Abstract
It has been well known that HLA-G molecules are present in a variety of human neoplastic diseases and the molecule may contribute to the escape of tumor cells from immune surveillance. Besides the studies that aim at elucidating the roles of HLA-G in immune regulation, the researches that focus on potential applications of HLA-G expression in cancer diagnosis represent another perspective in HLA-G research. This review summarizes those recent translational studies of HLA-G expression in the diagnosis of human cancer. Specifically, the promises and challenges for applying HLA-G expression to detect cancer in body fluids, to diagnose different types of human cancer and to predict clinical outcome in cancer patients will be briefly reviewed.
Introduction
Recent studies have provided an ample amount of evidence that HLA-G molecule participates in immune regulation, especially in immune tolerance in physiological process such as pregnancy. Shortly after the identification of HLA-G in extravillous (intermediate) trophoblastic cells [1], there has been increased interest in studying HLA-G expression in neoplastic diseases based on an attractive hypothesis that cancer cells may likely utilize HLA-G expression to escape host immunosurveillance similar to what extravillous (intermediate) trophoblastic cells do in the maternal-fetal interface. Indeed, an increasing number of studies have reported HLA-G expression in several human cancers such as ovarian carcinoma, gastric carcinoma, coetaneous melanoma, hematopoietic tumors, endometrial carcinoma, renal cell carcinoma, lung carcinoma, mesothelioma, breast carcinoma and trophoblastic tumors [2–8]. Furthermore, secreted HLA-G (sHLA-G) has also been detected in some of these tumor types [9–11]. These studies provide cogent in vivo evidence demonstrating HLA-G expression in human tumor tissues and support the view that HLA-G may participate in tumor development by suppressing immune regulation within tumor microenvironment (please see the review article [2] for details). Besides the active basic research on the roles of HLA-G in immunomodulation, the translational-type studies that focus on the potential applications of HLA-G expression in human cancer diagnosis represent another research front. This review will summarize those recent studies of HLA-G expression in human cancer, and more importantly will discuss the promise and challenges for applying the pattern of HLA-G expression to detect cancer in body fluids, to diagnose different cancer types and to predict clinical outcome in cancer patients.
sHLA-G as a biomarker for cancer detection in body fluid
As sHLA-G is a potential biomarker in body fluids, the measurement of sHLA-G levels in blood and effusion samples may have clinical values in differentiating malignant versus benign conditions. Rebmann et al. have elegantly applied ELISA to demonstrate an increased plasma sHLA-G level in patients with cutaneous melanoma, gliomas, breast and ovarian carcinoma [10]. In hematopoietic neoplasms, as compared to control patients, the concentration of sHLA-G in blood increased in multiple myeloma [12], acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages [13], in B-cell chronic lymphocytic leukemia and non-Hodgkin B and T cell lymphomas [14]. These reports provide preliminary evidence that sHLA-G is a surrogate tumor marker that awaits further validation for potential clinical applications.
Ascites and pleural effusions are commonly associated with a variety of diseases including infection, inflammatory disorders, cardiac, liver and renal diseases as well as neoplastic diseases. Diagnosis is usually based on cytology by examining representative cells obtained from the effusion fluid but the sensitivity of cytology has been estimated to be 60% at best [15]. The low sensitivity may be due to small numbers of tumor cells in the ascites or the presence of a large amount of leukocytes, mesothelial cells and blood that can obscure the malignant cells. For example, inflammation which is often associated with a malignant ascites can result in reactive changes in mesothelial cells, making their morphological distinction from carcinoma cells extremely difficult [15]. Therefore, molecular markers that are associated with malignant effusion would facilitate the diagnosis in this clinical setting.
Singer et al. applied a senstive ELISA to measure sHLA-G levels in the supernatant of peritoneal ascites from ovarian and breast carcinoma and benign controls [6]. In their study, the levels of sHLA-G were significantly higher in malignant ascites as compared to benign ascites. Interestingly, all but one malignant peritoneal ascites supernatant contained detectable sHLA-G including one specimen that had been missed on cytology. In order to detect ovarian and breast cancer in ascites using multiple cutoff values, the investigators used ROC curves to evaluate the performance of sHLA-G. The area under the ROC curve was 0.95 in assessing sHLA-G levels as the diagnostic tool to detect ovarian and breast cancer.
