Abstract
Morphine analgesic tolerance is heritable in both humans and rodents, with some individuals and strains exhibiting little and others exhibiting robust tolerance. 129S6/SvEv and 129P3/J mice reportedly do not demonstrate tolerance to morphine analgesia. Using our laboratory's standard morphine tolerance regimen and a between-subjects design, tolerance developed in the hot plate and tail withdrawal assays as indicated by a change in analgesic efficacy following a morphine challenge dose. Furthermore, the non-competitive NMDA receptor antagonist MK-801 (dizocilipine) blocked morphine tolerance in 129S6/SvEv and CD-1 mice in the hot plate assay. As previously reported, when a within-subjects design and cumulative dosing was employed, no tolerance was observed in the 129P3/J strain. However, using the same morphine regimen and a between-subjects design, comparable tolerance developed between 129P3/J and C57BL/6J strains following a single challenge dose of morphine. Spontaneous hyperalgesia was observed in the tail withdrawal assay following chronic morphine in C57BL/6J, but not 129P3/J mice. Additionally, morphine-tolerant C57BL/6J mice, but not 129P3/J mice, exhibited a large increase in the frequency of tail flicks during the first second following the baseline nociceptive response which may facilitate detection of the response during the tolerant state. We conclude that the method of tolerance assessment affects the ability to detect tolerance and thus, may affect the degree and pattern of heritability of this trait and this could have implications for gene mapping studies.
INTRODUCTION
Morphine analgesic tolerance can be observed in rodents and humans following chronic administration. Susceptibility to morphine tolerance is heritable, with some strains and individuals exhibiting robust tolerance, and others exhibiting very little tolerance (Foley 1993; Hoffmann et al. 1998; Kest et al. 2002; Liang et al. 2006; Mas et al. 2000). The identification of genes that contribute to the heritability of morphine tolerance could aid in the identification of susceptible patients and the development of concurrent treatments that limit tolerance and subsequently, drug dependence.
Using several inbred mouse strains and the tail withdrawal assay, it was demonstrated that morphine analgesic tolerance is heritable with some strains demonstrating robust tolerance (e.g., C57BL/6J), and others such as the 129P3/J strain showing no tolerance (Kest et al. 2002). A very recent report indicates a very different pattern of heritability of morphine tolerance in 7 of the same 11 mouse strains in which there is no correlation in tolerance liability rank (r=0.14; p>0.05; (Liang et al. 2006). Thus, the pattern of heritability of morphine tolerance in inbred mouse strains is not consistent across studies.
Co-administration of NMDA receptor antagonists with morphine disrupts the development of tolerance. Although the signaling pathway(s) that mediate the contribution of NMDA receptors to morphine tolerance is not clear, one hypothesis is that upon activation of protein kinase C, opioid receptor activation leads to NMDA receptor activation, calcium influx, and activation of second messenger systems that mediate changes in gene expression and neuroplasticity responsible for morphine tolerance (Trujillo 2002). Another possibility is that activation of NMDA receptors occurs indirectly in cells downstream of opioid receptors (Eitan et al. 2003) and adaptations in these cells contribute to morphine tolerance.
The 129S6/SvEv inbred mouse strain was first reported by Pasternak and colleagues to lack analgesic tolerance to morphine in the radiant heat tail flick assay (Kolesnikov et al. 1998) and unlike the outbred CD-1 mice, chronic exogenous NMDA co-administration did not accelerate the development of morphine tolerance in the 129S6/SvEv strain. This suggested a "defect" in NMDA receptors in this strain, possibly one which prevented NMDA-mediated neuroplasticity associated with tolerance. Subsequent data indicated that the lack of tolerance in 129S6/SvEv mice is associated with a decreased coupling of opioid receptors to stimulatory (Gs) G-proteins in the tolerant state (Crain & Shen 2000), suggesting an additional mechanism which might contribute to the lack of tolerance.
In the present study, we examined morphine tolerance in 129S6/SvEv and 129P3/J strains using two different regimens of morphine administration and tolerance assessment. Furthermore, we tested the effect of MK-801 (dizocilipine), a non-competitive NMDA receptor antagonist, on the development of morphine tolerance in 129S6/SvEv and CD-1 mice. Last, in the tail withdrawal assay, we examined the frequency of the baseline nociceptive response in 129P3/J and C57BL/6J mice during the naïve and morphine-tolerant state.
