Abstract
Objective:
To investigate vascular reactivity in heterozygous and homozygous offspring with a genetic predisposition for hypertension after postnatal cross fostering to mothers with the opposite genetic inheritance of the NOS3 knockout allele.
Study design:
Homozygous NOS3 knockout (C57BL/6J-NOS3−/−KO) and wild-type mice (NOS3+/+WT) were bred to obtain heterozygous litters with a paternally-derived (NOS3+/−pat) or maternally-derived (NOS3+/−mat) knockout allele. After delivery, heterozygous and homozygous litters were cross fostered to a mother with the opposite NOS3 gene status. Carotid arteries were placed in a wire myograph for isometric tension recording using contractile and relaxant agents. Statistical analysis with One-Way ANOVA and Neuman-Keuls post-hoc testing was performed.
Results:
Increased sensitivity to phenylephrine and absent relaxation to acetycholine in NOS3+/−mat was reversed with cross-fostering and vasorelaxation to isoproteronol was increased. Contraction to calcium was increased in the cross fostered paternally-derived and wild-type litters.
Conclusion:
Postnatal interventions may favorably alter the adult vascular profile resulting from an abnormal intrauterine environment.
Keywords: cross fostering, hypertension, fetal programming, vascular reactivity
Introduction
As hypothesized by Barker, stimuli or insults to the fetus during critical periods of development lead to ‘fetal programming’ and produce adaptive changes in the fetal anatomy, physiology and metabolism that have long-term consequences.1,2 While the epidemiological studies have demonstrated the role of the “in utero” environment in the fetal origins of adult diseases, a role for genetic factors cannot be excluded. Some investigators even argue that the association between low birth weight and adult disease is largely due to genetics rather than an unfavorable uterine environement.3
In prior studies from our lab, we used transgenic mice lacking functional endothelial nitric oxide synthase (NOS3) as an animal model of altered vascular adaptation to pregnancy in order to test the hypothesis regarding the fetal origin of adult vascular diseases. In the vasculature, NOS3 is the main isoform responsible for the production of nitric oxide (NO), a potent a vasorelaxant involved in the regulation of vascular tone.4,5 In pregnancy, NO is believed to play a significant role in maintaining adequate uteroplacental perfusion through vasodilation of the placental vasculature and alteration in NO production has been implicated in the onset of intrauterine growth restriction and hypertension.6 In the absence of NOS3, as seen in NOS3 knockout mice, arteries have been shown to exhibit increased contraction to phenylephrine and calcium, and absent or decreased relaxation to acetylcholine.7 To discern the contribution of a maternally-derived or paternally-derived knockout allele to the adult vascular profile, endothelial nitric oxide synthase-knockout (NOS3−/−KO) and wild-type (NOS3+/+WT) mice were bred to produce homozygous NOS3−/−KO, maternally-derived heterozygous (NOS3+/−mat; one abnormal allele derived from the mother), paternally-derived heterozygous (NOS3+/−pat; one abnormal allele derived from the father), and homozygous NOS3+/+WT litters.7
Despite being genomically similar, the vascular reactivity in the heterozygous adult offspring depended on the maternal genetic status and, by extension, their uterine environment. The vascular characteristics in the NOS3+/−mat offspring, which developed in a hypertensive NOS3−/−KO mother possessing an abnormal uterine environment, were similar to NOS3−/−KO offspring. Conversely, the vascular function in the genetically identical NOS3+/−pat offspring, having developed in a normal uterine environment of a NOS3+/+WT mother, were similar to NOS3+/+WT offspring.7 The results in this animal model of altered vascular adaptations to pregnancy demonstrated a ‘fetal programming’ effect of the abnormal uterine environment with impact on future vascular reactivity in the offspring.7 Further experiments using embryo transfer confirmed that the altered vascular function in the offspring was mostly the result of the abnormal uterine environment, rather than the parental origin of the NOS3 mutation.8
It is becoming evident that the postnatal period can also alter programming of adult diseases. ” In our NOS3 mice model of fetal vascular programming, both intrauterine and postnatal environments of the naturally fostered animals were determined by the maternal NOS3 status. Since the postnatal environment in our model may be affected by the maternal NOS3 status, we sought to determine the effect of cross fostering to the opposite maternal NOS3 status on vascular function in the offspring. Hence, our aim was to discriminate between the contributions of the intrauterine and postnatal environments to vascular function in adult life by cross fostering offspring to a mother with the opposite maternal NOS3 status. Lack of a functional NOS3 gene in the mother may affect the quality and quantity of postnatal feeding in addition to abnormal maternal behavior during rearing of offspring. We hypothesized that alteration of the postnatal environment through cross fostering to a foster mother with a NOS3 status that is opposite to the NOS3 status of the natural mother will alter the vascular profile of the heterozygous offspring in the direction of the cross fostered environment.
