Abstract
Dendritic cells (DC) can modulate the nature of immune responses in a stimulatory or tolerogenic fashion. Great attention has been given to the induction of immunity to tumour and infection. In this study, bone marrow-derived DC from healthy Lewis rats were pulsed in vitro with encephalitogenic myelin basic protein peptide 68–86 (MBP 68–86), and injected subcutaneously (1 × 106/rat) into normal Lewis rats. Upon observation of the rats pretreated in this way for 4 weeks, when no clinical signs of EAE occurred, these rats were immunized with MBP 68–86 and Freund's complete adjuvant. The pretreated rats failed to develop clinical EAE. This tolerance was associated with augmented proliferative responses, interferon-gamma secretion, inducible nitric oxide synthase (iNOS) expression and NO production. The frequency of apoptotic cells was increased in the rats receiving MBP 68–86-pulsed DC compared with unpulsed control DC. Few infiltrating inflammatory cells were observed in spinal cord sections from rats that had received MBP 68–86-pulsed DC. The data are compatible with the interpretation that MBP 68–86-pulsed DC induce tolerance to EAE possibly through up-regulation of iNOS expression and NO production, which mediate cell apoptosis, thereby reducing infiltration of inflammatory cells within the central nervous system.
Keywords: dendritic cells, tolerance, experimental allergic encephalomyelitis, nitric oxide, apoptosis
INTRODUCTION
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) of the immune system and are critically involved in the initiation of primary immune responses, graft rejection and autoimmune diseases [1]. They can modulate the nature of immune responses in stimulatory or tolerogenic fashion [2]. DC pulsed in vitro with tumour antigens have been successfully applied to induce specific cytotoxic T lymphocyte (CTL) responses to tumours [3,4], thereby leading to the rapid development of new therapeutic strategies against tumours. Most importantly, tumour peptide-pulsed DC can be used to elicit antigen-specific protective anti-tumour immunity in mice [5], and tumour antigen-pulsed autologous DC can elicit a clinically significant response in patients with B cell lymphoma [6]. These findings have important implications for the use of DC in immunotherapy of cancer.
Autoimmune diseases are initiated and maintained by sensitized and activated autoreactive T and B cells, which directly or indirectly destroy target cells harbouring corresponding tissue-specific antigens. EAE is the result of an autoimmune process mediated by autoreactive T cells with specificity for myelin antigens. Immunostimulatory functions of DC can be assumed to induce or worsen autoimmune processes, representing a failure to develop and maintain tolerance to self antigens. Whilst extensive studies have increased our understanding of the critical role of DC in the stimulation of primary immune responses, evidence has also accumulated that the activity of DC is not restricted to this niche of the immune system [7,8]. DC not only activate lymphocytes, but they also tolerize T cells to antigens, thereby minimizing autoimmune reactions [9]. In mice, in vivo treatment with Flt3L (a growth factor that expands DC in vivo) enhanced the induction of oral tolerance [10]. DC precultured with IL-10 induced a state of alloantigen-specific anergy in CD4+ T cells [11] and in CD8+ T cells by converting DC into tolerogenic APC [12]. Transfer of pancreas lymph node DC modulated autoimmunity and limited diabetes expression by the induction of regulatory cells in the non-obese diabetic (NOD) mouse [13]. Thus, the concept of ‘tolerogenic’ DC reflects an additional property of these important APC, which might be a useful tool in the therapy of patients with autoimmune diseases
In the present study, we successfully induced tolerance to actively induced EAE in Lewis rats by bone marrow (BM)-derived DC that had been pulsed in vitro with the encephalitogenic myelin basic protein peptide 68–86 (MBP 68–86).
MATERIALS AND METHODS
Animals and reagents
Male Lewis rats, weighing 150–180 g, were obtained from Zentralinstitut fur Versuchstierzucht (Hannover, Germany). Guinea pig MBP 68–86 (YGSLPQKSQRSQDENPV) was produced in an automatic Tecan-Syro Synthesizer (Multisytech, Bochum, Germany). Monoclonal anti-rat CD4 (W3/25) and anti-rat MHC class II (OX-6) antibodies were purified from culture supernatants of hybridomas [14]. Monoclonal anti-rat ED1, CD3, CD11c and Mac-1 antibodies were obtained from SeroTec (Oxford, UK). Monoclonal anti-rat B7-1 (CD80), B7-2 (CD86) and OX-42 antibodies were from PharMingen (San Diego, CA). Monoclonal anti-rat interferon-gamma (IFN-γ) (DB-1) and polyclonal anti-rat IFN-γ antibodies were from Innogenetics (Ghent, Belgium). Polyclonal anti-rat inducible NO synthase (iNOS) antibody was purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA).
Generation of DC from rat bone marrow
Bone marrow cells were flushed from femurs and tibias, depleted of erythrocytes with osmotic lysis, and suspended in serum-free Dulbecco's modification of Eagle's medium (Gibco, Paisley, UK) supplemented with 1% minimal essential medium (MEM) amino acids (Gibco), 2 mm glutamine (Flow Labs, Irvine, UK), 50 U/ml penicillin and 50 μg/ml streptomycin (Gibco) and 10 mm HEPES (Sigma, St Louis, MO). The cells (5 × 106/ml) were seeded in six-well plates (Costar, Fernwald, Germany). After 2 h, non-adherent cells were gently removed by swirling the plates and aspirating the medium, and plates were washed five times with serum-free medium. New medium containing 10% fetal calf serum (FCS; Gibco), supplemented with 10 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D, Wiesbaden, Germany) and 10 ng/ml of IL-4 (kindly provided by Dr P. van der Meide, Central Animal Laboratory, University of Utrecht, Utrecht, The Netherlands), was added to the wells, and exchanged for fresh medium after 3–4 days. After 7 days of culture, adherent DC were obtained and used in the present study. Adherent DC populations showed a restricted cell surface phenotype of DC derived from rat bone marrow, including high levels of CD11c (69%), MHC class II (85%), B7-1 (81%) and B7-2 (79%). CD3+ cells amounted to only 2·5%. We chose two MoAbs (clones OX-42 and Mac-1), which are suitable for the differentiation of DC and macrophages. Macrophage markers like OX-42 and Mac-1 were relatively low (OX42 13%, and Mac-1 16%) in enriched DC fractions.