METHODS
Drugs
Morphine sulfate was obtained from NIDA (Bethesda, MD). MK-801 (dizocilipine) was purchased from Sigma (St. Louis, MO). Drugs were dissolved in sterile 0.9% NaCl and administered in a volume of 10 ml/kg.
Animals
Male 129S6/SvEv mice were purchased from Taconic (Germantown, NY). Male and female 129P3/J and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Both male and female 129P3/J and C57BL/6J mice were used only in the experiments involving the regimen of Kest et al., (2002) because the investigators used both sexes in their study. The C57BL/6J strain was chosen as a reference strain, given that in contrast to 129P3/J mice, this strain was reported to exhibit robust tolerance (Kest et al. 2002). Male CD-1® mice were purchased from Charles River Laboratories (Raleigh, VA) and were chosen because it is the reference strain in the original finding indicating a lack of tolerance in 129S6/SvEv mice (Kolesnikov et al. 1998). All mice were 8–12 weeks old at the time of testing, housed four per cage in ventilated racks, and provided unrestricted access to food and water. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Institutional Animal Care and Use Committee. Separate mice were always used for each experiment and for each pain assay.
Tolerance regimens
Two different chronic morphine regimens were used in this study. In the first tolerance regimen (Bryant et al. 2006), mice were treated once daily for 6 days, with escalating doses of morphine (10, 10, 20, 20, 40, 40 mg/kg, s.c.) or saline (s.c.).
In the MK-801 experiment, morphine tolerance was induced in the same manner. An additional group receiving MK-801 and morphine (Bryant et al. 2006) received MK-801 (1 mg/kg, i.p.) immediately before morphine and 2h later (1 mg/kg, i.p.) in either the 129S6/SvEv or CD-1 strain ("MK+mor"). Mice receiving just morphine ("morphine") were given saline injections instead of MK-801 injections. Control mice ("saline") received saline for all injections during chronic treatment. An additional control group receiving chronic MK-801 alone ("MK-801") was included to examine the effect on subsequent acute morphine analgesia. These two strains were chosen for this particular experiment because they were used in the original study indicating an NMDA receptor defect in the 129S6/SvEv strain (Kolesnikov et al. 1998).
In the second chronic morphine regimen (Kest et al. 2002), mice were administered escalating doses of morphine three times daily over three days (10, 10, 10 mg/kg s.c. on day 1; 20, 20, 20 mg/kg s.c. on day 2; 40, 40, 40 mg/kg s.c. on day 3). In the case where a within-subjects design was employed on day 1, baseline measurements were recorded as described below and mice were injected and assayed for post-injection latencies every 30 min with different morphine doses (1, 2, 3.6, 6.5, 11.7, 21.0, 21.0, and 21.0 mg/kg). With respect to cumulative dosing, a greater number of injections were required to produce a sizable amount of analgesia in C57BL/6J mice (approximately 75% MPE). Thus, as previously employed, 129P3/J mice were given the same number injections and doses but not exposed to any further pain testing (Kest et al. 2002). Following tolerance induction, the same procedure for cumulative dosing was repeated on day 4 and the cumulative dose-response curves were compared to indicate the degree of tolerance as estimated by shift in ED50 values (see below).
In all tolerance experiments except for the cumulative dosing experiment tolerance was assessed via a difference in analgesic efficacy of a single challenge dose of morphine as indicated by %MPE and area-under-the-curve (challenge doses indicated below and in the Figure Legends).
Pain assays and assessment of morphine analgesia following chronic treatment
The pain assays and methodology used in assessing morphine analgesia are described in the sequential order for which the results are presented. Separate naïve mice were always used for each pain assay.
The 52.5°C hot plate assay (Eddy & Leimbach 1953) was first used to examine morphine analgesic tolerance in 129S6/SvEv (N=8) and 129P3/J mice. Following 6 days of chronic morphine or saline treatment and a between-subjects design (Bryant et al. 2006), for the hot plate (AccuScan Instruments; San Diego, CA), mice were placed on a hot metal surface inside an acrylic cylinder (7.5 cm diameter X 13 cm height) and the baseline latency to flick/lick the hindpaw or jump was recorded with a stopwatch to the nearest 0.1 s. Thirty min later, mice were injected with a challenge dose of morphine (7.5 mg/kg, s.c.) and tested for post-injection latencies every 30 min for 120 min. A cut-off latency of 60 s was employed as the endpoint of analgesia.
