Summary
Background
Intraflagellar transport (IFT) is a motility process operating between the ciliary/flagellar (interchangeable terms) membrane and microtubular axoneme of motile and sensory cilia. Multi-polypeptide IFT particles, composed of complexes A and B, carry flagellar precursors to their assembly site at the flagellar tip (anterograde) powered by kinesin, and turnover products from the tip back to the cytoplasm (retrograde) driven by cytoplasmic dynein. IFT is essential for the assembly and maintenance of almost all eukaryotic cilia and flagella, and mutations affecting either the IFT motors or the IFT particle polypeptides result in the inability to assemble normal flagella or in defects in the sensory functions of cilia.
Results
We found that the IFT complex B polypeptide, IFT27, is a Rab-like small G protein. Reduction of the level of IFT27 by RNA interference reduces the levels of other complex A and B proteins suggesting this protein is instrumental in maintaining the stability of both IFT complexes. Furthermore, in addition to its role in flagellar assembly, IFT27 is unique among IFT polypeptides in that its partial knockdown results in defects in cytokinesis and elongation of the cell cycle and a more complete knockdown is lethal.
Conclusion
IFT27, a small G protein, is one of a growing number of flagellar proteins that are now known to have a role in cell cycle control.
Introduction
Structurally, the cilium, or flagellum (interchangeable terms), consists of a microtubule-based axoneme surrounded by a specialized plasma membrane. The axoneme is assembled onto the basal body, a microtubule-organizing center derived from the centriole [1]. The assembly and maintenance of cilia depend on intraflagellar transport (IFT), a motility process occurring between the flagellar membrane and the axoneme [2] that transports flagellar precursors bound to IFT particles to their site of assembly at the distal tip and returns turnover products from the ciliary tip to the cell body [3]. IFT particles are composed of two large protein complexes, A and B, which contain approximately six and eleven polypeptides, respectively [4].
The requirement of IFT for assembly and maintenance of eukaryotic cilia was revealed by analysis of mutations in the genes encoding IFT polypeptides and the motors that move them [5, 6]. Some of these mutants were first generated in the bi-flagellate alga Chlamydomonas by insertional mutagenesis, in which flagella-defective mutants were screened to identify clones with deletions of genes encoding IFT polypeptides or motors. Such mutants had no, or very short, flagella [7–10]. Many Chlamydomonas IFT mutants were obtained in this fashion, but IFT27 mutants were not found after several attempts, hinting that such mutations were lethal. IFT27 was of particular interest because it is a Rab-like small G protein, without the protein:protein interactive motifs characteristic of other IFT polypeptides [11]. In this paper we show, using RNAi, that in addition to causing flagellar defects, a partial reduction of IFT27 inhibits cell division and a severe reduction of IFT27 expression is lethal. The block in cell division appears to occur prior to or during cytokinesis, as many cells contained duplicated nuclei but failed to separate. In addition, progression through the cell cycle in cells expressing little IFT27 requires 3 or more times longer than in wild type cells. IFT27 is, therefore, unique among the IFT polypeptides analyzed to date in that it is required for cell division and cell cycle progression. Recently, two Neks (NIMA-related expressed kinases) that are components of the flagella and basal bodies were also shown to be involved in cell cycle control [12, 13]. Likewise, mutations in polycystic kidney disease (PKD) gene products, polycystins 1 and 2, found in the ciliary membrane, are known to promote cell division. IFT27, the Neks, and polycystins are, therefore, among the first ciliary/flagellar proteins shown to be involved in cell cycle control.
Results
IFT27 is a Rab-like G protein
To identify the gene encoding IFT27, IFT27 was excised from an SDS-PAGE gel and subjected to MALDI-TOF sequencing (Univ. Mass. Med. School Proteomic Mass Spectrometry Lab.). The resultant peptide sequences were used to search the Chlamydomonas EST database and identified the predicted protein Chlre3:129193. The predicted cDNA was confirmed by sequencing an RT-PCR product and that was found to contain a 615 bp open reading frame encoding IFT27. (See S1 for details.)
Sequence alignments indicated that IFT27 is a Rab-like GTPase, distantly related to Rab proteins from mammals; the closest human orthologue identified in a BLAST search was a Rab-like protein gi30582469, RabL4 [14], E value = 3e−28. IFT27 orthologues are present in the genomes of zebrafish, mouse and human, but no cellular function has been assigned to IFT27 in any species.
IFT27 contains all five Ras-GTPase consensus sequences (Fig. 1) that are essential for GDP/GTP-binding and GTPase activity [15]. A GTP binding assay also confirmed that IFT27 can bind GTP and that binding is blocked by GDP (data not shown). However, IFT27 sedimented at 16S with other IFT particle proteins in spite of the addition of the following combinations: GTPγS, GTPγS plus AMPPNP, GTPγS plus ATP, GDP plus AMPPNP or GDP plus ATP in the membrane plus matrix extract (data not shown). It is likely, therefore, that IFT27 is constitutively associated with IFT particle complexes in either its GDP- or GTP- bound states. Furthermore, almost all the IFT27 in the membrane plus matrix cosediments with IFT particles (S2) so if IFT particles are regulated by binding and releasing IFT27 this does not appear to occur in the flagella.
