0002-8703(89)90410-9. [DOI] [PubMed] [Google Scholar]