In examining modulation of morphine tolerance with MK-801 in the hot plate assay, we used the same chronic regimen with extra treatment groups as described in the previous section (Bryant et al. 2006). Following baseline measurements on day 7, due to strain differences in acute morphine analgesic sensitivity, 129S6/SvEv mice (N=8) were administered 5 mg/kg (s.c.) and CD-1 mice (N=12–14) were administered a challenge dose of 10 mg/kg (s.c.). The reason 129S6/SvEv mice were given a slightly lower challenge dose than in the first experiment (7.5 mg/kg) is because all mice previously reached cut-off latency and thus, it would have been impossible to detect the possibility of enhanced or prolonged morphine analgesia following chronic MK-801 administration.
We also examined morphine tolerance in 129S6/SvEv (N=8) and 129P3/J mice (N=8) using a between-subjects design and the 49.0°C withdrawal assay (Ben-Bassat et al. 1959). Following 6 days of chronic morphine or saline treatment (Bryant et al. 2006), mice were placed momentarily in a cotton restraint immediately before the distal half of the tail was dipped into hot water provided by an electronic water bath (Lauda©) that was accurate to the nearest 0.1°C. The baseline latency for the mouse to flick the tail was recorded with a stopwatch to the nearest 0.1 s. Thirty min later, mice were injected with a challenge dose of morphine (7.5 mg/kg, s.c.) and tested for post-injection latencies every 30 min for 120 min. A 15 s cutoff latency was employed as the endpoint of analgesia. In the case where we wanted to measure the first and second analgesic response in 129P3/J mice in the tail withdrawal assay, the same procedure was employed. However, a lower dose of morphine was used (5 mg/kg, s.c.) so that cut-off latencies would not be reached and thus, the latency of both the first and second nociceptive response could be measured.
In using a separate previously published morphine regimen where the 49.0°C tail withdrawal assay and a within-subjects design were employed (Kest et al. 2002), baseline measurements and post-injection latencies were conducted exactly as described in the published study. Briefly, two baseline measurements were recorded, separated by 20 s. Immediately following the second measurement, mice (N=8) were injected with cumulative doses of morphine (doses indicated above and in the Figure Legend) and tested for analgesia every 30 min via two post-injection latencies, separated by 20 s. Successive cumulative doses were administered immediately following the second post-injection latency for each time point.
As a comparison, we employed the same exact tolerance regimen (Kest et al. 2002) but instead, used a between-subjects design where mice received either chronic morphine or saline. On test day 4, following baseline assessment, mice (N=8) were administered a challenge dose of morphine (129P3/J = 7.5 mg/kg, s.c.; C57BL/6J mice = 25 mg/kg, s.c.) and assayed for morphine analgesia every 30 min for 120 min.
In measuring the frequency of the nociceptive response following chronic morphine, we used the tail withdrawal assay, a between-subjects design, and the chronic regimen of Kest et al., (2002) (see above). Following chronic morphine, on test day 4, mice (N=4) were recorded with a digital camera for the first s during the baseline tail withdrawal response. Twenty s later, this procedure was repeated, and the flicks for the second baseline measurement during the 1 s period were totaled and averaged with the total from the first baseline measurement for each mouse. A flick was defined as a change in direction of tail movement and was scored by observing the video at 30 frames per second and counting the number of changes in the direction of tail movement during the 30 frames.
After measuring the frequency of the baseline nociceptive response following chronic morphine, we wanted to again, confirm tolerance in both strains under this regimen. We used the same challenge dose of morphine for 129P3/J mice (7.5 mg/kg, s.c.), but a lower dose for C57BL/6J mice (15 mg/kg, s.c.) as 25 mg/kg proved to be too high (i.e., too close to cut-off) in the previous experiment. Regardless of strain or chronic treatment, following a morphine challenge, mice did not exhibit any flicking during the 1 s following the first flick (data not shown).
Analysis
In examining morphine analgesia, in order to account for possible changes in baseline latencies following chronic administration, post-injection latencies were converted to percent maximum possible effect (%MPE) according to the following formula: %MPE = (post-injection latency - baseline latency) / (cut-off latency – baseline latency) * 100. Repeated measures ANOVA was used for analysis of experiments utilizing a time course for morphine analgesia. Area-under-the-curve (AUC; min x %MPE) from 0 to 120 min was calculated using the trapezoid method. Student's t-test were used to reveal tolerance in the AUC graphs. Two-way ANOVA was used for other analyses (see Results and Figure legends). ED50 calculations were estimated by converting the cumulative morphine doses to log values and curve fitting using Prism™ (GraphPad©, San Diego, CA). In the experiments using male and female 129P3/J and C57BL/6J mice, there was never a sex by treatment interaction in either strain; thus, the data were collapsed for each experiment.
