Abstract
Gametogenesis is a highly regulated process in all organisms. In the Drosophila ovary, signaling events between germline and somatic follicle cells establish the axes of the egg and the future embryo [1, 2]. A meiotic checkpoint which monitors double stranded DNA breaks (DSB) and involves the Drosophila ATR and Chk2 homologs, coordinates the meiotic cell cycle with these signaling events. Activity of the checkpoint affects the translation of the transforming growth factor-alpha like Gurken signaling molecule which normally induces dorsal cell fates in the follicle cells ([3], [4], [5]). We found that mutations in the Drosophila gene cutoff (cuff) affect germline cyst development and result in ventralized eggs due to reduced Grk protein expression. Surprisingly, we found that cuff mutations lead to a marked increase in the transcript levels of two retro-transposable elements, Het-A and Tart. Tagged Cuff protein shows a peri-nuclear localization in the nurse cells, similar to components of the RNAi machinery. We found that a small interfering RNA against the roo element is still produced in cuff mutant ovaries. These results indicate that Cuff is involved in the rasiRNA pathway, most likely acting downstream of siRNA biogenesis. The eggshell and egg laying defects of cuff mutants are suppressed by a mutation in chk2. We also found that mutations in aubergine (aub), another Drosophila gene implicated in the rasiRNA pathway, are also significantly suppressed by the chk2 mutation. Our results indicate that mutants in rasiRNA pathways lead to elevated transposition incidents in the germline, which activates a checkpoint that causes a loss of germ cells and a reduction of Gurken protein in the remaining egg chambers.
Results and Discussion
Cutoff is a female sterile mutation affecting eggshell polarity, karyosome formation and female fecundity
Cutoff (cuff) mutations were isolated in a large scale female sterile screen of Drosophila [6, 7], and one additional allele was identified in a P-element insertion screen [8]. Females trans-heterozygous for cuff alleles lay eggs with various degrees of ventralization (Table 1 and data not shown). Dorso-ventral polarity of egg and embryo depends on the levels of the Gurken (Grk) ligand, which is produced and secreted by the germline and activates the EGF receptor (Egfr) in the overlying follicle cells ([9], [10]). To determine whether Grk-Egfr signaling was affected, we analyzed the grk expression pattern in a strong cuff mutant background. In wild type egg chambers, at stage 9 of oogenesis, grk RNA becomes restricted to the future dorsal anterior side of the oocyte, forming a cap around the oocyte nucleus (Fig. 1A). Grk protein is translated from the tightly localized RNA, and is also spatially restricted to the membrane overlying the oocyte nucleus (Fig. 1B) [11, 12]. Cuff mutants do not significantly disrupt grk RNA localization (Fig. 1C). However, in many mid-stage egg chambers, the Grk protein level is greatly reduced, where between 10% to 40% of the egg chambers contain no detectable Gurken protein at all (Fig. 1D), consistent with defects in grk translation. In wild type egg chambers, by stage 3 of oogenesis, the oocyte nucleus forms a compact structure termed the karyosome (Fig. 1B inset). In cuff mutants, in 10–20% of the egg chambers, karyosome formation is affected, and instead, the DNA assumes various shapes and is often found in separate clumps (Fig. 1D inset).
Fig. 1. Grk expression in cuff mutant egg chambers.