MBP 68–86-pulsed DC in vitro
Collected DC (1 × 106/ml) were pulsed with 50 μg/0·5 ml volume of MBP 68–86 for 4 h at 37°C. Control unpulsed DC (1 × 106/ml) were set up in the absence of MBP 68–86. After pulsing, MBP 68–86-pulsed and unpulsed DC were washed three times with serum-free medium, and antigen presentation was detected. Briefly, DC were co-cultured with naive T cells (ratio 1:100) in the presence of MBP 68–86 (10 μg/ml). After 60 h of incubation at 37°C with 5% CO2 and humidified atmosphere, the cells were labelled for an additional 12 h with 10-μl aliquots containing 1 μCi of 3H-methylthymidine (specific activity 42 Ci/mmol; Amersham, Aylesbury, UK). Cells were harvested onto glassfibre filters and thymidine incorporation was measured. Cultures were run in triplicate and the results were expressed as ct/min. Ct/min of MBP 68–86-pulsed DC should be more than two-fold higher than ct/min of unpulsed DC. DC (1 × 106/ml) were injected subcutaneously into healthy Lewis rats in a total volume of 1 ml at four sites along the back. Control rats were injected subcutaneously with unpulsed DC (1 × 106 cells/ml) or PBS (1 ml).
Induction of EAE
Four weeks after s.c. injection of DC or PBS, rats were immunized in both hind footpads with 200 μl of inoculum containing 25 μg of MBP 68–86, 2 mg Mycobacterium tuberculosis (strain H37RA; Difco, Detroit, MI), 100 μl saline and 100 μl Freund's incomplete adjuvant (FIA; Difco). Clinical EAE scores were graded according to the following criteria: 0, asymptomatic; 1, loss of distal half of tail tonicity; 2, loss of entire tail tonicity; 3, hindlimb paresis; 4, hindlimb paralysis; 5, tetraplegia. Clinical observations were made in a blinded fashion by at least two investigators. All immunized animals injected with PBS (PBS-injected control EAE) developed clinical signs of EAE with onset at around day 10–12 post-immunization (p.i.). Clinical improvement occurred gradually and all PBS-injected control EAE rats had recovered on day 26 p.i.
Detection of IL-12 and IL-10 mRNA by in situ hybridization
Expression of IL-12 and IL-10 mRNA by DC was performed as described [15] and modified for mononuclear cells [16]. Aliquots containing 1 × 105 DC were dried onto restricted areas of glass microscope slides (ProbeOn slides; Fisher Scientific, Pittsburgh, PA). The cells were dried at 55°C for about 10 min and stored with silica in sealed boxes at −70°C until in situ hybridization (ISH). For each cytokine, a mixture of four different oligonucleotide probes was employed in order to increase the sensitivity of the method. The oligonucleotide sequences were obtained from GenBank and probes were designed using MacVector software. Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled with deoxyadenosine-5′-α-(thio)-triphosphate (S35) (Dupont Scandinavia, Stockholm, Sweden) terminated with deoxynucleotidyl transferase (Amersham). Cells were hybridized for 18 h at 42°C with 106 ct/min of labelled probe per 100 μl of hybridization mixture. After emulsion autoradiography, development and fixation, the coded slides were examined by dark field microscopy at ×20 magnification for positive cells containing >15 grains per cell in a star-like distribution. The intracellular distribution of the grains was always checked by light microscopy at ×20 magnification. Control probes were used in parallel with cytokine probes on the cells from each specimen. There were no difficulties in differentiating between cytokine mRNA-positive and -negative cells. All data were obtained in double-blind fashion.
Preparation of mononuclear cell suspensions
Rats were killed on day 14 p.i. Suspensions of mononuclear cells (MNC) from draining lymph nodes and spleen were prepared by grinding through a wire mesh. Erythrocytes were osmotically lysed. MNC were suspended in medium containing 10% FCS. The cells were washed three times and then diluted to a cell concentration of 2 × 106/ml. In some experiments, MNC were isolated from peripheral blood by density gradient centrifugation on Lymphoprep (Nyegaard, Oslo, Norway).
Proliferation assays
A radiometric proliferation assay based on 3H-thymidine incorporation was used. Briefly, 200-μl aliquots of MNC (2 × 106/ml) suspensions were applied into 96-well round-bottomed microtitre plates (Nunc, Copenhagen, Denmark) in the presence or absence of MBP 68–86 (10 μg/ml). After 60 h of incubation at 37°C with 5% CO2 and humidified atmosphere, the cells were labelled for an additional 12 h with 10-μl aliquots containing 1 μCi of 3H-methylthymidine. Cells were harvested onto glassfibre filters and thymidine incorporation was measured. Cultures were run in triplicate and the results were expressed as ct/min.