RESULTS
Morphine tolerance in 129S6/SvEv and 129P3/J strains in the hot plate and tail withdrawal assays following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006)
In Figure 1, following chronic morphine administration (Bryant et al. 2006), mice were tested on the hot plate assay or tail withdrawal assay for analgesic tolerance. 129S6/SvEv mice, but not 129P3/J mice, exhibited an increase in baseline latency in the hot plate assay following chronic morphine administration [t(14)=−2.3; p<0.05] (Table 1; "Bryant" regimen). 129S6/SvEv and 129P3/J mice treated for 6 days with escalating doses of morphine exhibited significant analgesic tolerance in the hot plate assay (F1,14=5.96; p<0.05; F1,14=19.91; p<0.001, respectively) (Figure 1A, B). This was confirmed in analyzing AUC [t(14)=2.8; p=0.01, t(14)=4.6; p<0.001, respectively] (Figure 1C, D).
FIGURE 1. Morphine tolerance in 129S6/SvEv and 129P3/J strains in the hot plate and tail withdrawal assays following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006).
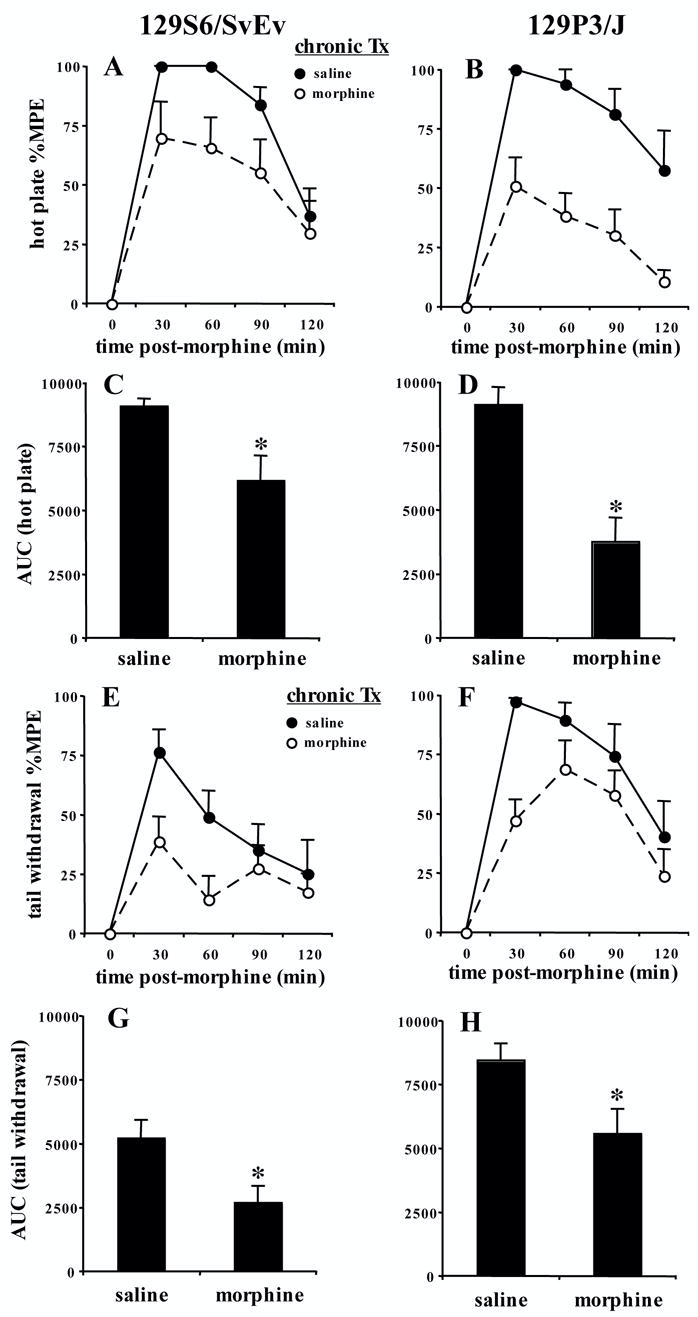
Male 129S6/SvEv (N=8) or 129P3/J mice (N=8) were treated once daily for 6 days with either saline (10 ml/kg, s.c.) or morphine (10–40 mg/kg, s.c.). On day 7, following baseline measurements, separate mice for each pain assay were tested for morphine analgesia (7.5 mg/kg, s.c.) from 30–120 min on either the 52.5°C hot plate or the 49.0°C tail withdrawal. A and B). Tolerance develops in 129S6/SvEv and 129P3/J strains in the hot plate assay. C and D). Tolerance develops in 129S6/SvEv and 129P3/J strains in the hot plate assay as indicated by area under the curve (AUC). E and F). Tolerance develops in 129S6/SvEv and 129P3/J strains in the tail withdrawal assay. G and H). Tolerance develops in 129S6/SvEv and 129P3/J strains in the tail withdrawal assay as indicated AUC. Close circles = chronic saline treatment. Open circles = chronic morphine treatment. Data are presented as the mean %MPE ± S.E.M. A p value of 0.05 was considered significant (*).