Materials and Methods
Animals
Mature cycling female and male mice (4-6 weeks old) homozygous for disruption of the NOS3 gene (NOS3−/−KO, strain B6.129P2-Nos3tm1Unc, stock no. 002684) and their age-matched wild-type controls (NOS3+/+WT, strain C57BL/6J, stock no. 000664) were obtained from Jackson Laboratory (Barharbour, Maine). The mice were housed separately in temperature and humidity controlled quarters with constant light:dark cycles of 12h:12h. They were provided with food and water ad libitum. The same chow was used for all animals, including the offspring post-weaning. Regular maintenance and care was provided by certified personnel and veterinary staff according to the guidelines of the Animal Care and Use Committee (ACUC).
Male and female homozygous wild-type and knockout mice were bred to produce heterozygous litters developing in either a normal (paternally-derived; NOS3+/−pat) or abnormal (maternally-derived; NOS3+/−mat) uterine environment (Figure 1). Within 24 hours of birth, the first generation homozygous and heterozygous offspring were cross fostered to an adult foster mother having the opposite homozygous genetic expression of the NOS3 gene when compared to their natural mother (Figure 1). As such, the NOS3+/−mat and NOS3−/−KO offspring were cross fostered to a NOS3+/+WT mother to produce NOS3FWT/−mat and NOS3FWT/−KO litters respectively, while NOS3+/−pat and NOS3+/+WT were cross-fostered to a NOS3−/−KO mother to produce NOS3FKO/−pat and NOS3FKO/+WT litters respectively. The male and female pups from each cross fostered litter were weighed jointly every other day until day 21 of life. At this time, they were removed from their foster mothers and separated into different cages according to their sex where they remained to feed and grow until 10-11 weeks of adult life. The pups were weighed weekly from weeks 4-9 of life. The findings in the four groups of cross fostered animals were evaluated in the context of our previously published findings in the naturally fostered first generation offspring.
Figure 1.
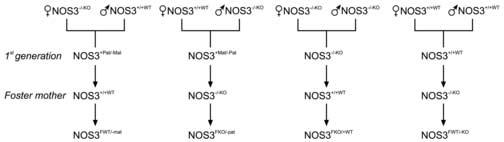
Schematic diagram of breeding of first generation heterozygous and homozygous mice from homozygous wild-type and knockout mice. The litters from the first generation were cross fostered to homozygous wild-type and knockout mothers to obtain the offspring used in the experiments.
In vitro experiments
At 10-11 weeks of life, the female animals were sacrificed by CO2 inhalation per the ACUC guidelines as well as the American Veterinary Medical Association guidelines. All procedures were carried out after approval by the ACUC of the University of Texas Medical Branch. Two-millimeter segments of the carotid arteries (usually 2-4 per animal) were mounted on a wire myograph (Model 410A, J.P. Trading I/S, Aarhus, Denmark) using 25-μm tungsten wires. The vessels were bathed in physiological salt solution maintained at 37°C, pH ∼7.4. A mixture of 95% O2 and 5% CO2 was bubbled continuously through the solution. The force was continuously recorded by an isometric force transducer and analyzed using PowerLab data acquisition and playback software (ADInstruments, Castle Hill, NSW, Australia).
The optimal diameter (OD) determined in each vessel was used for contractility studies. After stabilization of the tone, the vessels were contracted twice with 60 mmol/L KCl for 30 minutes to stabilize vascular responsiveness. The second contraction was used as the reference contraction in the final calculations. After one hour equilibration in physiological salt solution, contractile responses to the α1-adrenergic agonist phenylephrine (PE, 10−10-10−5 mol/L) were assessed in the presence and absence of a non-selective NOS inhibitor (Nω-nitro-L-arginine methyl ester, L-NAME). In addition, relaxant responses to endothelium-dependent acetylcholine (Ach, 10−10-10−5 mol/L) and β1-adrenergic agonist isoproteronol (Isop, 10−10-10−5 mol/L), were evaluated after precontraction of the vessels with PE (10−7-10−5 mol/L) in order to produce matching contractions in the different study groups. Finally, contractile responses to cumulative concentrations of Ca++ (0.05-5.0 mmol/L) were studied after equilibration of the vessels in a high-K+, Ca++-free solution (with EGTA added) in order to evaluate vascular smooth muscle responsiveness. Refer to Table 1 for contractile and relaxant agents used.