Figure 1. IFT27 is a Rab-like small G protein.

IFT27 contains all five G domains required for GDP/GTP-binding and GTPase activities, which are conserved across the Ras superfamily of small G proteins. The IFT27 sequence is shown in darkened boxes and below the consensus sequences are listed where “X” is an unspecified amino acid and (X)n represents a sequence of indeterminate length. The possible lipid modification motif at the C-terminus is also highlighted.
Generally, Rab proteins associate with cellular membranes through a prenyl (geranylgeranyl) group that is added after translation to a C-terminal prenylation motif, containing one or, more frequently, two cysteine residues. Chlamydomonas IFT27 contains a prenylation motif (Fig. 1); however, none of the IFT27 orthologues in zebrafish, mouse or human contains such a motif. Prenylated Rab proteins are hydrophobic and separate into the detergent phase after a Triton X-114 partition. In contrast, flagellar IFT27 remained in the aqueous phase indicating that it is not lipid modified (see S1).
IFT27::GFP distribution and movement is similar to that of other IFT particle proteins
GFP-tagged IFT27 transgene strains were generated to visualize the motility of IFT27 in the flagellum. IFT27::GFP was present in flagella of the transgene strains (Fig. 2A) and co-sedimented on sucrose density gradients with other IFT particle proteins, including native IFT27 (Fig. 2B). The total amount of IFT27 (IFT27::GFP plus native IFT27) in flagella from IFT27::GFP cells remained at the same level as native IFT27 alone in wild type flagella indicating that the stoichiometric level of IFT27 in an IFT particle is tightly controlled. When examined by immunofluorescence microscopy (Fig. 2C), the flagellar distribution of IFT27::GFP overlapped with IFT74/72 and in the cell body, IFT27::GFP was localized principally around the basal bodies in a pattern similar to that of FLA10 and other IFT proteins.
Figure 2. IFT27::GFP can substitute for endogenous IFT27 and incorporate into IFT complexes in the flagella.
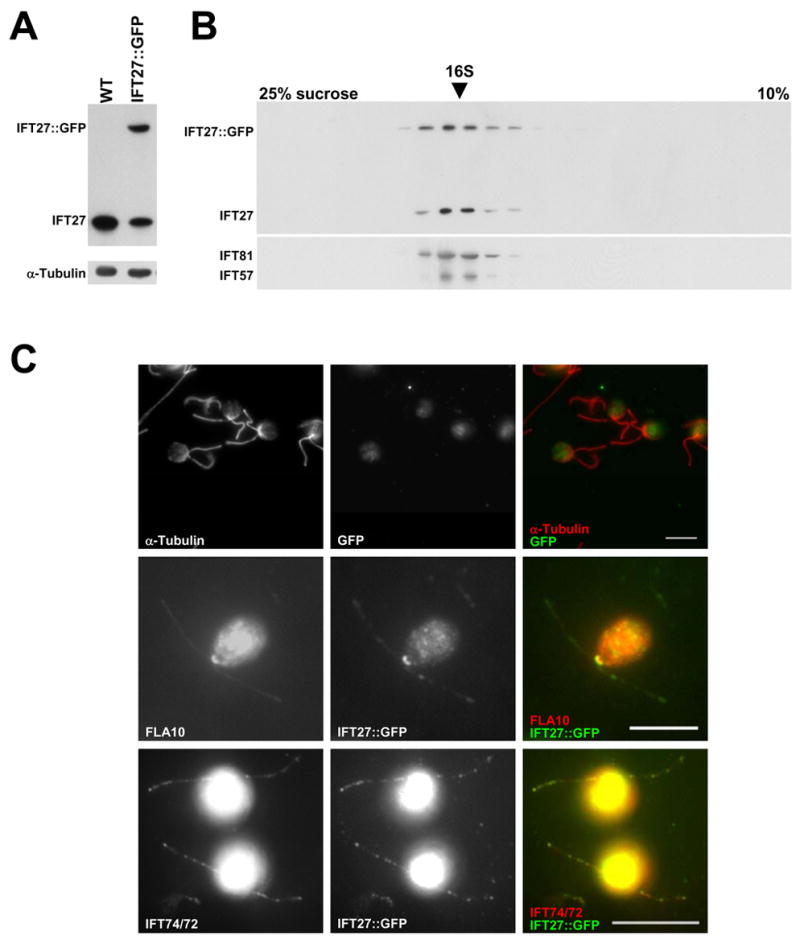
(A) Immunoblots of whole flagella from wild type and IFT27::GFP transgenic cells probed for IFT27 showing two forms of IFT27 in the transgenic cells. Alpha-tubulin is included as a loading control. (B) Immunoblots of gradients of soluble flagellar proteins from IFT27::GFP transgenic cells probed with antibodies against several IFT polypeptides. IFT27::GFP sediments with the other IFT particle proteins, including endogenous IFT27. (C) Immunofluorescence of wild type and IFT27::GFP transgenic cells. Wild type cells show no flagellar or basal body labeling with the α-GFP antibody (top row). The second and third rows show that IFT27 co-localizes with FLA10 around the basal bodies and with IFT74/72 in the flagella. The IFT74/72 panel was brightened in PhotoShop to make the flagella readily visible. Scale Bars = 10um.