TABLE 1. Changes in baseline latencies following chronic morphine administration.
Baseline latencies (± S.E.M.) are listed for each strain, treatment condition, and nociceptive assay following one of two published morphine regimens (Bryant et al. 2006; Kest et al. 2002). "B6" = C57BL/6J.
| Strain | Regimen | Treatment | Assay | Latency |
|---|---|---|---|---|
| 129S6 | Bryant | saline
morphine |
HP | 16.7±1.6
22.0±1.7* |
| 129S6 | Bryant | saline
morphine |
TW | 3.3±0.4
3.9±0.2 |
| 129P3 | Bryant | saline
morphine |
HP | 17.6±1.1
16.7±1.3 |
| 129P3 | Bryant | saline
morphine |
TW | 3.5±0.2
3.1±0.5 |
| 129P3 | Kest | day 1
day 4 |
TW | 2.9±0.2
2.7±0.3 |
| 129P3 | Kest | saline
morphine |
TW | 2.9±0.2
2.5±0.2 |
| B6 | Kest | day 1
day4 |
TW | 2.1±0.2
1.7±0.2* |
| B6 | Kest | saline
morphine |
TW | 2.3±0.2
1.5±0.08* |
| 129S6 | Bryant | saline
morphine MK+mor MK-801 |
HP | 14.9±1.6
16.1±1.1 20±1.2* 17.7±1.3 |
| CD-1 | Bryant | saline
morphine MK+mor MK-801 |
HP | 16.3±1.7
16.0±1.1 23.4±2.7* 25.7±1.9* |
significantly different from control mice receiving chronic saline or from day 1. A p-value of 0.05 was considered significant.
In the tail withdrawal assay, under this regimen, neither 129 strain demonstrated a change in baseline latency following chronic morphine administration (Table 1; "Bryant" regimen). Both 129S6/SvEv and 129P3/J mice exhibited significant tolerance in the tail withdrawal assay (F1,14=4.91; p<0.05; F1,14=5.11; p<0.05, respectively) (Figure 1E an F), which was confirmed in analyzing AUC [t(14)=2.5; p<0.05, t(14)=2.4; p<0.03, respectively] (Figure 1G, H).
Morphine tolerance to both the first and second nociceptive response in the tail withdrawal assay in 129P3/J mice following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006)
Given previous reports, we were quite surprised to observe tolerance in the 129 strains and given that the tail flick response is weaker under morphine, we thought perhaps, we might be measuring a different, less stringent, response from previous investigators (Kest et al. 2002). Thus, Figure 2 represents an examination of both the first and second nociceptive response following tolerance induction (Bryant et al. 2006) and a subsequent lower morphine challenge dose (5 mg/kg, s.c.). There was a main effect of treatment (F1,60=8.2; p<0.01) and response (F1,60=11.9; p<0.01), but no interaction. This indicates that tolerance developed to a similar degree to both the first and second nociceptive response (Figure 2A, B). In examining AUC, there was a main effect of treatment (F1,60=7.6; p<0.01) and response (F1,60=11.3; p=0.001), but no interaction, indicating tolerance with both responses (Figure 2C, D).
FIGURE 2. Morphine tolerance to both the first and second nociceptive response in the tail withdrawal assay in 129P3/J mice following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006).