Although sHLA-G may prove to be useful in assisting the diagnosis of malignant versus benign clinical conditions, it should be emphasized that body fluids from healthy individuals contain variable amount of sHLA-G probably derived from peripheral monocytes [16]. Thus, a large number of cases should be assessed for ROC curve analysis in order to determine the optimal cutoff of sHLA-G for potential clinical applications. The other challenge to apply sHLA-G ELISA for cancer detection is to demonstrate a higher performance of sHLA-G than previously published or pre-existing markers. Several protein markers have been studied in cancer and they include CA125, tissue polypeptide specific antigen, soluble interleukin-2 receptor alpha, soluble aminopeptidase N/CD13, alpha-fetoprotein, carcinoembryonic antigen, CA 19-9, CA 15-3 and several cytokines. Further studies should determine if a combination of selected secreted biomarkers including sHLA-G may provide a better approach to increase both sensitivity and specificity than single marker alone for cancer detection in body fluids. It will be necessary to compare the performance of the sHLA-G ELISA and routine cytological examination by testing a large number of cytology-negative but biopsy-positive samples. It will also be important to address how age, menopausal status, histological grade and other clinical parameters affect HLA-G levels in effusions.
The role of HLA-G in the diagnosis of human cancer on tissues
Diffuse pattern of HLA-G expression is associated with trophoblastic tumors and tumor-like lesions
As previously discussed, HLA-G was first identified as a molecule predominantly expressed in extravillous (intermediate) trophoblastic cells; therefore, it is likely that HLA-G expression should be detected in intermediate trophoblastic lesions. In fact, Singer et al. analyzed HLA-G immunoreactivity in human intermediate trophoblastic tumors including choriocarcinoma, placental site trophoblastic tumor and epithelioid trophoblastic tumor and tumor-like lesions including exaggerated placental site and placental site nodule. The researchers found that HLA-G immunoreactivity was detected in the majority of intermediate trophoblastic cells in all lesions. The diffuse staining pattern in virtually all intermediate trophoblastic lesions suggests that HLA-G immunoreactivity serves as a tissue marker to diagnose intermediate trophoblastic tumors and tumor-like lesions. As previously discussed, many human neoplastic diseases also express HLA-G, but the HLA-G immunoreactivity in those non-trophoblastic tumors is usually focal with <50% of tumor cells being positive for HLA-G. For example, although HLA-G immunoreactivity is observed in more than 50% of the endometrial carcinomas that histologically may resemble trophoblastic tumors and tumor-like lesions, the majority (>95%) of endometrial carcinoma cases exhibit positive HLA-G staining in less than 50% of tumor cells [17]. Therefore, diffuse HLA-G immunoreactivity within a tumor appears to be specific for intermediate trophoblastic cells in gestational trophoblastic diseases and can serve as a useful marker in the differential diagnosis of these lesions. The differential diagnosis between trophoblastic and non-trophoblastic lesions is important because patients are usually managed differently. For example, a placental site nodule which is a benign trophoblastic lesion can be morphologically confused with cervical squamous carcinoma of the uterus. With the HLA-G immunohistochemistry, a placental site nodule is diffusely positive for HLA-G while a cervical carcinoma is usually negative or very focally positive for HLA-G. Thus, application of HLA-G immunohistochemistry can be useful in this specific clinical setting. A placental site nodule is a benign lesion without a need for further treatment, while a cervical carcinoma is a malignant disease that required radical hysterectomy.