As expected for an IFT particle protein, time-lapse fluorescent microscopy showed IFT27::GFP undergoing typical IFT bi-directional transport in the flagella (Fig. 3 and Movie S3). The anterograde velocity (mean ± S.D.) was 1.93 ± 0.33 μm/sec (136 particles) and the retrograde movement was 3.28 ± 0.76 μm/sec (111 particles), comparable to the IFT velocities obtained in Chlamydomonas reinhardtii using DIC microscopy (anterograde: 2.2 ± 0.46 μm/sec, 32 particles; retrograde: 3.1 ± 0.51 μm/sec, 29 particle) (Movie S4) [16]. Most IFT27::GFP particles move continuously and only change their direction at the flagellar tip, though occasionally an anterograde IFT particle changes its direction before reaching the tip (Fig. 3B arrowheads).
Figure 3. IFT27::GFP undergoes IFT.
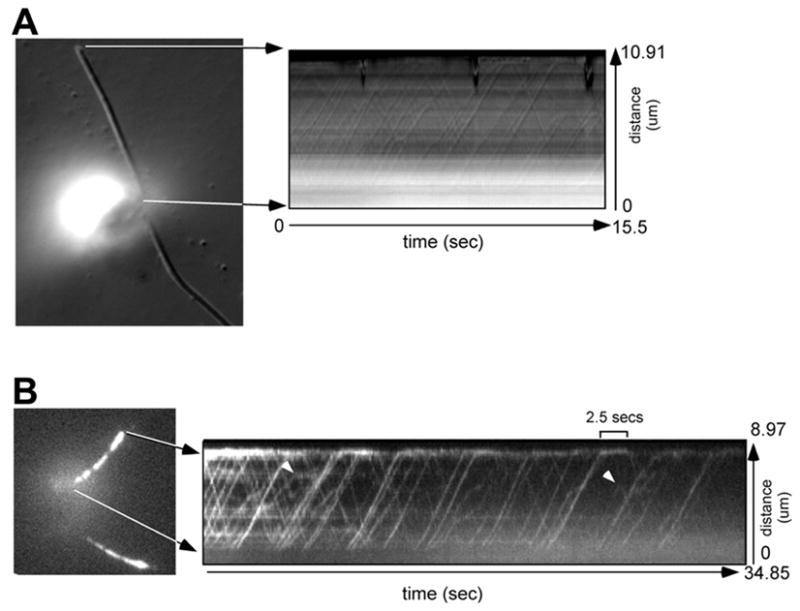
(A) The left panel is a DIC micrograph used for orientation. The kymograph (right panel) shows the trajectories of IFT particles moving bi-directionally in one flagellum of the cell. (B) The left panel is a fluorescence micrograph of a cell expressing IFT27::GFP. The kymograph (right panel) shows the movement of IFT27::GFP recorded by fluorescent microscopy. The lines in the kymographs were used to calculate the velocities of IFT particle or GFP movements. When IFT27::GFP reached the flagellar tip, it took up to a few seconds before reversing direction (one IFT27::GFP particle stayed at the tip for 2.5 secs). Some particles (arrow heads) reverse their direction before they reach the tip of the flagellum.
IFT27 RNAi knockdown cells show defects in cell division
Because a null IFT27 mutant was not found by screening several flagellar-defective mutant libraries [7, 8, 17], RNA interference (RNAi) was used to analyze the effect of IFT27 depletion on Chlamydomonas. Initially, we generated IFT27 knockdown cells using an RNAi construct comprising almost the full length IFT27 gene (RNAi-1). Later, to minimize the possibility that a non-related Rab gene was non-specifically targeted by the RNAi-1, a second construct (RNAi-2) was made including only the first exon of the IFT27 gene. This sequence shows very limited similarity to any other Ras protein in the genome of Chlamydomonas (S5).
The results of IFT27 knockdown, obtained from 5 transformation experiments (4 with RNAi-1, and 1 with RNAi-2), indicated that IFT27 was essential for cell growth. Many clones of transformants grown on plates initially appeared as tiny green colonies but then turned yellow and died. Moreover, some colonies from the original transformation plates could not be subcultured in solid or liquid media. For example, in the transformation with RNAi-2, of 70 colonies that grew on selective plates, 15 (21%) failed to grow after transfer to fresh plates. Thirty seven clones were transferred to liquid media and 8 (22%) failed to grow. Of the remaining 29 clones, 17 (59%) showed reduced levels of IFT27 on immunoblots of whole cell extracts and at least 8 of these grew very slowly compared to wild type cells and had a wide range of division defects (Fig. 4C).
Figure 4. Knock down of IFT27 causes cell division defects.
