Abstract
α2-Macroglobulin (α2M) is a proteinase inhibitor that functions by a trapping mechanism which has been exploited such that the receptor-recognized, activated form (α2M*) can be employed to target antigens to antigen-presenting cells. Another potential use of α2M* is as a drug delivery system. In this study we demonstrate that guanosine triphosphate, labeled with Texas red (GTP-TR) formed complexes with α2M* following activation by proteolytic or non-proteolytic reactions. Optimal incorporation occurred with 20μM GTP-TR, pH 8.0 for 5h at 50°C. NaCl concentration (100mM or 200mM) had little effect on incorporation at this pH or temperature, but was significant at sub-optimum temperature and pH values. Maximum incorporation was 1.2 mol GTP-TR/mol α2M*. PAGE analysis showed that 70-90% of the GTP-TR is bound in a SDS/2-mercaptoethanol resistant manner. Guanosine, adenosine, and imidazole competed with GTP-TR to form complexes with α2M*.
Keywords: α2-macroglobulin and drug delivery, α2-macroglobulin and non-proteolytic incorporation of nucleosides, α2-macroglobulin and incorporation of GTP-Texas red
The human plasma proteinase inhibitor α2-macroglobulin (α2M) is a homotetramer, molecular weight 718kDa, composed of two cage-like “half-molecules” linked by disulfide bonds. α2M inhibits proteinases by a “trapping” mechanism [1,2]. Proteinases first cleave native α2M at the “bait region”, resulting in receptor-recognized, “activated” α2M (α2M*), which has undergone a conformational change trapping the proteinase. These events sterically hinder access of substrates or antibodies to the proteinase [3,4]. Bait region cleavage results in greatly increased reactivity of internal β-cysteinyl-γ-glutamyl thiolesters in each α2M subunit. In this “nascent” state, the exposed thiolesters are labile and undergoes cleavage by nucleophiles [5,8]. Lysine-containing proteinases can form covalent linkages by nucleophilic substitution at the thiolester of the α2M subunits.
Several characteristics of α2M make it a pharmaceutically interesting protein. These include: the diversity of macromolecules that incorporate into α2M* including cytokines, chemokines, growth factors enzymes, or antigens [9-11]; receptor-mediated delivery to target cell types [11-13]; increased immunogeneicity of complexed antigens [7,8,10,14]; and development of a non-proteolytic alternative method of activating α2M [9,15]. Using this method, α2M* incorporates proteins as large as HIV gp 120 (molecular weight, ∼120kDa) or polypeptides as small as 1 kDa, as long as they contained nucleophilic amino acid side chains (Cianciolo and Pizzo, unpublished).
Practical applications of α2M* include it use in vaccines, where α2M functions both as a delivery system and as an adjuvant [8,10,14]. Molecules other than proteins or peptides may incorporate thus serving as a novel drug delivery system. Such an approach would be well suited for hepatic delivery since in vivo clearance studies demonstrate that α2M* is cleared in less than 10 minutes and that greater than 90% of this clearance mediated by hepatocytes and kupffer cells of the liver [16-18].
Nucleosides could be potential candidates for α2M* incorporation as amine groups present in the nucleoside could form covalent linkages by nucleophilic substitution at the thiolester as previously observed for other nucleophiles. Synthetic nucleosides derived from guanosine demonstrate immuno - modulatory and immuno-stimulatory properties and provide anti-tumor and -viral activity by the stimulation of endogenous cytokines [19-26].
The current studies were designed to probe whether guanosine nucleosides can incorporate into α2M* and to develop a general approach demonstrating incorporation of low molecular weight substrates into α2M*. We now report the incorporation into α2M* of fluorescently-labeled guanosine triphosphate (GTP) into α2M*, to date the smallest molecule reported to form complexes with α2M* at the thiolester. We demonstrate that the incorporation of the fluorescently-labeled GTP is both SDS and 2-mercaptoethanol resistant and is not dependent on the fluorescent probe. Furthermore we show that other nucleosides and bases, including guanosine, and imidazole, compete with the fluorescently-labeled GTP for binding with α2M*. This is the first demonstration of incorporation of nucleosides and nucleobases into α2M* and provides a model for studying the incorporation of related compounds of potential therapeutic application.