Table I.
Compounds used to investigate selected pathways involved in the regulation of vascular function.
| Compound | Type | Action | Dose |
|---|---|---|---|
| PE | Ά1-adrenergic agonist | Smooth muscle contraction | 10−10–10−5 M |
| L-NAME | NO antagonist | Nonselective NOS inhibitor | 10−4 M |
| Ach | Muscarinic-receptor agonist |
Endothelium-dependent- vasorelaxation |
10−10–10−5 M |
| Isop | β1-agonist | Endothelium-independent vasorelaxant |
10−10–10−5 M |
| Ca | Contractile agent | Smooth muscle contraction | 0.05–5.0 mM |
PE, Phenylephrine; Ach, Acetylcholine; Isop, Isoproteronol; Ca; Calcium
Data Analysis
Data are expressed as the mean ± SEM. Response to KCl was used as a reference to calculate the percentage of contraction achieved by the contractile agents studied, while phenylephrine precontraction was used to obtain the percentage of relaxation induced by the vasorelaxant agents used. For the vascular reactivity studies using acetylcholine, the area under the concentration response curve (AUC), logarithm of the concentration producing 50% of the maximal effect (log IC50, a measure of sensitivity to the agent), and the maximal effect were calculated. Data were compared between the various groups using one-way ANOVA followed by Student Neuman-Keuls test for multiple comparisons. A probability of < 0.05 was considered significant.
Drugs and solutions
The drugs were chosen based on their usual use to probe various vascular functions (Table I). They have been previously used in our laboratory in experiments with naturally fostered litters to establish dose-response protocols and the correct concentrations needed to evaluate vascular function in transgenic mice lacking functional NOS3. The drugs used include acetylcholine hydrochloride (Ach), phenylephrine hydrochloride (PE), Nω-nitro-L-arginine methyl ester (L-NAME), isoproteronol (Isop), and calcium (Ca2+) (Sigma, St. Louis, MO). Stock solutions of drugs at 10−2 mol/L were prepared with deionized water and stored at −20°C. Physiological salt solution was composed of 115 NaCl mmol/L, 5 KCl mmol/L, 1.2 NaH2PO4 mmol/L, 25 NaHCO3 mmol/L, 1.2 MgCl2 mmol/L, 2.5 CaCl2 mmol/L, 0.026 ethylenediaminetetraacetic (EDTA) mmol/L and 11 glucose mmol/L. For experiments with high-K+, Ca2+-free physiological salt solution (80 mmol/L K+), Ca2+ was omitted and NaCl was replaced by K+ in order to maintain osmolality of the solution.
Results
Average postnatal litter weight
Pups from each litter were weighed jointly (males and females) from birth to week 3 of life. There were no differences amongst the cross fostered groups in average weight per litter from birth to day 4 of life. The average weight was significantly higher in the NOS3FKO/+WT litters when compared to all other cross fostered groups from days 5-19 of life. The average weight was significantly higher in the NOS3FKO/−pat when compared to NOS3FWT/−mat and NOS3FWT/−KO from days 5-13 of life. The average weight was significantly higher in the NOS3FWT/−mat when compared to NOS3FWT/−KO from days 5-17 of life (Figure 2A).
Figure 2.
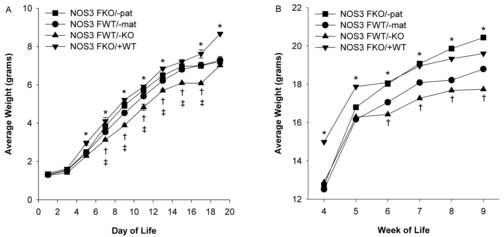
Figure 2A. Average daily weight (grams) of cross fostered offspring from day 1 to 19 of life in NOS3FKO/−pat (n=17), NOS3FWT/−mat (n=19), NOS3FWT/−KO (n=13), and NOS3FKO/+WT (n=10) male and female pups. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/+WT vs. all other groups, †P < 0.05 NOS3FKO/−pat vs. NOS3FWT/−mat and NOS3FWT/−KO, ‡P < 0.05 NOS3FWT/−mat vs. NOS3FWT/−KO.