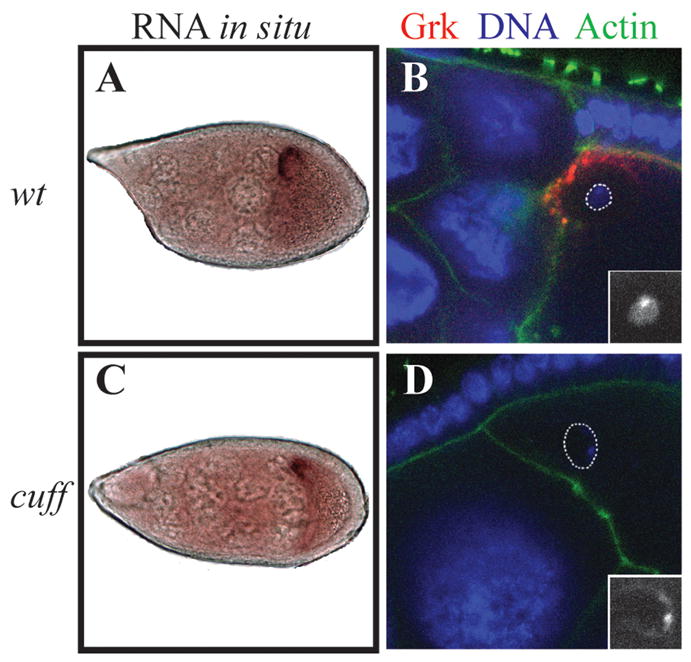
grk RNA in situ hybridization (A, C) and Grk antibody staining (B, D).
(A, B) Cuff heterozygous females were used as wild type control: In Stage 9 wild type egg chambers, grk transcripts form a tight cap around the oocyte nucleus at the dorsal cortex (A). Grk protein is translated from the localized mRNA, and is also restricted to the dorsal anterior region of the oocyte (B). At this stage of oogenesis, the chromatin of the oocyte nucleus forms a compact, round structure termed the karyosome (B inset).
(C, D) cuff WM25/KG05951. In Stage 9 cuff mutant egg chambers, grk transcript localization appears mostly normal (C), however, in 10–40% of the egg chambers, Grk protein is undetectable (D). Some 10–20% of the oocyte nuclei at this stage also assume a defective morphology. Often, the DNA seems to localize to the periphery of the nucleus (D inset). In B and D, the oocyte nucleus is marked by dotted lines.
Another prominent defect in cuff mutant females is a severely reduced fecundity (as reflected in the name of the gene). While cuff heterozygous females lay an average of around 20 eggs per day, newly eclosed cuff females of a strong allelic combination lay around 5 eggs per day. This phenotype becomes more severe as the females age. To address the cause of the reduced egg production, we analyzed the germaria, the anterior-most part of the ovarioles of the mutant females. In each ovariole, germ line stem cells divide asymmetrically, giving rise to another stem cell and a cystoblast. The cystoblast undergoes four rounds of mitosis with incomplete cytokinesis, forming an inter-connected 16 cell cyst which will differentiate into one oocyte and 15 associated nurse cells (for review of oogenesis see [13]). In the germarium, the 16 cells are connected by the fusome, a membraneous structure that connects the 16 cells of the cyst, which has been shown to be important for germ line cyst development [14, 15]. To assay the division of the germ line stem cells and the cystoblasts, we analyzed fusome branching in cuff mutants. In wild type germaria (either cn bw females, or cuff heterozygous females), using an antibody against alpha Spectrin, we always observed highly branched fusomes in region 1 and region 2 of the germaria (Fig. 2A, N=146). In newly eclosed cuff mutant females, we observed similar patterns. However, as the females aged, we noticed a sharp increase in the percentage of mutant germaria without cysts that contain highly branched fusomes (Fig. 2B). In cuff mutant females one week after eclosure, 54% of the germaria did not have highly branched fusomes (N=35); the number increases to 83% in mutant females two weeks after eclosure (N=43). Instead, in older cuff mutant females, the mutant cysts appear to arrest in early stages in the germaria containing spectrosome-like structures. We also stained cuff heterozygous and trans-heterozygous females with an antibody against C(3)G. C(3)G is a component of the synaptonemal complex [16] which starts assembly in region 2A of the germarium. In cuff mutant females one week after eclosure, 44% of the germaria did not contain any C(3)G positive cysts (Fig. 2D, N=50); the number increases to 72% in females two weeks after eclosure (N=50). We stained cuff heterozygous and trans-heterozygous females with an antibody against Fasciclin (Fas) III, which marks immature follicle cells before stage 4 as well as the polar cells after stage 4 of oogenesis. In wild type ovaries, we regularly observed developing germ line cyst in region 2b and/or region 3 of the germaria (Fig. 2E). However, in cuff mutant females one week after eclosure, 72% of the germaria did not contain cysts of normal morphology in region 2b or region 3 (Fig. 2F, N=44); the number increases to 84% in females two weeks after eclosure (N=44). In addition, in cuff mutant females, we often observed a germarium attached to a mature egg chamber or an empty ovariole with only the germarium at the tip (Fig. 2F inset).