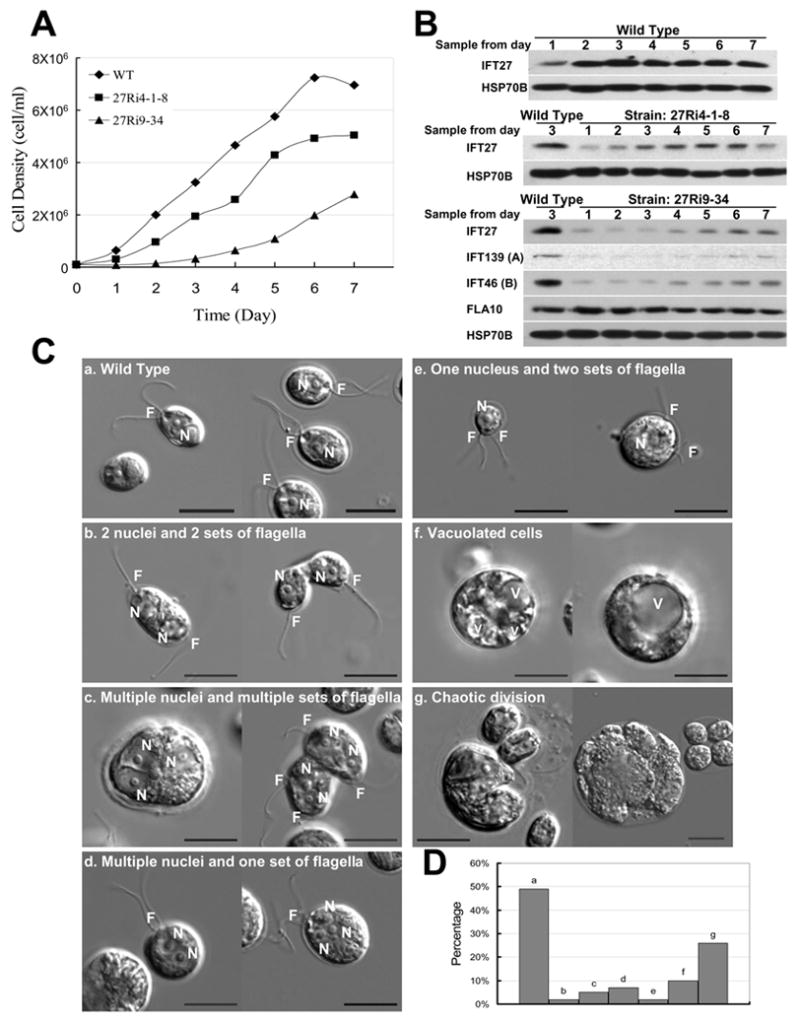
(A) Growth curves of wild type control (CC125) and two IFT27 RNAi knockdown clones (Ri 4-1-8 and Ri 9–34). (B) Immunoblots of whole cell extracts samples taken from the daily cultures in panel A. IFT27 was reduced in RNAi cells as were other IFT complex A and B proteins. FLA10 remained at the wild type level. HSP70B was used as a loading control. (C) DIC micrographs of wild type cells “a”, showing the normal cell shape and the position of nucleus “N” and flagella “F”; and IFT27 RNAi cells “b – g”, illustrating the major cell division defects seen in IFT27 knockdown cells. “V” = vacuole. (D) Plot of the percentages of the division defects for Ri 9–34 cells. The “a” bar represents the percentage of cells having one nucleus and one set of flagella. Many of the cells in this category had their flagella and nucleus positioned abnormally. “Set” refers to both pairs of flagella and unpaired singlet flagella. Scale bars = 10 μm.
Several IFT27 knockdown clones were chosen for detailed analysis and data for Ri 9–34 (RNAi-1) and 4-1-8 (RNAi-2) are shown. Both clones grew slower than wild type cells (Fig. 4A). This was especially true for Ri 9–34, which had the least amount of IFT27. The RNAi silencing effects on IFT27 imposed by RNAi-1 and RNAi-2 appeared to be unstable; thus, the IFT27 protein levels partially recovered in both knockdown clones during culture in liquid media over a period of days. Immunoblots of whole cell extracts showed that IFT27, which was at low levels on day 1, recovered to about 60% of wild type levels by day 5 in Ri 4-8-1 (Fig. 4B). Ri 9–34 recovered more slowly and as the IFT27 level increased the growth rate also partially recovered (Fig. 4A). The growth rate of the knockdown cells correlated with the amount of IFT27 present, indicating that the reduced IFT27 is the likely cause of growth inhibition.
In the above experiment the cells were not under selective pressure to express IFT27 RNAi. The plasmid used for RNAi-2 included an RNAi construct to knock down Maa-7, thereby conferring resistance to 5-fluoroindole on transformants [18]. When Ri 4-1-8 cells were cultured in liquid medium in the presence of 2.5 μM 5-fluoroindole, they failed to increase in density after two weeks. Thus, although IFT27 RNAi cells grown without selection gradually recovered both IFT27 expression and the ability to divide, when RNAi expression was maintained by negative selection, cell growth was repressed.
Cell division defects were prominent in IFT27 RNAi cells as can be seen from the representative DIC images shown in Fig. 4C. Cells from day 3 in the above growth curve experiments were used for detailed phenotypic analysis. In contrast to wild type cells, which contain one nucleus and one pair of flagella, 14% of Ri 9–34 cells had more than one nucleus; 26% were in large, irregular clumps, apparently due to incomplete cytokinesis; 2% had one nucleus but two sets of flagella, which were likely generated through asymmetric cell division; and 7 % were highly vacuolated, apparently undergoing cell death as the consequence of aberrant cell division (Fig. 4D). Dead cells, were commonly seen in this culture, something not seen in wild type cultures of similar density or in cultures of IFT52 RNAi knockdown cells (see below). Thus, the phenotypes of the IFT27 RNAi knockdown cells strongly support the conclusion that IFT27 plays an important role in cell division.