Figure 2B. Average weekly weight (grams) of female offspring from weeks 4 to 9 of adult life in NOS3FKO/−pat (n=7), NOS3FWT/−mat (n=12), NOS3FWT/−KO (n=8), and NOS3FKO/+WT (n=8). One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/+WT vs. all other groups, †P < 0.05 NOS3FKO/−pat vs. NOS3FWT/−mat and NOS3FWT/−KO.
From weeks 4-9 of life, the female offspring were separated from the male offspring and weighed weekly. The average weight of female offspring was significantly higher in the NOS3FKO/+WT in weeks 4 and 5 when compared to all other cross fostered groups. After week 5, the average weight of NOS3FKO/+WT was significantly higher than NOS3FWT/−mat and NOS3FWT/−KO. The average weight was significantly higher in the NOS3FKO/−pat from weeks 6-8 when compared to NOS3FWT/−mat and NOS3FWT/−KO. At week 9, NOS3FKO/−pat weighed significantly more than all other cross fostered groups (Figure 2B).
In vitro reactivity of the carotid arteries
Phenylephrine contraction and its maximal effect in carotid arteries from female offspring were significantly increased in the NOS3FWT/−KO compared to the other groups. NOS3FWT/−mat and NOS3FKO/−pat had similar contractile responses at all concentrations of phenylephrine with similar maximal effects. NOS3FKO/+WT had the most favorable response and a significantly lower maximal effect. In the presence of L-NAME, responses to phenylephrine in the NOS3FWT/−mat, NOS3FKO/−pat and NOS3FKO/+WT offspring increased and became similar to response in NOS3FWT/−KO (Figures 3A/B and Table 2).
Figure 3.
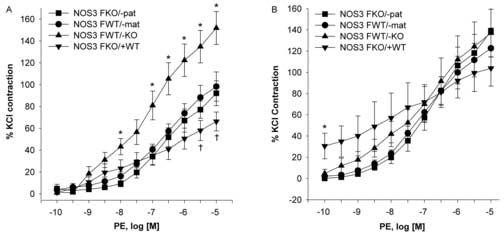
Figure 3A. Phenylephrine (PE) concentration-response curves in the carotid arteries of NOS3FKO/−pat (n=8), NOS3FWT/−mat (n=7), NOS3FWT/−KO (n=8), and NOS3FKO/+WT (n=6) female mice. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FWT/−KO vs. all other groups. †P < 0.05 NOS3FKO/+WT vs. all other groups.
Figure 3B. Phenylephrine (PE) concentration-response curves in the carotid arteries of NOS3FKO/−pat (n=8), NOS3FWT/−mat (n=7), NOS3FWT/−KO (n=8), and NOS FKO/+WT (n=6) female mice in the presence of the non-specific NOS inhibitor L-NAME. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/+WT vs. all other groups.
Table II.
Maximal Effect in the carotid arteries of the female cross fostered offspring at 10–12 wks of age (n = 4–10 mice in each group).
| Offspring | PE | L-NAME | Ach | Isop | Ca |
|---|---|---|---|---|---|
| NOS3FWT/−mat | 98.25±13.39 | 122.68±19.09 | −83.71±1.42‡ | −74.64±2.63 | 2.88±0.20 |
| NOS3FKO/−pat | 92.13±11.42 | 138.85±24.39 | −95.17±1.95 | −94.25±4.46§ | 4.17±0.24§ |
| NOS3FKO/+WT | 66.29±8.65† | 103.93±16.85 | −90.18±2.89 | −73.27±3.80 | 3.83±.047 |
| NOS3FWT/−KO | 151.88±15.04* | 137.03±22.40 | 18.28±5.03* | 63.59±6.35 | 3.08±0.62 |
PE, Phenylephrine; Ach, Acetylcholine; Isop, Isoproteronol; Ca; Calcium
Maximal effect is expressed as the percentage of the reference contraction to 60 mM KCl or PE precontraction as appropriate.
P < 0.05 for NOS3FWT/−KO vs. all.