Fig. 2. Germline cyst development in cuff mutants.
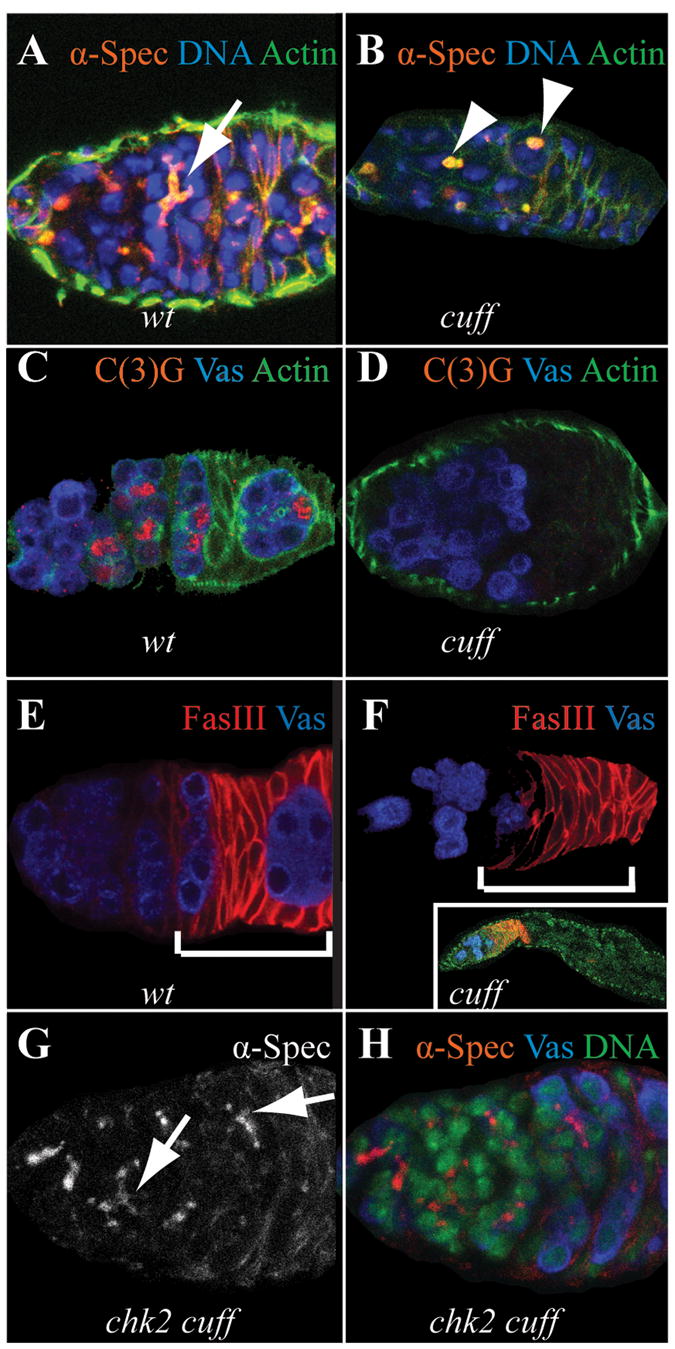
(A, B) Using an antibody against α-Spectrin (red), we always observed highly branched fusomes (arrow) in wild type germaria in the anterior regions (A). In contrast, in cuff mutant germaria, many of the cysts fail to divide normally and form branched fusomes, instead often retaining a round spectrosome-like fusome (arrowheads) (B).