The other application of HLA-G immunostaining is to facilitate the diagnosis among placental site trophoblastic lesions, particularly in the distinction between a placental site trophoblastic tumor and an exaggerated placental site. Double-staining technique using MIB-1 antibody to determine the Ki-67 proliferative index in HLA-G defined intermediate trophoblastic cells is a useful method in the differential diagnosis of exaggerated placental site (Fig. 1) versus placental site trophoblastic tumor, and placental site trophoblastic tumor versus choriocarcinoma. Without using HLA-G staining, assessment of Ki-67 (proliferation) index in tissue sections can be very difficult because proliferating T-cells and NK cells are also labeled with Ki-67 immunoreactivity that can confuse the counting of Ki-67 labeling index in trophoblastic cells. It has been reported that the Ki-67 index in intermediate trophoblastic cells of an exaggerated placental site was near zero. In contrast, the Ki-67 index in intermediate trophoblastic cells of a placental site trophoblastic tumor was elevated and in choriocarcinoma was even more significantly elevated.
Fig. 1.
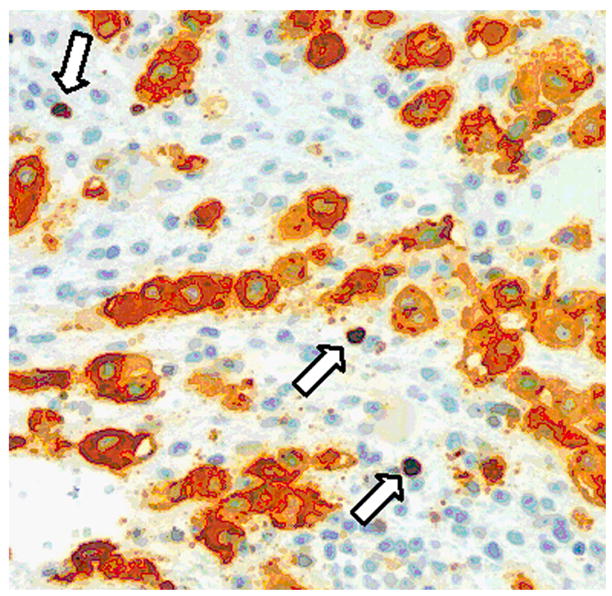
Immunohistochemistry of HLA-G and Ki-67 in an exaggerated placental site from an endometrial curettage specimen. HLA-G immunoreactivity is located in the cell membrane and cytoplasm while Ki-67 immunoreactivity is located exclusively in the nuclei. The HLA-G positive intermediate trophoblastic cells do not demonstrate Ki-67 nuclear staining. Therefore, the finding is consistent with an exaggerated placental site, a benign and physiological process during pregnancy. There are scattered Ki-67 nuclei in the filed, and they represent proliferating T-cells or NK-cells (arrows). Without HLA-G immunostaining, it would be difficult to determine the cell type (trophoblastic vs. non-trophoblastic) of the Ki-67 labeled cells.
HLA-G immunoreactivity as a diagnostic marker for distinguishing malignant versus benign neoplasms
In addition to its potential use as a marker in distinguishing intermediate trophoblastic tumors and tumor-like lesions, HLA-G immunoreactivity can also be useful to dinstinguish malignant versus benign neoplasms from patients. Although pathologic diagnosis based on unique morphological features of each tumor type is usually not a challenge, the diagnosis can be at times challenging, especially in final needle aspirates and peritoneal washing specimens where the amount of tumor cells is insufficient for optimal pathological assessment. For examples, HLA-G expression was reported in approximately 25% of invasive ductal carcinomas of the breast, but not in normal breast tissues and in benign ductal hyperplasia [3, 18]; therefore, HLA-G positive cells in a small fine needle biopsy specimen suggest the presence of carcinoma. Similarly, HLA-G expression is present in more than 50% of ovarian carcinomas but not in benign ovarian cysts and borderline tumors. For patients with adnexal masses, HLA-G positive cells in effusion or peritoneal washing samples likely indicate that patients may have an ovarian cancer in this specific clinical condition. It should be emphasized that, except for the differentiation between trophoblastic and non-trophoblastic tumors, there is little evidence that HLA-G alone would provide tissue-specific information regarding tumor origin since focal HLA-G immunoreactivity can be detected in many types of human cancers.