Assay of IFN-γ-secreting cells
Enzyme-linked immunospot (ELISPOT) assays were used for detection of IFN-γ secretion at the single-cell level. Nitrocellulose-bottomed microtitre plates (Millititre-HAM plates; Millipore, Bedford, MA) were coated with 100-μl aliquots of mouse anti-rat IFN-γ MoAb at 15 μg/ml. MNC (4 × 105 cells/200 μl) were added to individual wells in the absence or presence of MBP 68–86 (10 μg/ml). After 48 h of culture and washing, spots corresponding to IFN-γ-secreting cells were visualized by polyclonal rabbit anti-rat IFN-γ (1:400), biotinylated anti-rabbit IgG (1:500; Dakopatts, Copenhagen, Denmark) and ABC (1:200; Vector, Burlingame, CA). The red-brown immunospots, each representing an individual cell that had secreted IFN-γ, were counted in a dissection microscope.
Assay of nitrite
Because secreted NO quickly reacts with oxygen-yielding nitrite, the level of nitrite as a reflection of NO production in culture supernatants was measured using modified Griess reagent (Sigma). MNC (4 × 105/200 μl) were cultured in 96-well round-bottomed microtitre plates (Nunc) in the absence or presence of MBP 68–86 (10 μg/ml). After 48 h, 100 μl of supernatants from cultured cells were mixed with equal volume of Griess reagent. After a 10-min reaction at room temperature, absorbance at 540 nm was measured using an automated plate reader. Nitrite concentration was determined by comparison with a sodium nitrite standard curve in culture medium.
Assays of apoptotic cells in situ and in vitro
Apoptotic cells in lymph node sections were determined using FITC-conjugated dUTP (TUNEL) kit (Boehringer Mannheim, Mannheim, Germany). Lymph node sections (10 μm) were fixed with 4% paraformaldehyde for 30 min at room temperature, and then permeabilized with 0·1% Triton X-100 in 0·1% sodium citrate for 2 min on ice. TUNEL reaction mixture (50 μl) was added to samples for 60 min at 37°C in a wet dark box. A negative control (without terminal transferase) was included in each experiment set-up. Apoptotic cells among blood MNC were determined using Annexin-V-FLUOS kit (Boehringer Mannheim). MNC (4 × 105) were incubated in the presence of MBP 68–86 (10 μg/ml). After 48 h, cells were washed twice in PBS, and then incubated with Annexin-V-Fluos in a HEPES buffer containing propidium iodide (PI) for 15 min at room temperature. The samples were analysed on a FACScan flow cytometer using Cell Quest software.
Immunohistochemistry
The spinal cords of rats were dissected, frozen in liquid nitrogen and stored at −70°C for immunohistochemistry. Cryostat tissues were cut at 8 μm and fixed in acetone for 10 min. Endogenous peroxidase activity was inactivated with 0·3% H2O2 for 20 min. Non-specific binding sites were further blocked with 1% blocking reagent (Boehringer Mannheim). The sections were incubated with anti-rat CD4 (1:100) and anti-rat ED-1 (1:100) overnight at 4°C. Reactivity was detected using the ABC (Vector) and DAB (Vector) reaction systems. The specificity of the immunoreaction was tested by incubating sections without the primary antibodies or with non-specific rabbit IgG. For each animal, three spinal cord sections from different standardized parts of the cord were examined in a blinded fashion.
Statistical analysis
Data were expressed as means ±s.e.m. Statistical analysis was performed using the two-tailed t-test. P <0·05 was used to indicate a statistically significant difference.
RESULTS
Adherent DC expressed higher levels of IL-10 and lower levels of IL-12 mRNA
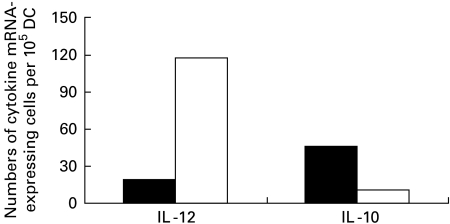
Profiles of cytokines of adherent and non-adherent DC were compared using in situ hybridization. Figure 1 shows that adherent DC used in the study exhibited higher levels of IL-10 mRNA expression and lower levels of IL-12 mRNA expression. In contrast, non-adherent DC had lower expression of IL-10 mRNA and higher expression of IL-12 mRNA. The results demonstrate that adherent DC could represent tolerogenic DC.
Fig. 1.
Expression of IL-10 and IL-12 mRNA by adherent (▪) and non-adherent dendritic cells (DC) (□) by in situ hybridization.
MBP 68–86-pulsed DC did not induce clinical EAE in susceptible Lewis rats, but caused tolerance to EAE
EAE in rodents is a T cell-mediated organ-specific autoimmune disease. Antigen-pulsed DC may therefore play an important role in eliciting primary immune responses of T cells in vivo. It is however, unclear whether DC prepared from BM of healthy rats, upon being pulsed in vitro with autoantigen, can elicit clinical EAE in susceptible Lewis rat. Here we observed, in four independent experiments, that rats injected subcutaneously with MBP 68–86-pulsed DC (1 × 106 DC/rat) failed to develop clinical EAE during an observation period of 4 weeks prior to immunization to induce EAE (Table 1 and Fig. 2a). There were also no differences in body weight changes between rats pretreated with MBP 68–86-pulsed DC compared with rats pretreated with unpulsed DC, or with PBS only, during the 4 weeks of observation prior to immunization (data not shown).
Table 1.