Additional evidence for a role of IFT27 in cell division was noted in cells expressing IFT27::GFP. Though some strains had no obvious growth defects, other strains, in which expression of native, untagged IFT27 was reduced, showed division defects similar to those seen in the IFT27 RNAi cells (S6). The severity of the phenotype paralleled the level of native IFT27 expression. By following individual cells immobilized in agarose, the time between cell divisions was found to be more than 48 hours in 90% of the cells compared to 12–14 hrs for wild type cells (S6) indicating that in addition to its role in cytokinesis, IFT27 also is involved in regulation of the cell cycle.
IFT27 RNAi knockdown cells show defects in flagellar assembly
Because mutations affecting other IFT proteins result in no, or very short, flagella, we expected that IFT27 RNAi knockdown cells would lack flagella. Flagellar lengths of Ri 9–34 cells from day 5 of Fig. 4A, in which IFT27 was about 20% of the wild type level, are shown in Fig. 5A (also see S7). Although some cells had full length flagella, the mean flagellar length was slightly shorter than wild type and, more strikingly, 20% of the cells had flagella of 5 μm or less (Fig. 5A). Furthermore, only about 60% of the cells possessed flagella compared to 89% of wild type cells. These results suggest that insufficient amounts of IFT27 impair the capacity of IFT to build normal length flagella.
Figure 5. Knockdown of IFT27 causes flagellar assembly and positioning defects.
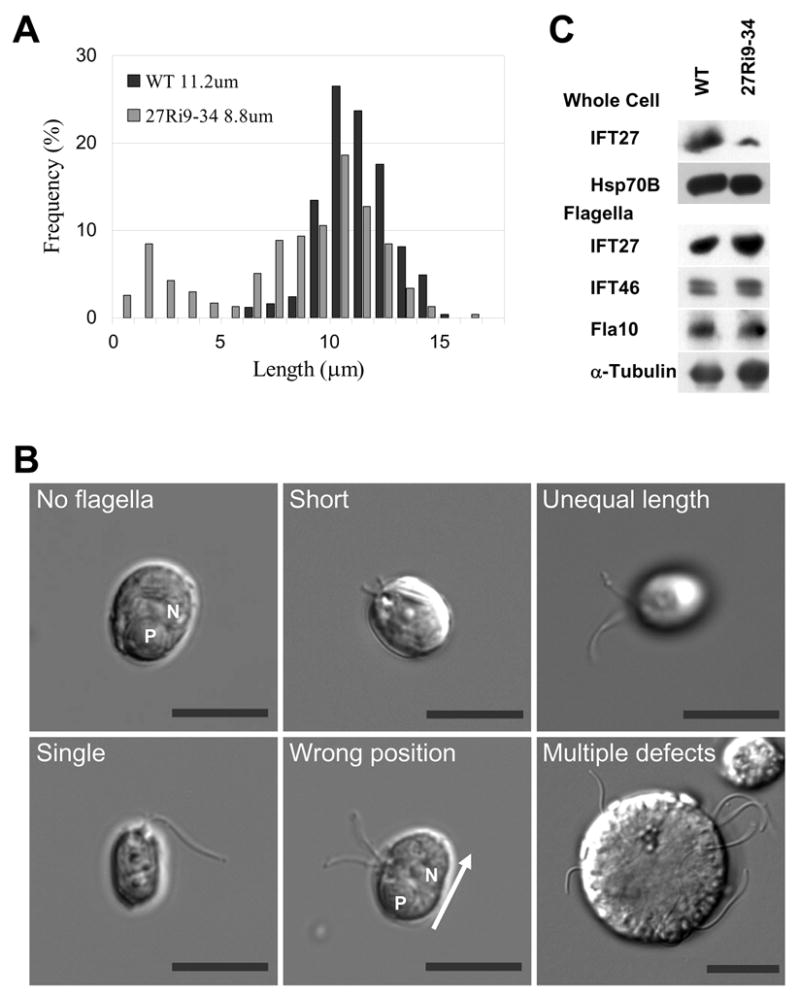
(A) Plots showing the length distribution of flagella from wild type and IFT27-depleted cells Ri 9–34 when the IFT27 level was 20% of wild type. Mean lengths are listed. (B) DIC micrographs illustrating the major flagellar defects in Ri 9–34. “P” = pyrenoid; “N” = nucleus; Bars = 10 μm. (C) Immunoblots comparing the levels of IFT proteins in whole cells and flagella of wild type and IFT27 knockdown cells. Although the level of IFT27 is greatly reduced in whole cells in the knockdown clone, the flagella of these cells have a normal complement of IFT proteins and FLA10.
The reason why many of the IFT27 knockdown cells had flagella could be that in a population with heterogeneous expression levels of IFT27, flagella may form on cells that have more than a threshold amount of IFT27. When flagella were isolated from IFT27 knockdown cells and probed on immunoblots, the level of IFT27 was indistinguishable from that of wild type flagella (Fig. 5C). Thus, the flagella that formed in IFT27 knockdown cells had a normal complement of IFT27.