Material and methods
Materials
Guanosine triphosphate - Texas red (guanosine 5'-triphosphate BODIPY ® TR) and sulforhodamine were from Invitrogen (Carlsbad, CA). Adenosine, adenine, guanosine, guanosine triphosphate guanine, imidazole, iodoacetamide, thymidine, HEPES, NaCl, and porcine pancreatic elastase were from Sigma-Aldrich (St. Louis, MO).
Preparation of human α2M and α2M complexes
Human α2M was purified using endotoxin-free plasma, columns, and buffers [9,15,27]. Activation to α2M* was achieved using ammonium bicarbonate [27]. α2M was also activated using elastase. Elastase and α2M were incubated at a ratio of 10:1 at room temperature for 45 min. Activated α2M* was carboxamidomethylated with10mM iodoacetamide for 15min at room temperature. GTP-TR (20,50 or 200 μM) was incubated with α2M or α2M* at 50°C for 5h or 37°C for 18h in HEPES buffer. The final concentrations of GTP-TR was ∼ 4-, 10-, and 40-fold in excess to α2M. HEPES (25mM) buffers were prepared at pHs of 6.5, 7.0, 8.0 or 9.0 with either 100mM or 200mM NaCl. Following incubation, unincorporated GTP-TR was removed using Micro Bio-Spin® P-30 tris chromatography columns (Bio-Rad, Hercules, CA). The fluorescence of GTP-TR was determined using the detector of a real-time quantitative PCR machine (MX300sp; Stratagene, LaJolla, CA) or a Storm 860 Phosphorimager® (Molecular Devices, Sunnyvale, CA). Fluorescence was converted to GTP concentration using a standard curve. Protein concentration was quantified using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL) and measuring the absorbance at λ = 280nm (extinction coefficient 0.893 M−1 cm−1).
Competition binding studies
GTP-TR was employed as a substrate to investigate whether other low molecular weight molecules form complexes with α2M*. A final concentration of 20μM GTP-TR was co-incubated at 50°C for 5h with adenosine, adenine, guanosine, guanosine triphosphate, guanine, thymidine, or imidazole at 1.0mM. Following incubation, free GTP-TR was separated from the complexes as described above and the fluorescence was quantified. A decrease in fluorescence indicates competition of the low molecular weight molecule with GTP-TR for complex formation with α2M*.
Polyacrylamide gel electrophorersis (PAGE)
Proteins were separated by SDS - PAGE, 4 - 20% polyacrylamide gels (pre-cast Ready Gel; Bio-Rad), using 25mM Tris, 192mM glycine, and 0.1% SDS (w/v; Bio-Rad). Non-denaturing 5% polyacrylamide gels (pre-cast Ready Gel; Bio-Rad) using 130mM tris, 45mM boric acid and 2.5mM ethylenediaminetetraacetic acid were also performed.
Data analysis
All incorporations were repeated a minimum of 5 times. Data presented are the mean ± 1 standard error (SEM). Statistcal analysis and non-linear regression were calculated using Prism 4 (GraphPad Software Inc, San Diego, CA) The stoichiometry of the α2M* – GTP-TR association is referred to as the resultant incorporation ratio and is cited as the number of mol GTP-TR/mol α2M*.
Results
Characterization of GTP binding to α2M*
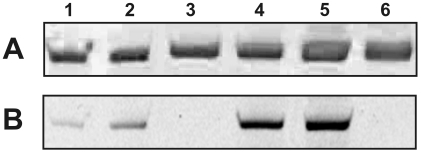
α2M* was incubated with a 100-fold molar excess of GTP-TR at 37°C for 18h. Following incubation, α2M*-GTP-TR complexes were separated from unbound GTP-TR. The samples were analyzed by non-denaturing PAGE (Figure 1) and the fluorescence was quantified. α2M*, because of its decreased Stokes radius, demonstrated greater mobility than native α2M in an electrophoretic field. The presence of GTP-TR was observed in non-proteolytically and proteolytically activated α2M* with ∼ twice the fluorescence in the non-proteolytically as compared to the proteolytically activated α2M* complexes (Figure 1B). Some GTP-TR associated with non-activated α2M, but this was only ∼ 10% of that present in activated α2M*.
Fig. 1.