MBP 68–86-pulsed dendritic cells (DC) induce clinical tolerance to EAE in Lewis rats
| Experiments | Incidence of disease | Clinical score* | Body weight (g)* |
|---|---|---|---|
| Exp. 1 | |||
| DC-tolerized EAE | 0/2 | 0 | 196 |
| Control EAE | 2/2 | 3·75 | 150 |
| Unpulsed DC-treated EAE | 2/2 | 3·5 | 182 |
| Exp. 2 | |||
| DC-tolerized EAE | 0/2 | 0 | 195 |
| Control EAE | 2/2 | 4 | 164 |
| Unpulsed DC-treated EAE | 2/2 | 4 | ND |
| Exp. 3 | |||
| DC-tolerized EAE | 0/2 | 0 | 194 |
| Control EAE | 2/2 | 4 | 148 |
| Unpulsed DC-treated EAE | 2/2 | 3 | ND |
| Exp. 4 | |||
| DC-tolerized EAE | 0/2 | 0 | ND |
| Control EAE | 2/2 | 3·25 | ND |
| Total | |||
| DC-tolerized EAE | 0/8 (0%) | 0 | 191 ± s.d. 4·3** |
| Control EAE | 8/8 (100%) | 3·75 ± s.d. 0·35 | 154 ± s.d. 11 |
| Unpulsed DC-treated EAE | 6/6 (100%) | 3·50 ± s.d. 0·29 | ND |
These data were obtained on day 14 p.i.
P < 0·001 (control EAE versus DC-tolerized EAE).
ND, Not done.
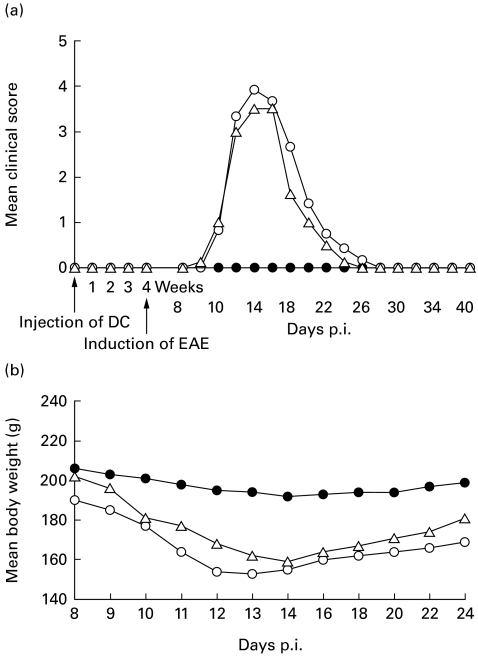
Fig. 2.
Lewis rats are protected from EAE by subcutaneous (s.c.) injection of myelin basic protein (MBP) 68–86-pulsed dendritic cells (DC) prepared from bone marrow of normal Lewis rats. Normal rats were injected subcutaneously with MBP 68–86-pulsed DC (•; 1 × 106 DC/rat) (DC-tolerized EAE), or unpulsed DC (Δ; 1 × 106/rat) (DC-control EAE), or with PBS (○; PBS-control EAE). Considering that DC may induce immunity, the observation period for clinical signs of EAE was performed over 4 weeks, before the three groups of rats were immunized with MBP 68–86 and Freund's complete adjuvant. (a) Mean clinical signs of EAE, and (b) mean body weight changes. These data are compiled from four independent experiments.
Since DC may not only activate lymphocytes, but also tolerize T cells to antigens, we examined the capability of MBP 68–86-pulsed DC to mediate tolerance to EAE. None of eight rats (0%) injected subcutaneously with MBP 68–86-pulsed DC (1 × 106 DC/rat) (DC-tolerized EAE) and 4 weeks later immunized with MBP 68–86 and Freund's complete adjuvant (FCA) developed clinical EAE during the subsequent 40 days of follow up (Fig. 2a). Eight of eight rats (100%) injected subcutaneously with PBS (PBS-control EAE) showed typical clinical signs of EAE (Table 1 and Fig. 2a). The body weight of the control EAE rats went down dramatically as the clinical signs became severe (Fig. 2b). In contrast, the DC-tolerized rats showed only slight body weight loss (Fig. 2b). None of eight rats (100%) injected subcutaneously with unpulsed DC (1 × 106; DC-control EAE) developed tolerance to EAE, but exhibited similar clinical severity of EAE and body weight loss compared with PBS-control EAE rats (Fig. 2a).
MBP 68–86-pulsed DC promoted spontaneous and specific antigen-induced proliferation
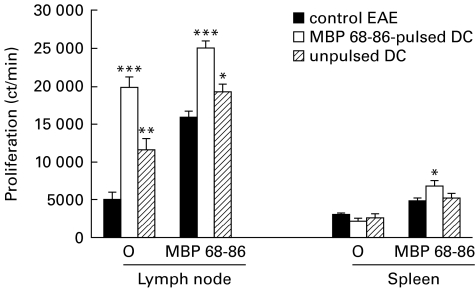
It was reported that antigen-pulsed DC can stimulate primary T cell proliferation in vitro [17] and spleen cell proliferation in vivo [18]. We therefore determined whether MBP 68–86-pulsed DC influenced proliferative response in DC-tolerized EAE rats. Spontaneous proliferation from lymph node and spleen as well as MBP 68–86-induced proliferation from spleen was higher in DC-tolerized EAE rats compared with PBS-control and DC-control EAE rats (Fig. 3). We also measured expression of MHC class II, B7-1 and B7-2 on MNC from blood, spleen and lymph node in DC-tolerized EAE rats and control EAE rats by flow cytometry. No differences were observed among three groups of EAE rats (data not shown).