(C, D) Using an antibody against C(3)G (red), we monitored meiotic progression in wild type and cuff mutant backgrounds. In wild type germaria, we consistently observed multiple cysts initiating meiosis (C); while in cuff mutant background, although germ cells are present (as marked by Vas staining in blue), the cysts lack C(3)G staining, indicating an early arrest (D).
(E, F) In wild type germaria, developing germline cysts migrate to the posterior of the germaria (E), and subsequently bud off, forming an egg chamber. In cuff mutants, this process is disrupted. In corresponding regions marked by the bracket (region 2b and 3 as marked by FasIII staining in red), there are few if any normal looking cysts in cuff mutant germaria (F).
(G, H) The defects in cyst development of cuff mutants is partially suppressed by a mutation in chk2. In chk2 cuff double mutants, we observed many highly branched fusomes (arrows in G), and females also lay many more eggs than the corresponding cuff mutant.
All flies were growing under optimal nutritional conditions. Cuff heterozygous females were used as wild type control, while cuff WM25/KG0595 and chk2 cuff WM25females were used for phenotypic analysis.
In older cuff mutant females, there is a gradual loss of germ cells even in the germaria. In mutant females three weeks after eclosure, around a third of the germaria did not contain any germ cells (N=74). Sometimes, we also observed germaria filled with cystoblast-like germ cells. Using the germline clone technique [17], we were able to show that Cuff is required in the germline for all of the functions described above, including Grk protein expression, reduced fecundity and karyosome phenotypes (data not shown).
Characterization of cutoff
We determined that cuff corresponds to CG13190. Sequencing of cuff mutants revealed several DNA changes in the mutant alleles (Fig. 3A). In particular, cuffWM25 is a strong allele based on the phenotypic analysis. In cuffWM25 mutants, the start codon of CG13190 is mutated, making it very likely to be a null allele (see Fig. 3A for details). We generated transgenes carrying either the cuff genomic region or the predicted cuff coding sequence under the control of a UAS enhancer. Both the genomic transgene as well as the cuff coding sequence driven by nanos-Gal4-VP16 fully rescued the eggshell defects of cuff mutants. The genomic transgene also rescues the female sterility.
Fig. 3. Characterization of cuff.
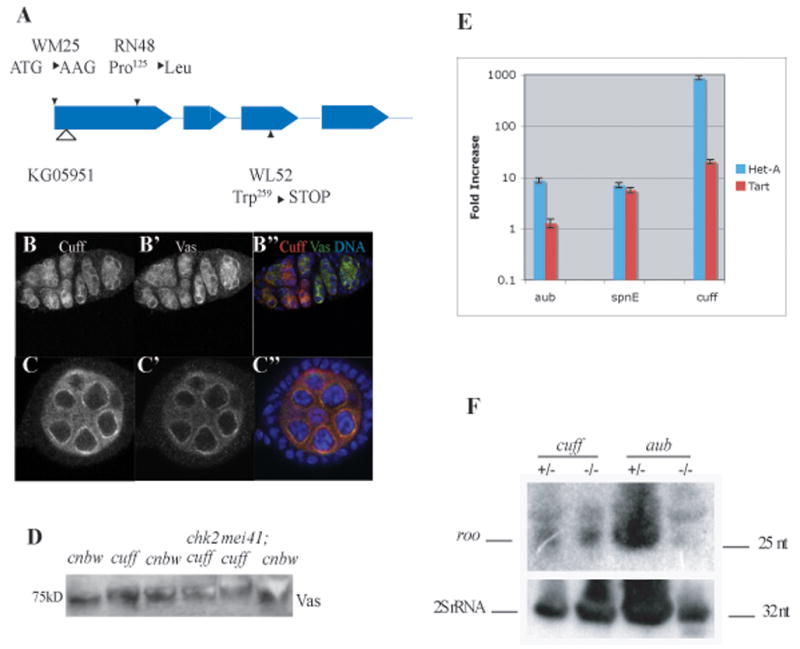
A. Sequencing of cuff mutants. cuffWM25, cuff RN48, and cuffWL52 were induced by EMS [6]. cuffKG05951 was derived by the BDGP Gene Disruption Project.