Electrophoretic analysis by non-denaturing 5% PAGE of complexes of GTP-TR and α2M* formed at 37°C for 18h (A) Coomassie brilliant blue and (B) fluorescence imaging. The lanes are as follows: lane 1 and 2, proteolytically activated α2M* with GTP-TR; lane 3, proteolytically activated α2M*; lane 4 and 5, non-proteolytically activated α2M* with GTP-TR; lane 6, non-proteolytically activated α2M*.
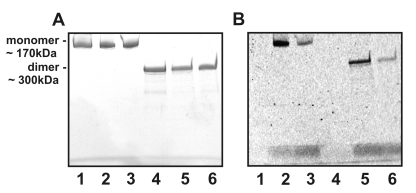
GTP-TR binding to α2M* was observed under SDS-nonreducing and –reducing PAGE (Figure 2). Under SDS-nonreducing conditions there was approximately a 20% decrease in fluorescence associated with α2M*. Under SDS-reducing conditions there was a further ∼40% decrease in fluorescence associated with α2M*. Under both SDS-nonreducing and –reducing conditions the fluorescence associated with carboxamidomethylated α2M* was approximately 70% less than that associated with α2M*. It was not possible to quantify the incorporation ratio of GTP-TR into α2M* using PAGE due to poor reproducibility in the quantification of a known GTP-TR concentration in the polyacrylamide gel. To reliably calculate the resultant incorporation ratio of GTP-TR to α2M* following separation of α2M*-GTP-TR complexes from unbound ligand, fluorescence was quantified using a RT-PCR fluorescence detector and protein concentration determined for each sample.
Fig. 2.
Electrophoretic analysis by nonreducing and reducing 4-20% SDS-PAGE of complexes of GTP-TR and α2M* formed at 37°C for 18h (A) Coomassie brilliant blue and (B) fluorescence imaging. The lanes are as follows: lane 1, nonreducing, non-proteolytically activated α2M*; lane 2, nonreducing, non-proteolytically activated α2M* with GTP-TR; lane 3, nonreducing, non-proteolytically activated carboxamidomethylated α2M* with GTP-TR; lane 4 reducing, non-proteolytically activated α2M*; lane 5, reducing, non-proteolytically activated α2M* with GTP-TR; lane 6, reducing, non-proteolytically carboxamidomethylated activated α2M* with GTP-TR
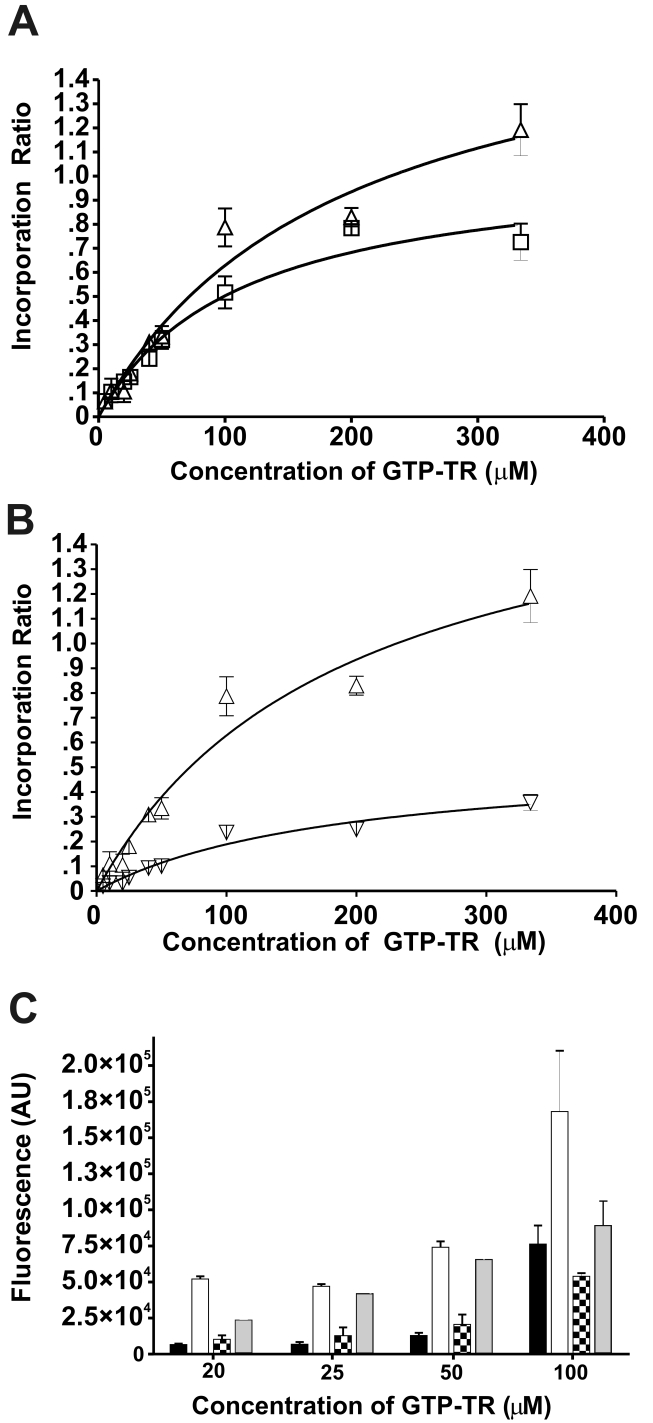
To optimize the conditions for formation of GTP-TR – α2M*, the effect of activation method, initial concentration of GTP-TR, temperature, NaCl concentration, and pH were investigated. The incorporation ratio was dependent on the initial concentration of GTP-TR (Figure 3). Following non-proteolytic activation, the greatest stoichiometry, 1.19 ± 0.11 mol GTP-TR/mol α2M*, was observed at 334 μM GTP-TR at 50°C for 5h (see Figure 3). Incorporation at incubation conditions of 50°C for 5h or 37°C for 18h was not significant (p<0.0001) until initial concentrations of GTP-TR were greater than 50 μM. Repeated separation and addition of GTP-TR (20μM) for four cycles, followed by incubation at 37°C for 18h did not increase the incorporation ratio (data not shown).