Fig. 3.
Spontaneous and myelin basic protein (MBP) 68–86-induced proliferation of lymph node and spleen mononuclear cells (MNC) from dendritic cell (DC)-tolerized, DC-control and PBS-control EAE rats on day 14 p.i. (cf. legend to Fig. 1). Results are expressed as mean ±s.e.m. of four rats from two independent experiments (*P < 0·05; **P < 0·01; ***P < 0·001).
Both MBP 68–86-pulsed and unpulsed DC induced IFN-γ secretion
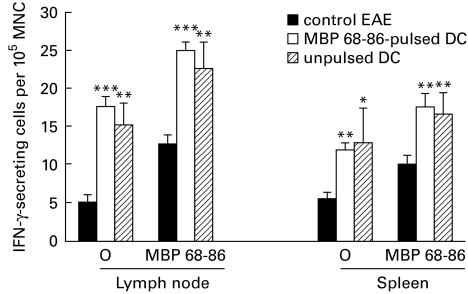
It was reported that IFN-γ was up-regulated in antigen-stimulated splenocytes, after DC were pulsed in vitro with ovalbumin and then transferred intravenously into rats [18]. Figure 4 shows that levels of both spontaneously and MBP 68–86-induced IFN-γ-secreting MNC from lymph node (P < 0·001) and spleen (P < 0·01) were in fact higher in both DC-tolerized and DC-control EAE rats compared with PBS-control EAE rats.
Fig. 4.
Spontaneously and myelin basic protein (MBP) 68–86-induced levels of IFN-γ-secreting cells among lymph node and spleen mononuclear cells (MNC) from dendritic cell (DC)-tolerized, DC-control and PBS-control EAE rats on day 14 p.i. (cf. legend to Fig. 1). Results are expressed as mean ±s.e.m. of four rats from two independent experiments (*P < 0·05; **P < 0·01; ***P < 0·001).
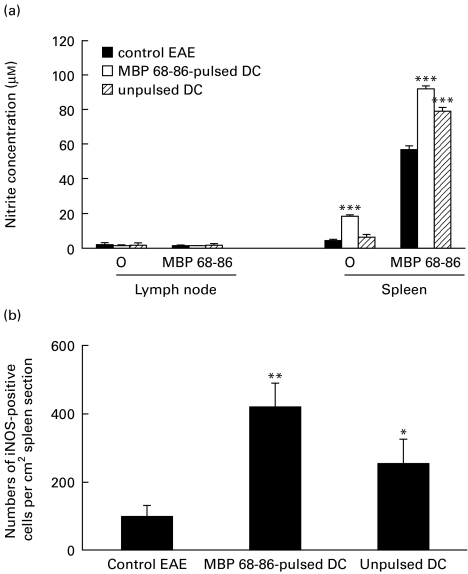
MBP 68–86-pulsed DC increased iNOS expression and NO production in splenocytes
IFN-γ can induce iNOS expression and NO production [19]. To test whether MBP 68–86-pulsed DC up-regulate iNOS expression and NO production, we determined nitrite concentration, an end product of NO. Nitrite concentrations in supernatants of both spontaneously and MBP 68–86-induced splenocytes from rats injected subcutaneously with MBP 68–86-pulsed DC were significantly augmented (Fig. 5a). In contrast, MNC from lymph nodes produced only low levels of nitrite both in the absence and presence of MBP 68–86. These results are consistent with our previous findings that MNC from lymph nodes did not produce, or could be induced to produce only low levels of nitrite in the presence of IFN-γ or lipopolysaccharide (LPS) (data not shown). iNOS+ cells were higher in spleen sections from DC-tolerized EAE rats compared with PBS-control and DC-control EAE rats (Fig. 5b). These results clearly demonstrate that MBP 68–86-pulsed DC can promote NO production by triggering iNOS expression.
Fig. 5.
Spontaneous and myelin basic protein (MBP) 68–86-induced nitric oxide (NO) production (a) in supernatants of cultured mononuclear cells (MNC) of lymph node and spleen, and inducible nitric oxide synthase (iNOS) expression (b) in spleen sections from dendritic cell (DC)-tolerized, DC-control and PBS-control EAE rats on day 14 p.i. (cf. legend to Fig. 1). Results are expressed as mean ±s.e.m. of four rats from two independent experiments (*P < 0·05; **P < 0·01; ***P < 0·001).
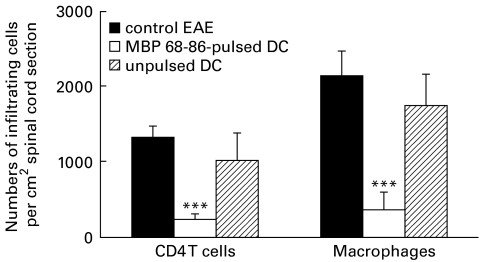
MBP 68–86-pulsed DC inhibited infiltration of CD4+ T cells and macrophages within the central nervous system
Based on the well-established observations that inflammatory cells (CD4+ T cells and macrophages) are accumulated within the central nervous system (CNS) in the Lewis rat EAE model, we examined whether DC-induced tolerance inhibits the infiltration of such inflammatory cells within the CNS. Immunohistochemistry staining of spinal cord sections revealed that the accumulation of CD4+ T cells and macrophages within the CNS was reduced (P < 0·001) in DC-tolerized EAE rats compared with PBS-control and DC-control EAE rats (Fig. 6).
Fig. 6.