B–C. In order to analyze Cuff localization, we expressed HA-tagged Cuff protein under the control of nanos-Gal4 VP16. When expressed in this manner, Cuff shows a prominent peri-nuclear pattern, co-localizing with Vas protein in the germarium (B) and early stage egg chambers (C).
D. In cuff mutants, Vas protein electorophoretic migration is slower than in wild type. A very similar phenotype was observed in spnB mutants (Ghabrial and Schupbach, 1999). The Vas migration defect is suppressed by a chk2 mutation, but not by a mutation in the Drosophila ATR homolog mei41.
E. Mutations in cuff lead to a strong up-regulation of Het-A and Tart retrotransposable elements in the Drosophila germline. In cuff mutants, Het-A displays a 800-fold increase in transcript levels, while Tart is up-regulated approximately 20 fold. A deregulation of these elements can also be detected in the germline of spnE and aub mutants (see also [31]). In spnE ovaries both Het-A and Tart are up-regulated approximately 10 fold, while in aub only Het-A levels are significantly increased.
F. rasiRNAs production is not affected in cuff mutant ovaries. Similar levels of the roo rasiRNA can be detected in ovaries heterozygous (lane 1) and homozygous (lane 2) mutant for cuff. In contrast, roo rasiRNAs are expressed in ovaries heterozygous mutant for aub (lane 3), but are absent from the homozygous mutant.
Genomic database searches identified the yeast gene Rai1 as a homolog of cuff. This gene has been shown to interact with a nuclear 5′-3′ exoribonuclease (Rat1) which is involved in rRNA processing and transcriptional termination [18–20]. A cytoplasmic homolog of Rat1, Xrn1, has also been described in yeast and vertebrates and has been implicated in mRNA regulation that is localized to cytoplasmic processing bodies [21, 22]. We generated an HA-tagged Drosophila Rat1 (CG10354) construct and overexpressed it with a fully functional FLAG tagged Cuff in the ovary. Using immuno-precipitation (IP), we failed to detect any interaction between the exo-ribonuclease and Cuff (data not shown). It is therefore possible that Drosophila Rat1 is not the correct partner for Cuff. This is also supported by our observation that over-expressed Rat1, as expected, localizes to the nucleus, whereas over-expressed Cuff localizes to the cytoplasm. We were not able to detect endogenous Cuff protein with an anti-Cuff antibody, presumably due to low levels of protein expression. However, over-expressed HA-tagged Cuff partially co-localizes with perinuclear puncta in the nurse cells in younger egg chambers. A similar localization pattern has been described for the helicase Vasa, and we found that Cuff partially co-localizes with Vasa in the cytoplasm (Fig. 3B, C). The peri-nuclear localization pattern, also designated as nuage in the germ cells and related to mammalian P- bodies [23], has been previously described for components of the RNAi machinery and for genes involved in RNA degradation [24, 25].