Fig. 3.
The effect of initial concentration of GTP-TR and temperature on the (A) resultant incorporation ratio, (B) under native conditions and (C) under nonreducing and reducing conditions. Complexes were prepared following non-proteolytic activation of α2M, by incubation at 37°C for 18h (□,■) or 50°C for 5h (△,▽,▲) . (A) The resultant incorporation ratio is shown as a function of initial GTP-TR concentration following incubation at 37°C for 18h (□) or 50°C for 5h (△). (B) The total incorporation ratio (△) and the SDS-sensitive associated GTP-TR incorporation (▽) are shown as a function of initial GTP-TR concentration following incubation at 50°C for 5h. (C) The fluorescence data are shown following SDS-PAGE analysis under non-reducing conditions at 37°C for 18h ( ) and 50°C for 5h (
) and 50°C for 5h ( ) or under reducing conditions at 37°C for 18h (
) or under reducing conditions at 37°C for 18h ( ) and 50°C for 5h (
) and 50°C for 5h ( ) as a function of initial concentration of GTP-TR. All data points are the mean ± 1 SEM, with n=6.
) as a function of initial concentration of GTP-TR. All data points are the mean ± 1 SEM, with n=6.
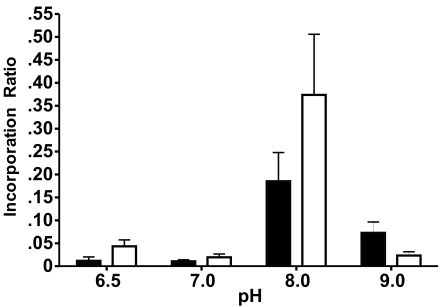
There was greater GTP-TR fluorescence associated with α2M* under nonreducing and reducing conditions following incubation at 50°C for 5h compared to incubation at 37°C for 18h (Figure 3C). Following incubation at 37°C for 18h, with 20μM, 25μM, and 50μM GTP-TR, the fluorescence associated under nonreducing conditions was approximately twice that observed under reducing conditions. Following incubation at 50°C for 5h, with 20μM, 25μM, and 50μM GTP-TR, the fluorescence associated under non-reducing conditions was approximately tenfold of that observed under reducing conditions. At both temperatures and durations of incubation, increasing the initial concentration to greater than 50μM increased the proportion of fluorescence observed under reducing conditions. The optimal pH for incorporation was 8.0. NaCl concentration did not effect the resultant incorporation ratio (p<0.0003) (Figure 4).