Other flagellar defects were seen in IFT27-depleted cells: after 3 days in culture, 8% of the flagellated Ri-9–34 cells had flagella of unequal length (Fig. 5B); 13% had a single flagellum; and 18% had a flagellar positioning defects, with flagella positioned abnormally around the cell body (Fig. 5B) instead of at the anterior of the cell (Fig. 4C). These positional defects may arise secondarily from aberrant cell division.
We examined the level of several additional IFT particle proteins by immunoblotting whole cell extracts of IFT27 knockdown clones. Surprisingly, all IFT particle proteins tested, including proteins of both complex A and B (Fig. 4B, and IFT52 and IFT81, not shown), were dramatically decreased when IFT27 was knocked down, and increased in parallel with IFT27 as it recovered during culture (Fig. 4B). In contrast, the IFT motor FLA10 remained at the wild type level throughout, despite the decrease of IFT particle proteins. Thus, IFT27 appears to be involved in maintaining the expression level of the other IFT particle proteins.
IFT52 knockdown does not affect cell division but does affect flagellar assembly
As a control for IFT27 RNAi, we also used RNAi to knockdown the expression of IFT52, another complex B protein. Results from several IFT52 knockdown clones are shown in Fig. 6. Although the level of IFT52 in these clones was 40% to 20% of wild type levels (Fig. 6A and not shown), they all grew as well as wild type cells (Fig. 6B) and showed no defects in division. Inhibition of division in IFT27 knockdown cells, therefore, is specific to the IFT27 RNAi construct, not a bi-product of RNAi.
Figure 6. Knock down of IFT52 causes flagellar assembly defects.
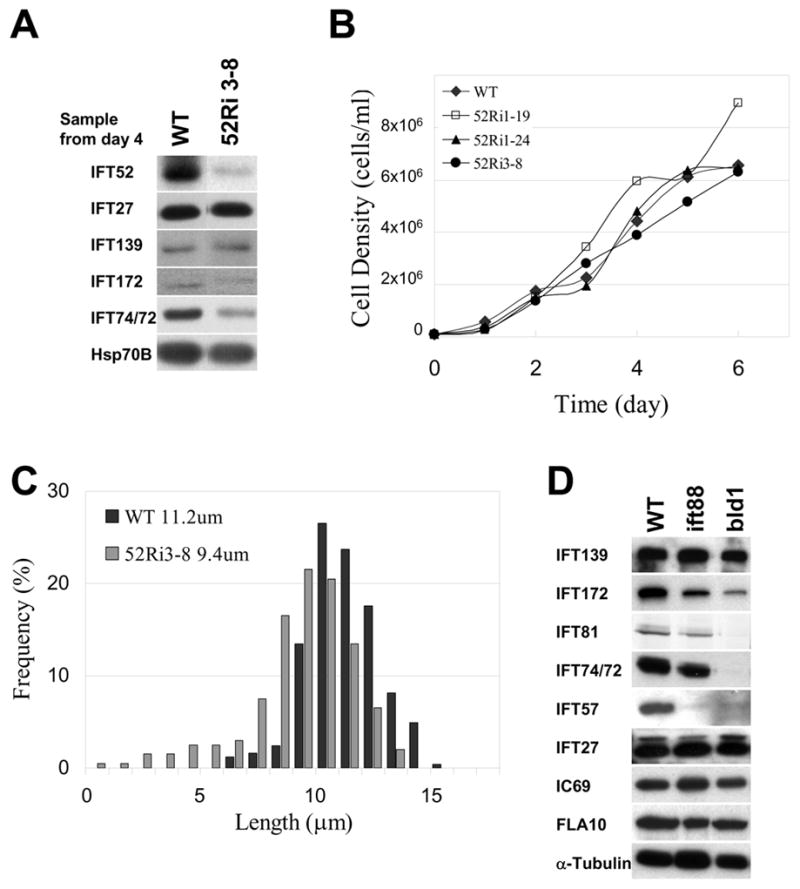
(A) Immunoblots of wild type and IFT52 RNAi knockdown cells probed with antibodies against IFT polypeptides. Some IFT complex B proteins were reduced in the RNAi cells, but IFT27 and complex A protein IFT139 were not. HSP70B was included as a loading control. (B) Growth curves of wild type control (CC125) and three IFT52 RNAi knockdown clones. (C) Plots showing the length distribution of flagella in wild type and IFT52-depleted cells, 52Ri3–8, when the IFT52 level was 20% of wild type. Mean lengths are listed. (D) Immunoblots comparing the levels of IFT proteins in whole cells of wild type and mutants lacking IFT88 or IFT52 (bld1). Despite the reduction of most complex B proteins in these mutants, IFT27 and complex A proteins remained at wild type levels.
The flagellar phenotype of IFT52 knockdown cells was very similar to that seen in the IFT27 knockdown cells: at day 4 of growth (Fig. 6B) the mean flagellar length was about 80% of wild type and more cells had short flagella (Fig. 6C and S7). It appears, therefore, that even though cells lacking IFT52 (bld1) cannot assemble flagella [10], flagellar assembly does occur in partial knockdowns of IFT52.