Given the eggshell ventralization and the karyosome defect, cuff has similar mutant phenotypes as a group of mutants known as the spindle class genes [26]. Several members of this group encode DNA repair genes [27, 28] for instance spindle(spn) B (XRCC3), and okra (DmRad54). In these mutants, the DSBs that are created during recombination persist, activating Chk2 through the Drosophila ATR homolog mei-41. The activity of these kinases negatively regulates the translation of Grk, possibly through a post-translational modification of Vasa, which in turn, leads to ventralization of the eggs laid by mutant females [4, 29]. Inactivation of the checkpoint, for instance through mutations in chk2 or mei-41, suppresses the egg shell defects of the spindle class DNA repair mutants. In addition, in double mutants of the DNA repair genes and the genes required for initiating the DSBs, such as c(3)g, mei-W68 or mei-P22, DSBs are not generated, therefore the checkpoint is not activated, and the egg shell morphology is normal, even in the presence of the repair mutants. To check whether Cuff is involved in the repair of DSBs initiated in prophase of meiosis I, we generated mei41; cuff and cuff; c(3)g double mutants. While both mutations suppress the eggshell defect of spnB or okra to wildtype morphology [3]neither suppresses the eggshell defect of cuff, indicating that Cuff does not function in the meiotic repair pathway. To our surprise, however, a mutation in chk2 partially suppresses the eggshell defect of cuff (Table 1) as well as the defects in cyst development (Fig. 2G, H). chk2 cuff double mutants lay mostly wild type looking eggs (Table 1), they have cysts with highly branched fusomes in the germaria (N=52) (Fig. 2G, H), and the females lay more eggs compared to cuff single mutants although the rescue is not 100% (data not shown). In certain allelic combinations, we were able to observe a dominant effect in the chk2 suppression of cuff eggshell defect. To check whether the eggshell defect of other RNAi machinery components can be suppressed by chk2, we generated double mutants of chk2 and aub. The chk2 mutation partially suppresses the eggshell defect (Table 1) as well as the laying defect of aub mutants (data not shown). As in chk2 cuff double mutants, we also observed a partial dominant suppression in the chk2 aub combination. Compared with aub mutants, chk2 aub/+ aub flies lay more eggs, a higher percentage of which are of wild type-like morphology. In contrast, no significant suppression of the aub egg shell phenotype was observed in combination with two alleles of mei-41. However, it should be noted that mei41; aub, mei41; cuff and cuff; c(3)g females continued to lay very few eggs, such that a small percentage of suppression would have gone unnoticed.
Previously, work from our lab suggested that the DNA repair checkpoint, upon activation, regulates Grk translation through a post-translational modification of Vas, resulting in slower Vas electrophoretic mobility [29]. To address whether in cuff mutants, the checkpoint acts in the same manner, we assayed Vas mobility in cuff mutant combinations. In cuff mutants, Vas migrates slightly slower compared with wild type control consistent with the modification seen in the DNA repair mutants. The mobility is not changed in mei41; cuff double mutant background, which is consistent with the fact that mei41 mutants do not significantly suppress the egg shell phenotype of cuff. However, Vasa mobility is restored to wild type in the chk2 cuff double mutant (Fig. 3D). This suggests that although in cuff mutant, the checkpoint is activated through a different sensing mechanism, upon activation, the checkpoint involves Chk2 and acts through similar pathways to affect Gurken translation in the egg chambers that escape the early arrest.