Fig. 4.
The effect of pH and NaCl concentration on the resultant incorporation ratio of GTP-TR with α2M* Complexes were prepared following non-proteolytic activation of α2M, by incubation of α2M* with a 40-fold molar excess GTP-TR at 37°C for 18h, in 25mM HEPES buffer solutions of pH 6.5, 7.0, 8.0 and 9.0 with NaCl concentration of 100mM ( ) or 200mM (
) or 200mM ( ). All data points are the mean ± 1 SEM, with n=6.
). All data points are the mean ± 1 SEM, with n=6.
Incorporation of low molecular weight molecules into non-proteolytically activated α2M*
Non-proteolytically activated α2M* was incubated with sulforhodamine at 37°C for 18h. Following separation from unbound sulforhodamine, no fluorescence was observed using the fluorescence detector. Using PAGE analysis, sulforhodamine association with α2M* was less than 4% of the GTP-TR equivalent control. No fluorescence was observed under SDS- nonreducing conditions (data not shown).
GTP-TR was used as an indicator to investigate whether other small molecular weight molecules can compete to form complexes with non-proteolytically activated α2M*, we compared the fluorescence observed following incubation of α2M* with 20μM GTP-TR to the fluorescence observed following the co-incubation of α2M* with 20μM GTP-TR and the competing low molecular weight molecule. Competition with GTP, guanosine, imidazole, and thymidine resulted in a significant decrease in GTP-TR fluorescence (Table I). Comparative experiments were performed in which α2M* was omitted and no decrease in fluorescence was observed (data not shown). SDS-PAGE analysis showed that competition with 1mM guanosine and imidazole resulted in a reduction in fluorescence associated with α2M* under SDS- non-reducing and reducing conditions. The reduction in fluorescence associated with α2M* under SDS nonreducing conditions was twice that under reducing conditions (data not shown).
Table 1.
Competition of low molecular weight molecules with GTP-TR for formation of complexes with α2M*. A 50-fold molar excess (1mM) of competing molecule was co-incubated with GTP-TR (20μM) and non-proteolytically activated α2M* for 5h at 50°C in 25mM HEPES pH 8.0, 200mM NaCl following which unbound ligand was separated and the fluorescence of the α2M* complex was quantified. A decrease in fluorescence indicates competition of the low molecular weight molecule with GTP-TR for formation of a complex with α2M*. All data points are the mean ± 1 SEM, with n=6.
| Compound | Percentage fluorescence | P- value |
|---|---|---|
| GTP-TR | 100 | - |
| GTP | 50 | 0.001 |
| Guanosine | 65 | 0.01 |
| Guanidine | 79 | 0.12 |
| Adenosine | 68 | 0.44 |
| Adenine | 89 | 0.14 |
| Thymidine | 74 | 0.02 |
| Imidazole | 74 | 0.02 |
Discussion
These studies were undertaken to determine if the receptor-recognized form of α2M, α2M*, could incorporate small molecules such as nucleosides. Such incorporation is not only of mechanistic interest, but also would suggest that α2M* could serve as a delivery system for nucleoside-derived drugs. Here we demonstrate that the fluorescently-labeled nucleoside analogue GTP-TR incorporates into α2M* made either through a proteolytic activating mechanism or by nucleophilic exchange. Moreover, a simple technique has been developed that utilizes fluorescently-labeled GTP incorporated in α2M* to demonstrate, by competition analysis, that other nucleoside derivatives can also incorporate into α2M*.