The effect of the IFT52 knockdown on other IFT proteins was analyzed on immunoblots of whole cell extracts (Fig. 6A). Several IFT complex B proteins were reduced in IFT52 RNAi clones, but IFT27 and complex A protein IFT139 were not. This relationship holds true in several mutants affecting IFT proteins: in mutants lacking IFT88, IFT52 (Fig. 6D) or IFT46 (Hou and Witman, personal communication), the level of IFT27 is unchanged though the levels of several other complex B proteins are reduced along with the mutant protein. Thus, IFT27 appears to be unique in that its concentration has a global effect on the levels of complex A and B proteins, but its level is not affected by the loss of other complex B proteins.
Discussion
In this report we show that IFT27, one of the “core” polypeptides of IFT particle complex B [19], is a Rab-like small G protein. GFP-tagged IFT27 moves at the rate of IFT in both anterograde and retrograde directions in the flagella, it sediments on sucrose gradients and it co-localizes by immunofluorescence with the other IFT polypeptides in the flagella and, principally, around the basal bodies. These are all constant features of IFT polypeptides. However, IFT27 has none of the numerous protein-protein interacting motifs characteristic of other IFT polypeptides [11] although like all Rab proteins it must bind an effector protein. In addition, unlike other IFT proteins, no orthologue of IFT27 was identified in the genomes of C. elegans or Drosophila. In these organisms the cells requiring IFT for ciliary assembly are sensory neurons at an end-state in differentiation and, therefore, are not dividing, consistent with IFT27 being related to cell proliferation. Functionally, IFT27 is unlike any of the other IFT polypeptides described so far [5, 6]: in addition to its role in flagellar assembly, IFT27 is required for the normal completion of cell division.
This division phenotype is specific to IFT27 depletion. Co-suppression of related genes is a potential hazard of RNAi technology, especially when dealing with a member of a superfamily like Ras. However the 5′ fragment of the IFT27 cDNA used to make the double stranded RNA shared little similarity to other Ras cDNAs in Chlamydomonas, so it is unlikely these homologues were affected. The specificity of the observed phenotype to IFT27 was corroborated by second, complementary technique: cells expressing IFT27::GFP, in which native IFT27 was reduced, displayed the same division defects seen in the IFT27 RNAi cells (see S6 for details). Thus, this phenotype was not due to co-suppression of a spurious target gene by RNAi.
As a member of the Rab family, IFT27 may play a direct role in the final stages of cytokinesis as some members of the Rab family are involved in the vesicle trafficking required to complete this process. Human Rab35 [20], for example, regulates membrane flux needed for the final abscission of dividing cells and Rab5 and Rab11 may also be active in this process. With this in mind it is of interest that unlike in the flagellum where all the IFT27 sediments with complex B proteins, in the cell body about half of the soluble IFT27 does not cosediment with complex B (Qin and Rosenbaum, unpublished data). Therefore, a portion of the IFT27 in the cell body might have a role in cytokinesis, apart from its role in flagellar assembly.
The phenotype of IFT27-depleted cells is reminiscent of the phenotype seen when a maternal centriole protein, centriolin, is silenced [21], both proteins appearing to affect cytokinesis. Interestingly, centriolin is localized to the maternal centriole [21] from which a primary cilium is assembled and has been shown to function in membrane trafficking in cytokinesis [22]. Given that IFT27 is a Rab-like small GTPase, it may function in membrane dynamics in the final stage of cytokinesis.
Incomplete or asymmetric cytokinesis can account for many of the defects seen in IFT27-depleted cells including aberrant numbers of flagella, basal bodies and nuclei and could also contribute to retarded growth and increased cell death; but the very slow growth of IFT27 knockdown cells under continued selection and the death of many RNAi clones suggest that IFT27 has another role in the cell cycle. Indeed, cells expressing IFT27::GFP in which expression of native IFT27 was reduced required 3 or more times as long as wild type cells between divisions.
The effect of IFT27 on the cell cycle could be mediated through its affect on flagella. A wide variety of channels, receptors and signaling molecules have been identified in cilia. Recently it has been shown that the TRPV channels in the membranes of sensory cilia in C. elegans move within the plane of the membrane, powered by the IFT system underlying the membrane [23]. In such a situation, rather than being associated with vesicle motility or fusion as described for many Rabs, IFT27 would be associated with a planar ciliary membrane as an IFT particle component. The movement and/or placement of such channels may affect cell division in some way that is dependent on IFT27.
Cilia house cell signaling components including elements of the sonic hedgehog pathway involved in early vertebrate development [24], and polycystin 1 and 2, the defective gene products of autosomal dominant polycystic kidney disease (ADPKD). In the latter case, the polycystins compose cation channels positioned in the membranes of the mechano-sensitive primary cilia of kidney tubules [25, 26]. Mutations in the polycystins, their failure to target to cilia, or the inability to assemble cilia results in uncontrolled cell division and PKD (reviewed in [27]). This pathway demonstrates another link between the primary cilia and cell cycle control.
There are several reports that suggest a role for the cilium in the control of the cell cycle based on the fact that most vertebrate cells have primary cilia in the G0/G1 phase of the cell cycle, and that these cilia are resorbed during the G1 phase or before or during S-phase [28]. The implication in these reports is that exiting G0 or passing through mitosis may depend on resorption of cilia, at least in part to allow the basal body/centriole to participate in spindle formation. However, no causal relationship has ever been demonstrated between ciliary resorption and mitosis [28].