Numbers of infiltrating cells in sections of spinal cord from dendritic cell (DC)-tolerized, DC-control and PBS-control EAE rats on day 14 p.i. (cf. legend to Fig. 1). Three different sections of each spinal cord were detected. Results are expressed as mean ±s.e.m. of CD4+ and ED-1+ cells per 1 cm2 tissue area and obtained from four rats in two independent experiments (***P < 0·001).
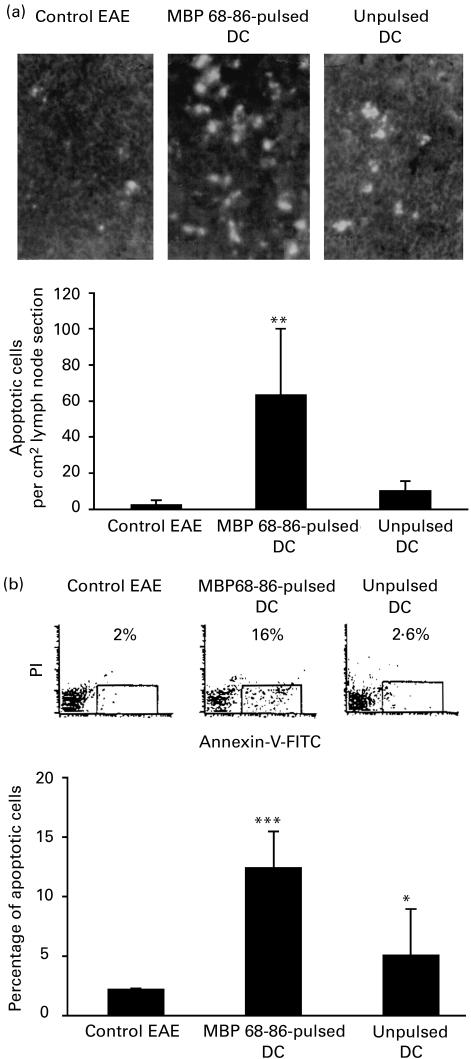
MBP 68–86-pulsed DC increased cell apoptosis
In rats injected subcutaneously with MBP 68–86-pulsed DC, nitrite concentrations in supernatants of cultured spleen MNC were elevated, and infiltration of CD4+ T cells and macrophages in spinal cord sections was reduced. To define factor(s) by which CD4+ T cells and macrophages were inactivated during the course of EAE, cell apoptosis was evaluated, since high levels of NO can induce apoptosis of CD4+ T cells [20]. Numbers of apoptotic cells in lymph node sections (Fig. 7a) and among blood MNC (Fig. 7b) were higher in DC-tolerized EAE rats compared with PBS-control and DC-control EAE rats, indicating that NO-mediated apoptosis may be involved in DC-tolerized EAE rats.
Fig. 7.
Apoptotic cells in lymph node sections on day 14 p.i. and among blood mononuclear cells (MNC) on day 7 p.i. from dendritic cell (DC)-tolerized, DC-control and PBS-control EAE rats (cf. legend to Fig. 1). (a) Numbers of apoptotic cells in lymph node sections are expressed as mean ±s.e.m. from two independent experiments. Photos (upper panels of (a)) represent apoptotic cells stained with TUNEL kit from control EAE, DC-tolerized and DC-control EAE, respectively. (b) Numbers of apoptotic cells among blood MNC are expressed as mean ±s.e.m. of four rats from two independent experiments. The dot plots (upper panels of (b)) show apoptotic cells stained with Annexin-V. (*P < 0·05; **P < 0·01; ***P < 0·001.)
DISCUSSION
A major goal regarding the pathogenesis of autoimmune diseases is to define the triggering events that lead to activation of previously indifferent but potentially autoreactive T cells. DC may break the state of ignorance and trigger the autoimmune response by providing costimulatory signals for naive T cells. The potency of DC in eliciting primary immune responses in vivo has been demonstrated by studies in which immune responses against alloantigens [21] and HY antigens [22] were induced with as few as 104−105 DC. Due to their high immunostimulatory capacity, the use of DC pulsed with tumour peptides or crude tumour protein homogenates has been proposed as an efficient immunotherapeutic means of inducing immune responses against tumours. Although DC initially break the state of ignorance of autoreactive naive T cells [23], it is still unclear whether such a process can result in clinical disease in experimental autoimmune disease models like EAE. Recently, Ludewig et al. [24] reported that adoptive transfer of DC expressing the immunodominant cytotoxic T lymphocyte epitope of the lymphocytic choriomeningitis virus glycoprotein (LCMV-GP) resulted in autoimmune diabetes in RIP-GP mice. Considering the effect of DC in the induction of EAE, we first studied whether MBP 68–86-pulsed DC can induce clinical EAE in susceptible Lewis rats. Neither clinical signs of EAE nor body weight changes were registered during an observation period of 4 weeks upon s.c. injection of DC. The discrepancy between the results of Ludewig et al. [24] and ours may be due to the different animal models employed as well as the different routes of DC administration adopted. We used the EAE model in Lewis rats and injected DC by the s.c. route, while Ludewig et al. [24] studied an autoimmune diabetes model in RIP-GP mice and transferred DC by the venous route. Most importantly, in the present study we collected adherent DC which express high levels of IL-10 mRNA and low levels of IL-12 mRNA compared with non-adherent DC, suggesting that adherent DC may represent tolerogenic DC. Suppression of endogenous IL-10 gene expression in DC enhances antigen presentation for specific Th1 responses [25]. DC pretreated with IL-10 induce tolerance by converting immature DC into tolerogenic DC [11]. Thus, different DC subsets may provide T cells with the different cytokine microenvironment that determines the type of immune response [26].