Cutoff mutations lead to an increased level of retro-transposable elements in the female germ line
Several of the Spindle Class genes, such as spnE and aub, have been shown to be essential components of the RNAi machinery [30]. Since over-expressed Cuff has a peri-nuclear localization, we wanted to check whether Cuff might also be required in RNAi pathways. Recently, a specific branch of the RNAi pathways, the repeat associated small interfering RNA (rasiRNA), has been implicated in the control of retro-transposable elements in the Drosophila germline [31]. Using qRT-PCR we studied the level of Het-A and Tart, two of the retro-transposable elements responsible for maintaining the telomere in Drosophila. Previously, it has been shown that in spnE and aub mutants, HetA and Tart transcripts are de-repressed, resulting in a marked elevation in the transcripts level. Compared with heterozygous controls, in spnE homozygous mutant females, Het-A and Tart transcripts are up-regulated by approximately ten-fold, while in aub mutants only Het-A is significantly upregulated. In cuff mutant females, the elevation for both transcripts is even more pronounced. Compared with the heterozygous control, HetA levels are elevated by more than 800-fold in cuff mutants, and Tart transcript levels increase by more than 20-fold (Fig. 3E). Transposable elements are normally silenced in the Drosophila germline by the rasiRNA pathway. This silencing process appears to be strongly impaired in the cuff mutants. We further tested whether the upregulation of the transposable elements in cuff mutants could be due to a reduction in the level of rasiRNAs. However, we found that the levels of the 25 nt long roo interfering RNA are not reduced in cuff mutant ovaries, in contrast to ovaries mutant for aub (Fig 3F). This indicates that Cuff is not involved in the biogenesis of the rasiRNAs and points to a function for Cuff in the actual silencing process. Since high transcript levels of the retro-transposable elements in the germline are correlated with elevated transposition incidents, which in turn lead to decreased chromosomal integrity [31, 32], it is possible that such chromosomal defects activate the checkpoint involving chk2. Such a model has recently been proposed by Klattenhoff et al. [33]. In addition, since transposable elements are involved in chromatin structure regulation [34], it is also possible that a chromatin checkpoint exists that involves Chk2 activity. Once Chk2 is activated, either by the mutants in DNA repair pathways, or by RNAi components such as Cuff and Aub, Chk2 activity leads to post-translational Vas modification, and a negative regulation of Grk translation. However, unlike DNA repair mutants, cuff and aub mutations are not suppressed to wildtype morphology and fecundity by mutations in mei41, suggesting that they activate the checkpoint through a different, or additional, sensing mechanism. Furthermore, most of the mutants in DNA repair pathways do not cause defects in cyst development or germline stem cell maintenance. These additional defects seen in cuff mutants could be due to the timing of checkpoint activation. DNA repair mutants activate the meiotic checkpoint during prophase of meiosis which initiates after the formation of the 16 cell cyst, while cuff and aub mutants appear to act earlier in oogenesis, given that they have effects already during the mitotic cycles preceding the onset of meiosis. The transposon activated checkpoint leads to not only translational arrest of Grk, but also mitotic cell cycle arrest. Many of the arrested germline cells and cysts eventually undergo apoptosis, leading to gradual loss of germline stem cells and developing cyst in cuff mutants. However, germ cells that escape the early arrest encounter the second checkpoint effect that leads to a reduction in Gurken translation.
It was recently discovered that there are a large number of different small RNAs generated in the germline of both mammals and flies [35–38]. Many of them are associated with Piwi family proteins, and most have no known functions. Since the germline represents a special cell type that will pass its DNA on to future progeny, it is possible that selfish elements have developed a high propensity to remobilize in the germ line. It is further very plausible that in most organisms, the germline has evolved sophisticated mechanisms to defend itself against such transposable elements. Many of the small RNAs found in the germline may be involved in the defense against transposable elements, as well as in the regulation of transcription and translation. When the machinery to generate these small silencing RNAs or the effector complexes that are responsible for transcript degradation are disrupted, chromosomal integrity might be at risk. Here we have found that in Drosophila a checkpoint involving the conserved Chk2 kinase monitors the RNAi mediated events in the germline, and ensures the genomic integrity of the progeny. Chk2 therefore acts as a surveillance factor for both transposon generated problems as well as DNA repair problems in the germline. Whether Chk2 has a similar role in the mammalian germline will be interesting to investigate in the future.