P < 0.05 is for NOS3FKO/+WT vs. all.
P < 0.05 is for NOS3FWT/−mat vs. all.
P < 0.05 is for NOS3FKO/−pat vs. all.
Acetylcholine did not relax carotid arteries from NOS3FWT/−KO. NOS3FWT/−mat had significantly decreased relaxation to acetylcholine when compared to NOS3FKO/−pat and NOS3FKO/+WT, but overall had a similar profile as NOS3FKO/−pat. The maximal effect in NOS3FWT/−mat was significantly decreased when compared to NOS3FKO/−pat and NOS3FKO/+WT. In addition, the AUC and IC50 for acetylcholine was significantly decreased in NOS3FWT/−mat when compared to NOS3FKO/−pat and NOS3FKO/+WT. NOS3FKO/−pat and NOS3FKO/+WT had a similar response to acetylcholine and maximal effects (Figure 4 and Tables 2 and 3).
Figure 4.
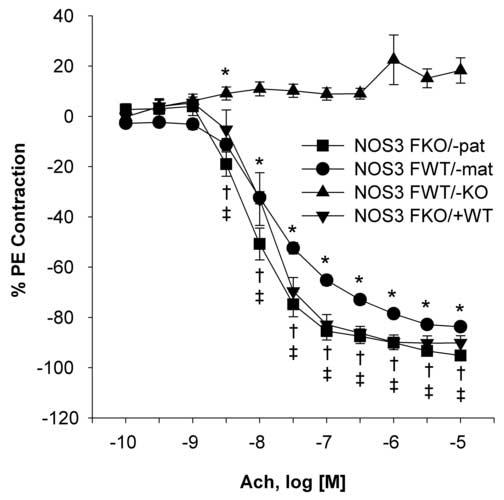
Acetylcholine (Ach) concentration-response curves in the carotid arteries of NOS3FKO/−pat (n=10), NOS3FWT/−mat (n=10), NOS3FWT/−KO (n=7), and NOS3FKO/+WT (n=4) female mice. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/−pat vs. NOS3FWT/−mat and NOS3FWT/−KO, †P < 0.05 NOS3FKO/+WT vs. NOS3FWT/−mat and NOS3FWT/−KO, ‡P < 0.05 NOS3FWT/−mat vs. NOS3FWT/−KO.
Table III.
Area under the acetylcholine concentration-relaxation curves (AUC; arbitrary units) and logarithm of the molar concentration that produces 50% relaxation (log IC50; a measure of sensitivity) in the carotid arteries of cross fostered female offspring (n = 4–10 mice in each group).
| Offspring | AUC | Log IC50 |
|---|---|---|
| NOS3FWT/−mat | 229.65±5.78* | 7.66±0.04* |
| NOS3FKO/−pat | 286.73±11.80 | 8.07±0.09 |
| NOS3FKO/+WT | 262.81±13.57 | 7.91±0.13 |
AUC and IC50 were not calculated for the NOS3FWT/−KO because of the contractile response to acetylcholine.
P < 0.001 for NOS3FWT/−mat vs. NOS3 FWT/−pat.
Response to the vasorelaxant β-adrenoreceptor agonist isoproteronol was significantly lower in NOS3FKO/+WT when compared to the other groups. NOS3FWT/−KO and NOS3FWT/−mat had more relaxation to isoproteronol, but the maximal effects were similar to NOS3FKO/+WT. NOS3FKO/−pat had significantly more relaxation to isoproteronol than the other three groups (Figure 5 and Table 2).
Figure 5.
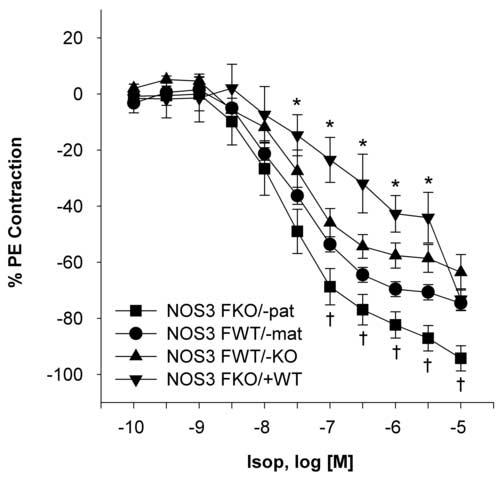
Isoproteronol (Isop) concentration-response curves in the carotid arteries of the NOS3FKO/−pat (n=8), NOS3FWT/−mat (n=7), NOS3FWT/−KO (n=8), and NOS FKO/+WT (n=5) female mice. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/+WT vs. all other groups. †P < 0.05 NOS3FKO/−pat vs. all other groups.