The predictive value of HLA-G expression for clinical outcome in cancer patients
Several studies have demonstrated a correlation between HLA-G expression and patient’s clinical outcome including overall survival and the risk to develop metastatic diseases. It should be noted that such correlation depends on the cancer types and specific clinical settings as HLA-G expression can be associated with either a favorable or an unfavorable clinical outcome. In ovarian carcinomas, HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma [19]. In that study, HLA-G expression in tumor cells was significantly lower in effusions obtained during or following chemotherapy. The presence of HLA-G-positive tumor cells in effusions obtained prior to the institution of chemotherapy significantly correlated with better overall survival. The above findings suggest that the HLA-G-expressing cells are more susceptible to elimination by the immune response or treatment. Similarly, Ishigami et al. found that the 5-year survival rate in gastric cancer patients whose tumors were positive for HLA-G was 78%, which was significantly higher than 51% in the HLA-G negative group [20]. In that study, HLA-G positive group had a more differentiated histology, less nodal invasion and earlier clinical stage than HLA-G negative patients.
In contrast to the above findings, HLA-G expression has been reported to be associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia [21]. In this study, patients with more than 23% HLA-G positive cells had significantly shorter progression-free survival time than patients with 23% or fewer positive cells. In multivariate analysis, HLA-G expression was an even better independent prognostic factor than the ZAP-70 and CD38 status. In endometrial carcinomas, HLA-G immunoreactivity was associated with metastatic disease and the performance of HLA-G staining to distinguish metastatic versus non-metastatic endometrial carcinoma is acceptable as the area under the ROC curve was 0.75 [17]. The researchers in that report argued that HLA-G may serve as a clinical marker for the preoperative prediction of metastatic endometrial carcinoma. In addition, Kleinberg et al. reported a trend of HLA-G expression and shorter disease-free interval in breast carcinoma, although the difference was not statistically significant [3].
The major challenge for applying HLA-G as a predictive marker for clinical behavior in cancer patients is the lack of large series of clinical studies to confirm those preliminary studies as discussed in the previous sections. It should be noted that the predictive value from the published studies will probably not be as high when applied to other sets of patients in different institutions, especially when more representative control subjects are included in prospective cohort studies. It is expected that an algorithm should be developed by incorporating HLA-G with other biomarkers and clinical parameters to better predict the clinical behavior in cancer patients.
Conclusion
HLA-G is a tumor-associated molecule that is expressed by a variety of neoplastic diseases. The diffuse pattern of HLA-G immunoreactivity in trophoblastic lesions suggests that HLA-G can be used as a tissue biomarker in the differential diagnosis of trophoblastic versus nontrophoblastic diseases. Furthermore, as HLA-G is exclusively expressed in malignant tissues of epithelial origin, HLA-G expression can assist the diagnosis of malignant versus benign lesions in small biopsies. Besides, the sHLA-G can be used as a surrogate biomarker in body fluid for cancer detection as malignant cases contained much higher levels of sHLA-G than the benign specimens did. Finally, HLA-G immunoreactivity may prove to be useful to predict clinical behavior in cancer patients and provide oncologists a new molecular approach to better manage their cancer patients. Despite the promise, several issues need to be addressed before HLA-G can be used in the future clinical applications. One of the most critical tasks is to assess the usefulness of HLA-G in large clinical studies from multiple institutions as reproducibility is the key factor for a successful implementation of a biomarker in clinical use. The multi-institutional studies would either corroborate or invalidate the findings from the reported preliminary studies. Nevertheless, it is foreseeable that the enthusiasm in pursing translational research in HLA-G in cancer diagnosis will continue and it is expected that HLA-G alone or in combination with other molecular markers will prove to be useful in future clinical practice.