Supplementary Material
Supplemental Data include Experimental Procedures
Acknowledgments
The authors would like to thank Julius Brennecke, Alexej Aravin and Greg Hannon for technical advice regarding rasiRNAs analysis. Scott Hawley for providing fly stocks; Scott Hawley and Scott Page for providing antibodies; Leontine Galante, Rong Lu, Scott Terhune and Nir Yakobi for technical advice regarding qRT-PCR, Gail Barcelo for assistance with in situ hybridization, and Joe Goodhouse for help with confocal microscopy. We are very grateful to Martha Klovstad, and Scott Ferguson for critical comments on the manuscript and to members of the Schüpbach and Wieschaus laboratories for helpful discussions and suggestions. This work was supported by the Howard Hughes Medical Institute and US Public Health Service Grant PO1 CA41086.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 2.Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol. 2004;14:R438–449. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Ghabrial A, Schupbach T. Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nat Cell Biol. 1999;1:354–357. doi: 10.1038/14046. [DOI] [PubMed] [Google Scholar]
- 4.Abdu U, Brodsky M, Schupbach T. Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol. 2002;12:1645–1651. doi: 10.1016/s0960-9822(02)01165-x. [DOI] [PubMed] [Google Scholar]
- 5.Staeva-Vieira E, Yoo S, Lehmann R. An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. Embo J. 2003;22:5863–5874. doi: 10.1093/emboj/cdg564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- 10.Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- 11.Neuman-Silberberg FS, Schupbach T. The Drosophila TGF-alpha-like protein Gurken: expression and cellular localization during Drosophila oogenesis. Mech Dev. 1996;59:105–113. doi: 10.1016/0925-4773(96)00567-9. [DOI] [PubMed] [Google Scholar]
- 12.Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- 13.Spradling AC. Germline cysts: communes that work. Cell. 1993;72:649–651. doi: 10.1016/0092-8674(93)90393-5. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Dev Genet. 1995;16:6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 16.Page SL, Hawley RS. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 2001;15:3130–3143. doi: 10.1101/gad.935001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y, Bai X, Lee I, Kallstrom G, Ho J, Brown J, Stevens A, Johnson AW. Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Mol Cell Biol. 2000;20:4006–4015. doi: 10.1128/mcb.20.11.4006-4015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, Buratowski S. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature. 2004;432:517–522. doi: 10.1038/nature03041. [DOI] [PubMed] [Google Scholar]
- 20.Sydorskyy Y, Dilworth DJ, Yi EC, Goodlett DR, Wozniak RW, Aitchison JD. Intersection of the Kap123p-mediated nuclear import and ribosome export pathways. Mol Cell Biol. 2003;23:2042–2054. doi: 10.1128/MCB.23.6.2042-2054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orban TI, Izaurralde E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. Rna. 2005;11:459–469. doi: 10.1261/rna.7231505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 25.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Reyes A, Elliott H, St Johnston D. Oocyte determination and the origin of polarity in Drosophila: the role of the spindle genes. Development. 1997;124:4927–4937. doi: 10.1242/dev.124.24.4927. [DOI] [PubMed] [Google Scholar]
- 27.Ghabrial A, Ray RP, Schupbach T. okra and spindle-B encode components of the RAD52 DNA repair pathway and affect meiosis and patterning in Drosophila oogenesis. Genes Dev. 1998;12:2711–2723. doi: 10.1101/gad.12.17.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdu U, Gonzalez-Reyes A, Ghabrial A, Schupbach T. The Drosophila spn-D gene encodes a RAD51C-like protein that is required exclusively during meiosis. Genetics. 2003;165:197–204. doi: 10.1093/genetics/165.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghabrial A, Schupbach T. Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nat Cell Biol. 1999;1:354–357. doi: 10.1038/14046. [DOI] [PubMed] [Google Scholar]
- 30.Kennerdell JR, Yamaguchi S, Carthew RW. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 2002;16:1884–1889. doi: 10.1101/gad.990802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 2006;20:345–354. doi: 10.1101/gad.370206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 33.Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE. Drosophila rasiRNA Pathway Mutations Disrupt Embryonic Axis Specification through Activation of an ATR/Chk2 DNA Damage Response. Dev Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, Elgin SC. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 35.Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–2222. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 37.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 38.Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuman-Silberberg FS, Schupbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457–2463. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- 41.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 42.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include Experimental Procedures