Molecules can bind to α2M by a variety of mechanisms. For example: (i) cytokine binding is mediated by hydrophobic interactions, disulfide linkages, and interactions with zinc; (ii) proteinase binding is both non-covalent and is also mediated by ε(γ-glutamyl)lysine linkage; and (iii) virus binding involves the terminal sialic acids on carbohydrate side chains [12]. We have previously presented the mechanism by which molecules incorporate into α2M* [27,28]. Incorporation is meditated through nucleophile exchange at Gln952 or thiol disulfide exchange at Cys949. Under reducing conditions ligands bound to the Cys949 residue dissociate, but ligands bound to the Glx remain associated. 70-90% of GTP-TR was associated to α2M* in a SDS and 2-mercaptoethanol-resistant manner. Previous studies of the structure of α2M* have indicated that the thiol ester is a site for covalent attachment to α2M*; for example, the attachment of the protease to the inhibitor [29-31]. Our data indicate that GTP-TR associates with α2M* in a similar covalent manner. This covalent incorporation is independent of the initial concentration of GTP-TR and of incubation conditions of either 50°C for 5h or 37°C for 18h. α2M* incubated with iodoacetamide is modified by carboxyaimdomethylation at Cys949 residue. Non-denaturing PAGE and SDS/PAGE of α2M* and carboxamidomethylation α2M* revealed that the majority of GTP-TR is bound to the thiol groups. The binding of GTP-TR to the thiol ester differs from the incorporation of cadaverine (a diamine molecule, molecular weight, 102) where studies showed that the majority was not bound to the thiol ester but to the Gln residues [32]. Increasing the initial concentration of GTP-TR above 50μM resulted in a greater proportion of the GTP-TR being associated with the Cys949 residue. Temperature and duration of incubation also affected the distribution of GTP-TR to the Gln952 or the Cys949 residues; at a concentration of 20-50 μM and a temperature of 50°C, a greater proportion of GTP-TR was associated with the Glx, whereas at 37°C the distribution between the Gln952 and Cys949 residue was approximately equal.
A non-proteolytic method of activating of α2M has been developed by our laboratory. This method of activation of α2M would be significantly cheaper and easier to implement for pharmaceutical use than the use of proteolytic enzymes to activate α2M [9,15]. The resultant incorporation ratio of GTP-TR/α2M* is dependent on the method of activation of α2M. Following non-proteolytic activation there was greater GTP-TR incorporation than observed following proteolytic activation. The method of activation of α2M is known to influence the final incorporation ratio of ligands [9]. Using GTP-TR as an indicator to investigate whether guanosine derivatives can form complexes with α2M*, there was the possibility that the fluorescent indicator, Texas red (TR), could itself have been responsible for the association with α2M*. Small amounts of sulforhodamine, the parent fluorophore of Texas Red, were shown to associate with α2M*, but this association was SDS-sensitive. As shown in Figure 2 however, GTP-TR forms SDS and 2-mercaptoethanol resistant covalent associations with α2M* and thus the fluorescent probe is not responsible for the SDS and 2-mercaptoethanol resistant covalent binding of the GTP-TR. As shown in Table 1, guanosine and other nucleosides and nucleobases were able to compete with GTP-TR to form complexes with α2M*. This indicates that it is neither the Texas red nor the phosphate groups that are responsible for the association. The GTP-TR fluorescence decreased from 100% to approximately 70% with each competing molecule. This is equivalent to an incorporation ratio of non-fluorescently labeled nucleosides or bases that of approximately 0.03.
Using SDS-PAGE under nonreducing or reducing conditions it was possible to determine that guanosine competes with GTP-TR for binding both at the Cys949 residue and the Gln952 residue. Possible interactions between α2M* and guanosine could be due to nucleophilic attack by: the primary amine at C2, the 2′ and 3′ hydroxyl groups, or the amide at C6. The competition for complex formation may be due not only to nucleophilic attack but to additional interactions: for example (and not limited to), hydrogen bond formation with the 2′-hydroxyl group. To investigate the nature of the interaction with α2M*, imidazole was co-incubated with α2M * and GTP-TR. Imidazole does not contain either the sugar or the amide group. Co-incubation with imidazole resulted in decreased fluorescence similar to that observed following co-incubation with guanosine. This suggests that the amide group performs a role in the competition for complex formation with α2M*. Nonreducing and reducing SDS-PAGE analysis of imidazole competition complexes showed that imidazole, like guanosine competes for association with α2M*, with equal competition at the Glx residue and the Cys949 residues.
In this report we have demonstrated that nucleosides and bases compete with GTP-TR for formation of complexes with α2M*. Although the incorporation ratios of the nucleosides or bases was low, covalent association with α2M* was demonstrated using PAGE analysis following incubation with SDS or SDS and 2-mercaptoethanol. With optimization of incubation conditions, and by chemical modification of the nucleosides, the association with α2M* should be capable of being improved, allowing α2M* to be considered as a novel drug delivery system.