Acknowledgments
This study is supported by a research grant from the National Institute of Health (CA-103937).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 2.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65(22):10139. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 3.Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, Nesland JM, Shih Ie M, Davidson B. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch. 2006;449(1):31. doi: 10.1007/s00428-005-0144-7. [DOI] [PubMed] [Google Scholar]
- 4.Hansel DE, Rahman A, Wilentz RE, Shih Ie M, McMaster MT, Yeo CJ, Maitra A. HLA-G upregulation in pre-malignant and malignant lesions of the gastrointestinal tract. Int J Gastrointest Cancer. 2005;35(1):15. doi: 10.1385/ijgc:35:1:015. [DOI] [PubMed] [Google Scholar]
- 5.Singer G, Kurman RJ, McMaster M, Shih I-M. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol. 2002;26 (7):914. doi: 10.1097/00000478-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, Grosse-Wilde H, Cheng CC, Kurman RJ, Shih Ie M. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9(12):4460. [PubMed] [Google Scholar]
- 7.Bukur J, Rebmann V, Grosse-Wilde H, Luboldt H, Ruebben H, Drexler I, Sutter G, Huber C, Seliger B. Functional role of human leukocyte antigen-G up-regulation in renal cell carcinoma. Cancer Res. 2003;63(14):4107. [PubMed] [Google Scholar]
- 8.Urosevic M, Kurrer MO, Kamarashev J, Mueller B, Weder W, Burg G, Stahel RA, Dummer R, Trojan A. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159(3):817. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebmann V, Pfeiffer K, Passler M, Ferrone S, Maier S, Weiss E, Grosse-Wilde H. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53(1):14. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- 10.Rebmann V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13(5):371. doi: 10.1016/s1044-579x(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 11.Rebmann V, van der Ven K, Passler M, Pfeiffer K, Krebs D, Grosse-Wilde H. Association of soluble HLA-G plasma levels with HLA-G alleles. Tissue Antigens. 2001;57(1):15. doi: 10.1034/j.1399-0039.2001.057001015.x. [DOI] [PubMed] [Google Scholar]
- 12.Leleu X, Le Friec G, Facon T, Amiot L, Fauchet R, Hennache B, Coiteux V, Yakoub-Agha I, Dubucquoi S, Avet-Loiseau H, Mathiot C, Bataille R, Mary JY. Total soluble HLA class I and soluble HLA-G in multiple myeloma and monoclonal gammopathy of undetermined significance. Clin Cancer Res. 2005;11(20):7297. doi: 10.1158/1078-0432.CCR-05-0456. [DOI] [PubMed] [Google Scholar]
- 13.Gros F, Sebti Y, de Guibert S, Branger B, Bernard M, Fauchet R, Amiot L. Soluble HLA-G molecules increase during acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages. Neoplasia. 2006;8(3):223. doi: 10.1593/neo.05703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebti Y, Le Friec G, Pangault C, Gros F, Drenou B, Guilloux V, Bernard M, Lamy T, Fauchet R, Amiot L. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol. 2003;64(11):1093. doi: 10.1016/j.humimm.2003.08.345. [DOI] [PubMed] [Google Scholar]
- 15.Motherby H, Nadjari B, Friegel P, Kohaus J, Ramp U, Bocking A. Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20(6):350. doi: 10.1002/(sici)1097-0339(199906)20:6<350::aid-dc5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64(11):1017. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 17.Barrier BF, Kendall BS, Sharpe-Timms KL, Kost ER. Characterization of human leukocyte antigen-G (HLA-G) expression in endometrial adenocarcinoma. Gynecol Oncol. 2006;103(1):25. doi: 10.1016/j.ygyno.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Singer G, Rebmann V, Y-C C, Liu H-T, Ali SZ, Reinsberg J, McMaster M, Pfeiffer K, Chan DW, Wardelmann E, Grosse-Wilde H, Kurman RJ, Shih I-M. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460. [PubMed] [Google Scholar]
- 19.Davidson B, Elstrand MB, McMaster MT, Berner A, Kurman RJ, Risberg B, Trope CG, Shih Ie M. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005;96(1):42. doi: 10.1016/j.ygyno.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Ishigami S, Natsugoe S, Miyazono F, Nakajo A, Tokuda K, Matsumoto M, Okumura H, Douchi T, Hokita S, Aikou T. HLA-G expression in gastric cancer. Anticancer Res. 2006;26(3B):2467. [PubMed] [Google Scholar]
- 21.Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood. 2005;105(4):1694. doi: 10.1182/blood-2004-08-3335. [DOI] [PubMed] [Google Scholar]