Responses to cumulative concentrations of Ca2+ were significantly higher in NOS3FKO/−pat when compared to NOS3FWT/−mat. NOS3FWT/−KO and NOS3FWT/−mat had less contraction to calcium (Figure 6 and Table 2).
Figure 6.
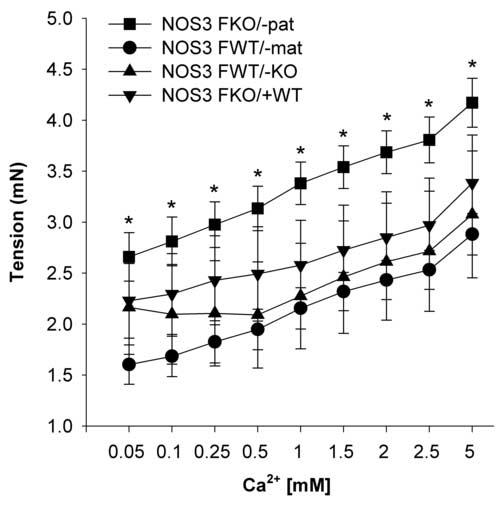
Calcium (Ca2+) concentration-response curves in the carotid arteries of NOS3FKO/−pat (n=7), NOS3FWT/−mat (n=8), NOS3FWT/−KO (n=4), and NOS3FKO/+WT (n=3) female mice. One-way ANOVA followed by Newman-Keuls tests were used.
*P < 0.05 NOS3FKO/−pat vs. NOS3FWT/−mat.
Comment
The contributions of the uterine environment and genetics to fetal development is well established. Abnormalities of the uterine environment can lead to fetal insults during critical periods of development, which in turn lead to changes in fetal structure, physiology and metabolism. These adaptive compensations induce permanent changes in the structure or function of developing organs/organ systems. Consequently, these alterations may lead to adult disease. Genetics also play a role, specifically when the abnormal genetic traits or polymorphisms inherited are maternally-derived. Genetics may also affect the offspring phenotype differently according to which parent has passed on the abnormal trait, which is referred to as genomic imprinting.9,10 Hypertensive disorders of pregnancy are a good illustration of this concept. Maternal hypertension can cause inadequate vascular adaptations during pregnancy along with altered utero-placental circulation.11,12 These events can ultimately lead to the development of adult disease..
The postnatal environment is another potential factor in determining the offspring's phenotype through alterations in breastfeeding, parenteral behaviors, nutritional intake, and environmental exposures, especially when the abnormalities are passed on from the mother. To assess the role of the postnatal environment, cross fostering animal litters at birth is used. It is well established that behavioral and nutritional interactions between mother and pups influences offspring development.13 It is also known that transgenic animals do not exhibit normal maternal behavior, i.e. variations in activity, aggression, anxiety, attention given to their pups. As a result, cross fostering the offspring of knockout mothers to a normal mother is utilized in an attempt to compensate for certain developmental abnormalities that may be passed on the heterozygous or homozygous offspring from a knockout mother during gestation.14
In the animal model of hypertension used in these experiments, homozygous knockout mice for the NOS3 allele and their counterparts, the wild-type mice, were bred to produce both homozygous and heterozygous offspring that could be cross fostered to a mother with the opposite inheritance for the NOS3 allele. Studies by Longo, et al. in naturally fostered animals have shown that the vascular, mechanical and contractile properties of the carotid arteries in the heterozygous adult offspring obtained from breeding NOS3−/−KO with NOS3+/+WT depends on whether the mother is NOS3−/−KO or NOS3+/+WT, and therefore developed in an abnormal or normal uterine environment respectively.15 The vascular profile of the female adult heterozygous offspring that inherits the mutated NOS3 allele from the mother (NOS3+/−mat) and therefore develops in an abnormal uterine environment, is similar to female homozygous NOS3−/−KO mice who have no functional NOS3 alleles. In contrast, the vascular profile of the female adult heterozygous offspring that inherits the non-functional NOS3 allele from the father (NO3+/−pat), and therefore develops in a NOS3+/+WT mother, is similar to female NOS3+/+WT control mice. Overall, the carotid arteries of the NOS3−/−KO and NOS3+/−mat adult offspring mice displayed increased rigidity of the vessel wall, altered vessel elastic properties, and increased contractility of the vessel when exposed to K+, Ca2+, phenylephrine and serotonin.15,16 Also, the NOS3−/−KO and NOS3+/−mat mice did not exhibit appropriate vascular relaxation when exposed to acetylcholine, and in fact showed vasoconstriction.15,16