In the present study, we demonstrate that DC prepared from BM of healthy Lewis rats and pulsed in vitro with the encephalitogenic MBP 68–86 peptide can completely abrogate the development of clinical EAE in all recipient animals when later immunized with MBP 68–86 and FCA. However, DC pulsed in vitro with the encephalitogenic MOG peptide 35–55 or PLP peptide 139–151 did not suppress the development of EAE induced with MBP 68–86. There were also no differences between immune responses of unpulsed DC and MOG peptide 35–55-pulsed DC or PLP peptide 139–151-pulsed DC (data not shown), indicating that DC-mediated tolerance is antigen-specific. Immunohistochemistry staining of spinal cord sections showed only a low degree of CD4+ T cell and macrophage infiltration in the DC-tolerized rats, suggesting that autoantigen-pulsed DC were able effectively to inhibit the autoaggressive inflammatory process in the target organ.
The mechanisms behind DC-mediated tolerance are not completely understood. A regulatory molecule of interest in tolerance induction is NO [27]. NO is produced by macrophages and also by a subpopulation of myeloid DC in response to IFN-γ or endotoxin, or upon interaction with allogeneic T cells [28]. Apoptosis is a major mechanism for elimination of T cells and macrophages within the CNS during inflammation. NO has the potency to induce apoptosis of autoreactive T cells [29], myelin-reactive T cells [30], mouse splenic T cells [31] and mouse thymocytes [32]. Downing et al. [33] proposed a nitrergic mechanism of DC-mediated T cell elimination. Antigenic stimulation of T cells resulted in their production of IFN-γ, and then induced DC to express iNOS and to produce NO. The NO mediated a lethal growth inhibition of co-cultured T cells. Antibodies to IFN-γ and inhibitors of iNOS were both effective at interrupting this cascade [33]. The present study clearly demonstrates that, in DC-tolerized rats, there is augmentation of NO production that may have the potential to eliminate autoreactive T cells and/or macrophages, and to reduce infiltration of inflammatory cells within the CNS through induction of apoptosis. Though the number of apoptotic cells in lymph node sections and among MNC was higher in DC-tolerized EAE rats compared with control DC as well as PBS-injected EAE rats, the number of apoptotic cells was not different in the sections of spinal cord from the three groups of rats, suggesting that the effector site of MBP 68–86-pulsed DC is in the periphery.
A frequently proposed cascade for the development of autoimmune disease involves the induction and expansion of Th1 cells in response to antigens. However, several parts of this concept have been repeatedly challenged. IL-12 protects from Th1-mediated experimental autoimmune uveitis (EAU) [34]. It has been demonstrated that both IFN-γ and NO are two potentially disease-suppressing molecules in EAE [35–37]. IFN-γ suppressed EAE by inducing iNOS and subsequently NO production [38]. IL-12 protects from EAU by curtailing development of uveitogenic effector T cells, involving hyperinduction of IFN-γ and up-regulation of NO production, which at least in part trigger apoptotic deletion of antigen-specific T cells at a critical time point during antigen priming [34]. A positive correlation between recovery from EAE and the levels of NO was observed [20,37]. Interestingly, when recovered Lewis rats are treated with N-methyl-L-arginine acetate (inhibitor of NO production), 100% of animals develop a relapse of EAE [37], suggesting a central role for NO in the immunoregulation of EAE. It has also to be noted that correlation among proliferation, IFN-γ, NO and apoptosis was observed here. There is however, no definite evidence that the three events are connected. In addition, proliferation, IFN-γ and NO production in rats receiving unpulsed DC were also increased compared with PBS-pretreated control EAE. However, the extent of proliferation, IFN-γ and NO production in rats receiving MBP 68–86-pulsed DC was higher than in rats receiving unpulsed DC. The role of NO in immune responses comprises both effector (e.g. tissue destruction or apoptosis of autoreactive T cells) and regulatory (e.g. modulation of cytokine responses) functions, depending on concentration and/or target cells in the local microenvironment.
In contrast to the tolerance that can be induced by oral or nasal administration of soluble antigen, MBP 68–86-pulsed DC induced active tolerance to EAE. Thus, spontaneous as well as specific antigen-induced proliferation of MNC from spleen and lymph node were enhanced in DC-tolerized EAE rats. It has previously been shown that DC pulsed with microbial antigens (e.g. leishmania and histoplasma antigens) are capable of inducing proliferative responses of autologous CD4+ lymphocytes [39]. Influenza virus-infected DC [40] or DC from the NOD mouse [41] also induced T cell proliferation in vivo. Finkelman et al. [2] reported that DC can present antigen in either a stimulatory or tolerogenic manner. After ovalbumin pulsing of DC and adoptive transfer, freshly isolated DC preferentially stimulated Th2-dependent IgG1 responses [18]. Whether the increased DC proliferation observed in the present study results from Th2 cell expansion remains to be determined.
In conclusion, the present study shows that MBP 68–86-pulsed DC do not induce clinical EAE in susceptible Lewis rats, but induce tolerance to EAE. The mechanism of this phenomenon may involve enhanced proliferation and IFN-γ secretion, causing up-regulation of iNOS expression and NO production which result in apoptosis of CD4+ T cells and/or macrophages/DC at a critical time point during immune responses, and reduce infiltration of inflammatory cells within the CNS. The mechanism of DC-induced tolerance is likely to be dependent upon initiation of a complex set of changes to T cell responses. Such information will assist vaccine design and aid in designing regimens aimed at applying immune tolerance as a treatment for human disease.