Acknowledgements
This work was supported by NHLBI, National Institutes of Health, Grant HL-24066.
Abbreviations Used
- α2M
α2- macroglobulin
- TR
Texas red
Footnotes
This work was supported by NHLBI, National Institutes of Health, Grant HL-24066.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swenson RP, Howard JB. Structural characterization of human α2-macroglobulin subunits. J. Biol. Chem. 1979;254(44):52–4456. [PubMed] [Google Scholar]
- 2.Barrett AJ, Starkey PM. The interaction of α2-macroglobuiln with proteinases: characteristics and specifity of the reaction, and a hypothesis concerning it molecular mechanism. Biochem J. 1973;122:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barret AJ, Brown MA, Saayer CA. The electrophoretically ‘slow’ and ‘fast’ forms of the α2-macroglobulin molecule. Biochem J. 1979;181:401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sottrup-Jensen L, Lonblad PB, Stepanick TM, Petersen TE, Magnusson S, Jornvall H. Primary structure of the ‘bait’ region for proteinases in α2-macroglobulin: nature of the complex. FEBS Lett. 1981;127(1):67–173. doi: 10.1016/0014-5793(81)80197-4. [DOI] [PubMed] [Google Scholar]
- 5.Sottrup-Jensen L, Petersen TE, Magnusson S. Trypsin-induced activation of the thiol esters in α2-macroglobulin generates a short-lived intermediate (‘nascent’ α2-macroglobulin) that can react rapidly to incorporate not only methylamine or putrescine but also proteins lacking proteinase activity. FEBS Lett. 1981;128:123–126. doi: 10.1016/0014-5793(81)81096-4. [DOI] [PubMed] [Google Scholar]
- 6.James K. Interactions between cytokines and α2-macroglobulin. Immunol. Today. 1990;11:163. doi: 10.1016/0167-5699(90)90067-j. [DOI] [PubMed] [Google Scholar]
- 7.Chu CT, Pizzo SV. Receptor-mediated antigen delivery into macrophages. Complexing antigen to α2-macroglobulin enhances presentation to T cells. J. Immunol. 1993;150:48–58. [PubMed] [Google Scholar]
- 8.Liao H-X, Cianciolo GJ, Staats HF, Scearce RM, Lapple DM, Stauffer SH, Tomasch JR, Pizzo SV, Montefiori DC, Hagen M, Eldridge J, Haynes BF. Increased immunogenicity of HIV envelope subunit complexed with α2-macroglublin when combine with monosphoryl lipid A and GM-CSF. Vaccine. 2002;20:2396–2403. doi: 10.1016/s0264-410x(02)00090-7. [DOI] [PubMed] [Google Scholar]
- 9.Grøn H, Pizzo SV. Nonproteolytic incorporation of protein ligands into human α2-macroglobulin; implications for the binding mechanisms of α2-macroglobulin. Biochemistry. 1998;37:6009–6014. doi: 10.1021/bi973027c. H. [DOI] [PubMed] [Google Scholar]
- 10.Cianciolo GJ, Enghild JJ, Pizzo SV. Covalent complexes of antigen and α2-macroglobulin: evidence for dramatically-increased immunogenicity. Vaccine. 2001;20:554–562. doi: 10.1016/s0264-410x(01)00361-9. [DOI] [PubMed] [Google Scholar]
- 11.Pizzo SV, Gonias SL. Receptor-mediated protease regulation. In: Conns PM, editor. The Receptors. Academic Press; Orlando: 1984. pp. 177–211. [Google Scholar]
- 12.Borth W. α2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 13.Chu CT, Howard GC, Mirsa UK, Pizzo SV. α2-Macroglobulin: a sensor for proteolysis. Ann. NY. Acad Sci. 1994;10:291–307. doi: 10.1111/j.1749-6632.1994.tb44319.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu CT, Oury TD, Enghild JJ, Pizzo SV. Adjuvant-free in vivo targeting. Antigen delivery by α2-macroglobulin enhance antibody formation. J. Immunol. 1994;15:1538–1544. J.J. [PubMed] [Google Scholar]
- 15.Grøn H, Thorgersen I, Enghild JJ, Pizzo SV. Structural and functional analysis of spontaneous re-formation of the thiol ester bind in human α2-macroglobulin, rat α1-inhibitor-3 and chemically modified derivatives. Biochem J. 1996;318:539–545. doi: 10.1042/bj3180539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic “fast” forms of human α2-macroglobulin. J. Biol. Chem. 1981;256:8134–8139. [PubMed] [Google Scholar]