Results from Chlamydomonas suggest a key role for ciliary loss prior to cell cycle completion. Two Neks (a family of cell cycle related kinases), Fa2p and Cnk2p [28, 29], which are localized on the ciliary apparatus (ie. basal bodies and/or axonemes), function in normal ciliary resorption and in cell cycle progression [28]. The kinase Fa2p plays a role in flagellar resorption/detachment that occurs prior to cell division, and also affects the G2/M transition of the cell cycle [30]. Likewise, the flagellar Nek Cnk2p affects the regulation of flagellar shortening and cell size [12]. Both kinases and IFT27 may be in the same signaling pathway, ultimately affecting the cell cycle.
Other ciliary proteins that may have a role in cell cycle control are inversin [31], Bardet-Biedl Syndrome 4 (BBS4) [32], centrin [33, 34] and katanin [35–37]. Inversin, an Anaphase Promoting Complex (APC) binding protein is found in the cilium, but the relevance of this localization to the cell cycle control is still unknown [31]. BBS 4, which is related to a number of developmental pathologies, is localized principally at the basal body/centriole [38]. Cytologically, depletion of BBS4 by RNAi results in disorganization of the cytoplasmic microtubule network [32], possibly because of primary effects on the ability of the basal body/centriole to organize microtubules, and, more importantly, the cells cannot complete cell division [32]. Since both BBS and IFT27 affect cell division, and because the ciliary basal body where they are localized will ultimately form the poles of the mitotic apparatus [39], the centriole/centrosome will probably be the most important organelle to focus on in terms of the role of IFT27 and BBS4 in cell cycle control.
It remains to be determined in which cell cycle signaling pathway IFT27 functions, and future work will be directed toward this question. The overriding question is how and why an IFT polypeptide, moving rapidly along the length of the cilium in particles involved in ciliary assembly/disassembly, is involved in cell cycle control. Our current hypotheses center around the possibility of IFT27 acting as a cell cycle repressive checkpoint protein, notifying ciliated cells, including most G0 cells in our body, that the cilium is present, and therefore to hold up the cell cycle until the cilia are resorbed and the centrioles can be used in cell division. Whether or not IFT27 is involved in this flagellar shortening is still not known, but recent results (Wang and Rosenbaum, unpublished) indicate that IFT27 and other IFT polypeptides decrease for a period just before cell division begins and then recover. This pre-division decrease in IFT may, in turn, be responsible for the requisite shortening of the flagella prior to division, and its increase would allow cell division to be completed and new cilia/flagella to be assembled by IFT.
Supplementary Material
Acknowledgments
The authors thank Y. Hou, G. Witman, and G. Pazour for sharing unpublished results, P. Hegemann, S. Dutcher and W. Snell for plasmids and C. Beck for antibodies. This work was supported by NIH grant GM14642 to JR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dutcher SK. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic. 2003;4:443–451. doi: 10.1034/j.1600-0854.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 2.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 7.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr Biol. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 11.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–442. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 12.Bradley BA, Quarmby LM. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J Cell Sci. 2005;118:3317–3326. doi: 10.1242/jcs.02455. [DOI] [PubMed] [Google Scholar]
- 13.Mahjoub MR, Montpetit B, Zhao L, Finst RJ, Goh B, Kim AC, Quarmby LM. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J Cell Sci. 2002;115:1759–1768. doi: 10.1242/jcs.115.8.1759. [DOI] [PubMed] [Google Scholar]
- 14.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 15.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 16.Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J Cell Biol. 2005;170:649–659. doi: 10.1083/jcb.200412021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou Y, Pazour GJ, Witman GB. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol Biol Cell. 2004;15:4382–4394. doi: 10.1091/mbc.E04-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohr J, Sarkar N, Balenger S, Jeong BR, Cerutti H. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 2004;40:611–621. doi: 10.1111/j.1365-313X.2004.02227.x. [DOI] [PubMed] [Google Scholar]
- 19.Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- 20.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, Blomberg M, Doxsey S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 25.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 26.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 28.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13:221–228. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 30.Mahjoub MR, Qasim Rasi M, Quarmby LM. A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol Biol Cell. 2004;15:5172–5186. doi: 10.1091/mbc.E04-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- 32.Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 33.Biggins S, Rose MD. Direct interaction between yeast spindle pole body components: Kar1p is required for Cdc31p localization to the spindle pole body. J Cell Biol. 1994;125:843–852. doi: 10.1083/jcb.125.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 35.McNally FJ, Thomas S. Katanin is responsible for the M-phase microtubule-severing activity in Xenopus eggs. Mol Biol Cell. 1998;9:1847–1861. doi: 10.1091/mbc.9.7.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dymek EE, Lefebvre PA, Smith EF. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot Cell. 2004;3:870–879. doi: 10.1128/EC.3.4.870-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohret TA, Zhao L, Quarmby LM. Cloning of Chlamydomonas p60 katanin and localization to the site of outer doublet severing during deflagellation. Cell Motil Cytoskeleton. 1999;43:221–231. doi: 10.1002/(SICI)1097-0169(1999)43:3<221::AID-CM5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 39.Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 2001;11:413–419. doi: 10.1016/s0962-8924(01)02085-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