Acknowledgments
This work has been supported by grants from the Swedish MS Society (NHR), the Swedish Medical Research Council and Karolinska Institute Research Funds.
REFERENCES
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–6. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–14. [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in anti-tumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–10. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 4.Toes RE, van der Voort EI, Schoenberger SP, et al. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160:4449–56. [PubMed] [Google Scholar]
- 5.Zitvogel L, Mayordomo JI, Tjandrawan T, Deleo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependent on T cells, B7 costimulation, and T helper cell-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwisnski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 7.Krasteva M, Kehren J, Horand F, et al. Dual role of dendritic cells in the induction and down-regulation of antigen-specific cutaneous inflammation. J Immunol. 1998;160:1181–90. [PubMed] [Google Scholar]
- 8.Steptoe RJ, Thomson AW. Dendritic cells and tolerance induction. Clin Exp Immunol. 1996;105:397–402. doi: 10.1046/j.1365-2249.1996.d01-779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Viney JL, Mowat AM, O'malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 11.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 12.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8 (+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 13.Clare-Salzier MJ, Brooks J, Chai A, van Herle K, Anderson C. Prevention of diabetes in nonobetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–8. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmdahl R, Olsson T, Moran T, Klareskog L. In vivo treatment of rats with monoclonal anti-T cell antibodies. Immunohistochemical and functional analysis in normal rat and experimental allergic neuritis. Scand J Immunol. 1985;22:157–69. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 15.Dagerlind Å, Friberg K, Bean AL, Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992;98:39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- 16.Link J, Söderström M, Olsson T, Höjeberg B, Ljungdahl Å, Link H. Increased transforming growth factor-beta, interleukin-4 and interferon-gamma in multiple sclerosis. Ann Neurol. 1994;36:379–86. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- 17.Konecny P, Stagg AJ, Jebbar H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–11. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, Holt PG. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–31. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz CJ, Mansfield JM. IFN-gamma-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell Immunol. 1999;192:24–32. doi: 10.1006/cimm.1998.1429. [DOI] [PubMed] [Google Scholar]
- 20.Xiao BG, Huang YM, Xu LY, Ishikawa M, Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68–86 in Lewis rats: a role for dendritic cells in inducing apoptosis of CD4+ T cells. J Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 21.Knight SC, Mertin J, Stackpoole A, Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci USA. 1983;80:6032–5. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boog CJ, Kast WM, Timmers HT, Boes J, de Waal LP, Melief CJ. Abolition of specific immune response defect by immunization with dendritic cells. Nature. 1985;318:59–62. doi: 10.1038/318059a0. [DOI] [PubMed] [Google Scholar]
- 23.Nossal GJ, Herold KC, Goodnow CC. Autoimmune tolerance and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:S49. doi: 10.1007/BF00586279. [DOI] [PubMed] [Google Scholar]
- 24.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igietseme JU, Ananaba GA, Bolier J, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–9. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today. 1999;20:27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-γ, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 29.Williams MS, Noguchi S, Henkart PA, Osawa Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol. 1998;161:6526–31. [PubMed] [Google Scholar]
- 30.Zettl UK, Mix E, Zielasek J, Stangel M, Hartung HP, Gold R. Apoptosis of myelin-reactive T cells induced by reactive oxygen and nitrogen intermediates in vitro. Cell Immunol. 1997;178:1–8. doi: 10.1006/cimm.1997.1113. [DOI] [PubMed] [Google Scholar]
- 31.Okuda Y, Sakoda S, Shimaoka M, Yanagihara T. Nitric oxide induces apoptosis in mouse splenic T lymphocytes. Immunol Letters. 1996;52:135–8. doi: 10.1016/0165-2478(96)02597-7. [DOI] [PubMed] [Google Scholar]
- 32.Fehsel K, Kroncke KD, Meyer KI, Huber H, Wahn Vand V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–65. [PubMed] [Google Scholar]
- 33.Downing JE, Virag L, Perry ME. Nitrergic mechanism of DC-mediated T-cell elimination. Immunol Today. 1998;19:190–1. doi: 10.1016/s0167-5699(97)01223-1. [DOI] [PubMed] [Google Scholar]
- 34.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. IL-12 protects from a Th-1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon-γ, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramashaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–7. [PubMed] [Google Scholar]
- 36.Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88:1–8. doi: 10.1016/s0165-5728(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 37.O'brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163:6841–7. [PubMed] [Google Scholar]
- 38.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–86. [PubMed] [Google Scholar]
- 39.Ahuja SS, Mummidi S, Malech HL, Ahuja SK. Human dendritic cell (DC)-based anti-infective therapy: engineering DCs to secrete functional IFN-γ and IL-12. J Immunol. 1998;161:868–76. [PubMed] [Google Scholar]
- 40.Oh S, McCaffery JM, Eichelberger MC. Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogenic T-cell proliferation at low, but not high, doses of virus. J Virol. 2000;74:5460–9. doi: 10.1128/jvi.74.12.5460-5469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radosevic K, Casteels KM, Mathicu C, Van Ewijk W, Drexhage HA, Leenen PJ. Splenic dendritic cells from the non-obese diabetic mouse induce a prolonged proliferation of syngeneic T cells. A role for an impaired apoptosis of NOD T cells? J Autoimmun. 1999;13:376–82. doi: 10.1006/jaut.1999.0338. [DOI] [PubMed] [Google Scholar]