After cross fostering, the observed difference in sensitivity to phenylephrine shown in NOS3+/−mat and NOS3−/−pat was abolished. The NOS3FWT/−mat, NOS3FKO/−pat had essentially the same response to phenylephrine. In both the naturally fostered and cross fostered studies, the homozygous knockout offspring were still highly sensitive to phenylephrine and the wild type offspring had a normal response to phenylephrine, an expected finding. In fact, when NO production was inhibited by L-NAME in all groups, the differences previously seen in the cross fostered groups was abolished. After cross fostering, NOS3FWT/−mat regained their relaxation to acetylcholine and responded similarly to NOS3FKO/−pat NOS3FKO/+WT. Although the maximal effect was similar amongst the three cross fostered groups, the IC50 was significantly higher in NOS3FWT/−mat than NOS3FKO/−pat indicating lower sensitivity. Again, NOS3FWT/−KO failed to relax in the presence of acetylcholine. After cross fostering, NOS3FWT/−KO and NOS3FWT/−mat exhibited improved relaxation to isoproteronol that was similar to NOS3FKO/−pat. However, of note, NOS3FKO/+WT had a significantly decreased response to isoproteronol that was not observed in the naturally fostered offspring. We believe that this is an interesting finding that deserves further investigation.
Responses to cumulative concentrations of calcium in the naturally fostered offspring was significantly increased in NOS3+/−mat and NOS3−/−KO, compared to NOS3−/−pat and NOS3+/+WT. One likely explanation is that fetal programming induces changes in the vessels' mechanical properties, which become more rigid and have hypertrophy of the smooth muscle. After cross fostering, NOS3FKO/−pat and NOS3FKO/+WT contracted significantly more and NOS3FWT/−mat and NOS3−/−KO contracted appropriately. This shows that the postnatal environment has affected the response of smooth muscle in these pups to calcium.
The postnatal weight gain did vary amongst the four cross fostered groups. Overall, NOS3FKO/+WT had greater postnatal weigh gain, followed by NOS3FKO/−pat, NOS3FWT/−mat, and lastly NOS3FWT/−KO. From weeks 4-9, NOS3FKO/+WT had the most postnatal weight gain overall. These findings suggest that postnatal cross fostering does not alter the postnatal weight gain as the homozygous wild-type and paternally-derived heterozygous pups have the most weight gain and the homozygous knockout and maternally-derived heterozygous pups have less weight gain, which is expected. When comparing these findings to the findings in the in vitro vascular reactivity studies, it becomes apparent that although postnatal growth was not affected by cross fostering, vascular reactivity was. As a result, we hypothesize that postnatal nutrition and/or environmental exposures have a much more profound effect on vascular development perhaps through effects on the neuroendocrine and metabolic systems.
Our findings suggest that the postnatal environment has a significant role in modulating the adult vascular phenotype. Although the cross fostered homozygous offspring did not appear to be affected significantly by postnatal cross fostering, the cross fostered heterozygous offspring were. It appears that the homozygous genotypes (complete presence or absence of nitric oxide), as well as development in the homozygous mother, are not altered through postnatal manipulations. However, heterozygous genotypes, with the presence of one functional NOS3 allele, can have their adult vascular phenotype altered with postnatal rearing by a mother with the opposite genetic inheritance of the NOS3 allele. More specifically, NOS3FWT/−mat exhibited the most profound alteration with overall improvement in vascular profile. Therefore, development in a knockout mother can be positively affected by more favorable postnatal environment.