- 17.Gliemann J, Larsen TR, Sottrup-Jensen L. Cell association and degradation of α2-macroglobulin-trypsin complexes in hepatocytes and adipocytes. Biochim. Biophys. Acta. 1983;756:230–237. doi: 10.1016/0304-4165(83)90096-x. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SR, Rosenburg MR, Ney KA, Michalopoulos G, Pizzo SV. Binding of α2-macroglobulin to hepatocytes: Mechanism of in vivo clearance. Biochem. Biophys. Res. Comm. 1985;128:795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara K, Anderson JD, Kini GD, Dalley K, Larson SB, Smee DF, Jin J, Sharma BS, Jolley WB, Robins RK, Cottam HB. Thiazole[4,5-d]pyrimidine nucleosides. The synthesis of certain 3-β-D-ribofuranosylthiazolo[4,5-d]purimidines as potential immuno-therapeutic agents. J. Med. Chem. 1990;33:407–415. doi: 10.1021/jm00163a064. [DOI] [PubMed] [Google Scholar]
- 20.Testerman TL, Gerster JF, Imberston LM, Reter MJ, Miller RL, Gibson SJ, Wagner TL, Tomai MA. Cytokine induction by the immunomodulators imiquimod and S27609. J. Leukoc. Biol. 1995;58:365–372. doi: 10.1002/jlb.58.3.365. [DOI] [PubMed] [Google Scholar]
- 21.Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 2001;48:751–755. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 22.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nature Immunol. 2002;3:196–200. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizama H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR-7 MyD88-dependent signaling pathway. Nature Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Chuang T-H, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer T, Nindl I, Schmook T, Ulrich C, Sterry W, Stockfelth E. Induction of apoptosis by Toll-like receptor 7 agonist in tissue cultures. Br. J. Dermatol. 2003;149:9–13. doi: 10.1046/j.0366-077x.2003.05632.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, Gibson SJ, Imberston LM, Jacobson JR, Knafla RT, Maye PV, Nikolaides N, Oneyemi FY, Parkhurst GJ, Pecore SE, Reiter MJ, Scribner LS, Testerman TL, Thompson NJ, Wagner TL, Weeks CE, Andre J-D, Lagain D, Bastard Y, Lupu M. Synthesis and structure – activity – relationships of 1H-imidazol[4,5,-c]quinolines that induce interferon production. J. Med. Chem. 2005;48:3481–3491. doi: 10.1021/jm049211v. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee G, Grøn H, Pizzo SV. Incorporation of non-proteolytic proteins by murine α2-macroglobulin. Biochim. Biophys. Acta. 1999;1432:49–56. doi: 10.1016/s0167-4838(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 28.Chu CT, Pizzo SV. Biology of disease α2-macroglobulin, complement, and biological defense” Antigens, growth factors, microbial proteases and receptor ligation. Lab. Invest. 1994;71:792–812. [PubMed] [Google Scholar]
- 29.Swenson RP, Howard JB. Characterization of alkylamine-sensitive site in α2-macroglobulin. Proc. Natl. Acad. Sci. USA. 1979;76:4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant JB, Vermeulen M, Swenson RP. The temperature-sensitive bond in human α2-macroglobulin is the alkyamine-reactive site. J. Biol. Chem. 1980;255:3820–3823. [PubMed] [Google Scholar]
- 31.Harpel P, Hayes MB, Hugli TE. Heat-induced fragmentation of human α2-macroglobulin. J. Biol.Chem. 1979;254:8669–8678. [PubMed] [Google Scholar]
- 32.Mortensen SB, Sottrup-Jensen L, Hansen HF, Rider D, Petersen TE, Magnusson S. Sequence location of putative tranglutaminase crosslinking site in human α2-marcoglobulin. FEBS Lett. 1981;129:314–317. doi: 10.1016/0014-5793(81)80191-3. [DOI] [PubMed] [Google Scholar]