The exact mechanisms responsible for these changes induced by an altered postnatal environment need further investigation. In naturally fostered litters, development in a knockout mother and rearing by the same mother ultimately affect the development of the adult vascular phenotype through in utero fetal programming and postnatal maternal behaviors and nutrition in the form of breast milk. After cross fostering, the factors changed are postnatal maternal behaviors and nutrition. Therefore, investigation into whether certain maternal behaviors or components of breast milk are responsible is warranted. The potential modulating role of postnatal nutrition in our model is supported by the findings of Cook et al.17 These investigators found that NOS3+/− mice fed a normal diet had normal insulin sensitivity and were normotensive. However, when fed a high-fat diet over an 8-week period, they developed exaggerated arterial hypertension and had fasting hyperinsulinemia and a 35% lower insulin-stimulated glucose utilization than control mice. In addition to a mechanistic explanation, these findings point to the significant impact of the postnatal environment on diseases in later life, and the exciting opportunity for preventive strategies, particularly in predisposed offspring either due to a genetic or intrauterine alteration.
In conclusion, the postnatal environment appears to play a significant role in the development of the adult vascular phenotype by significantly improving the NOS3FWT/−mat vascular profile. The fact that it is this particular heterozygote that is affected by postnatal cross fostering is significant in that it is this genotype that develops in a knockout mother. Ideally, translational research is needed in human pregnancies in order to see how the human vascular profile can be altered through improvement in postnatal nutrition and/or exposure to altered maternal behaviors.
Footnotes
Supported by NHLBI R01 HL080558-02
CONDENSATION
The postnatal environment alters fetal programming of vascular function in an animal model of adverse uterine environment induced by absence of endothelial nitric oxide synthase.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJP, Osmond C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJP. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–74. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 3.Moore T. Genetic conflict, genomic imprinting and establishment of the epigenotype in relation to growth. Reproduction. 2001;122:185–93. doi: 10.1530/rep.0.1220185. [DOI] [PubMed] [Google Scholar]
- 4.Wu KK. Inducible cyclooxygenase and nitric oxide synthase. Adv Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- 5.Forstermann U, Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch Pharmac. 1995;352:351–64. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- 6.Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003;9:359–80. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 7.Longo M, Jain V, Anderson G, Vedernikov Y, Garfield RE, Saade GR. Effect of in-utero environment and genetic imprinting on future vascular reactivity in transgenic mice lacking a functional endothelial nitric oxide synthase. Am J Obstet Gynecol. 2002;186:S19. [Google Scholar]
- 8.Longo M, Jain V, Langenveld J, Vedernikov Y, Bukowski R, Anderson GD, Garfield RE, Saade GR. Embryo transfer prevents development of abnormal vascular function in offspring of endothelial nitric oxide synthase knockout mice. J Soc Gynecol Investig. 2004;11(2 Suppl 1):294A. Abstract. [Google Scholar]
- 9.Hurst LD. Is multiple paternity necessary for the evolution of genomic imprinting? Genetics. 1999;153:112–24. doi: 10.1093/genetics/153.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tycko B, Morrison IM. Physiological functions of imprinted genes. J Cell Physiol. 2002;192:245–58. doi: 10.1002/jcp.10129. [DOI] [PubMed] [Google Scholar]
- 11.Rotmensch S, Liberati M, Luo JS, Kliman HJ, Gollin Y, Bellati U, et al. Color Doppler flow patterns and velocity waveforms of the intraplacental fetal circulation in growth-retarded fetuses. Am J Obstet Gynecol. 1994;171:1257–64. doi: 10.1016/0002-9378(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 12.Henrikson T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–24. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- 13.McCarty R, Lee JH. Maternal influences on adult blood pressure of SHRs: a single pup cross-fostering study. Physiol Behav. 1996;59:71–6. doi: 10.1016/0031-9384(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 14.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel T. Infant vocalization, adult aggression and fear behavior of an oxytocin null mutant mice. Horm behave. 2000;37:145–55. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 15.Longo M, Jain V, Vedernikov Y, Bukowski R, Garfield RE, Hankins GDV, Anderson GD, Saade GR. Fetal origins of adult vascular dysfunction in mice lacking endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2004;288(5):R1114–21. doi: 10.1152/ajpregu.00367.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kodja G, Laursen J, Ramasamy S, Kent J, Hurz S, Burchfield J, et al. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206–13. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- 17.Cook S, Hugli O, Egli M, Menard B, Thalamann S, Sartori C, et al. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes. 2004;53:2067–72. doi: 10.2337/diabetes.53.8.2067. [DOI] [PubMed] [Google Scholar]