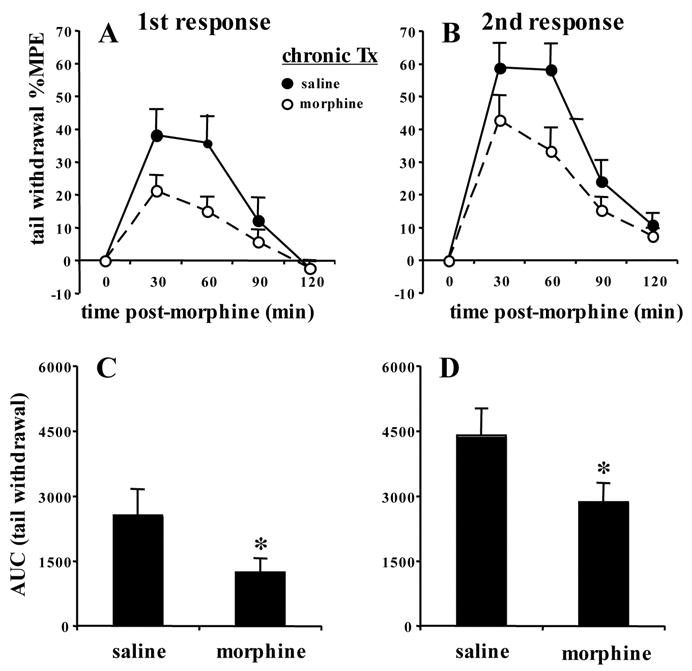
Male 129P3/J mice (N=16) were treated once daily for 6 days with either saline (10 ml/kg, s.c.) or morphine (10–40 mg/kg, s.c.). On day 7, following baseline measurements, mice were administered a challenge dose of morphine (5 mg/kg, s.c.) and tested for analgesia, measuring both the first and second analgesic response from 30–120 min in the 49.0°C tail withdrawal assay. A and B). Tolerance develops to the first and second analgesic response in 129P3/J mice. C and D). Tolerance develops to the first and second nociceptive response as indicated by AUC. Data are presented as the mean %MPE ± S.E.M. A p-value of 0.05 was considered significant ("*").
Disruption of morphine tolerance by MK-801 in 129S6/SvEv and CD-1 mice in the hot plate assay following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006)
In Figure 3, following chronic co-administration of MK-801 with morphine (Bryant et al. 2006), mice were tested for modulation of tolerance on the hot plate assay using a single morphine challenge dose. With respect to 129S6/SvEv mice, in examining changes in baseline latencies, there was a main effect of treatment (F3,28=2.9; p=0.05). Mice that received chronic MK-801 plus morphine ("MK+mor") demonstrated significantly higher baseline latencies than control mice (p<0.05) (Table 1). In examining modulation of morphine tolerance, due to the fact that all control mice reached cut-off latency in the first experiment (Figure 1A) and because we wanted to be able to detect any change in morphine sensitivity following chronic MK-801, we used a lower morphine challenge dose (5 mg/kg, s.c.). There was a main effect of treatment (F3,28=6.09; p<0.05), time (F3,3=28.69, p<0.05), and an interaction of treatment with time (F3,9=4.21; p<0.05). Subsequent one-way ANOVA followed by Fisher’s PLSD indicated that significant tolerance developed at 30 min post-injection (“morphine” < “saline”; F3,28=6.6; p<0.05) but not at later time points (p>0.05). The shorter lasting analgesia (and subsequently, tolerance) compared to Figure 1 could be due to a combination of both a lower challenge dose used in this experiment (5 mg/kg versus 7.5 mg/kg, s.c.) and cross-tolerance of stress-induced analgesia across all groups (Lewis et al. 1981) because this regimen required three times as many injections and concomitant handling. Co-administration of MK-801 with morphine completely blocked the development of morphine tolerance exhibited at 30 min in the 129S6/SvEv strain as these mice demonstrated significantly greater analgesia than morphine-tolerant mice (p<0.05) but did not differ from control mice (p>0.05) (Figure 3A). Chronic administration of MK-801 alone ("MK-801") had no effect on acute morphine analgesia at 30 min because this group did not differ significantly from control mice (p>0.05). However, there was a significant prolongation of morphine analgesia at 60 min (F3,28=6.41; p<0.05) and 90 min (F3,28=4.18; p<0.05) in both groups that previously received MK-801 as compared with control mice (p<0.05) (Figure 3A).
FIGURE 3. Disruption of morphine tolerance by MK-801 in 129S6/SvEv and CD-1 mice in the hot plate assay following a between-subjects design and a once daily regimen of escalating morphine doses (Bryant et al., 2006).
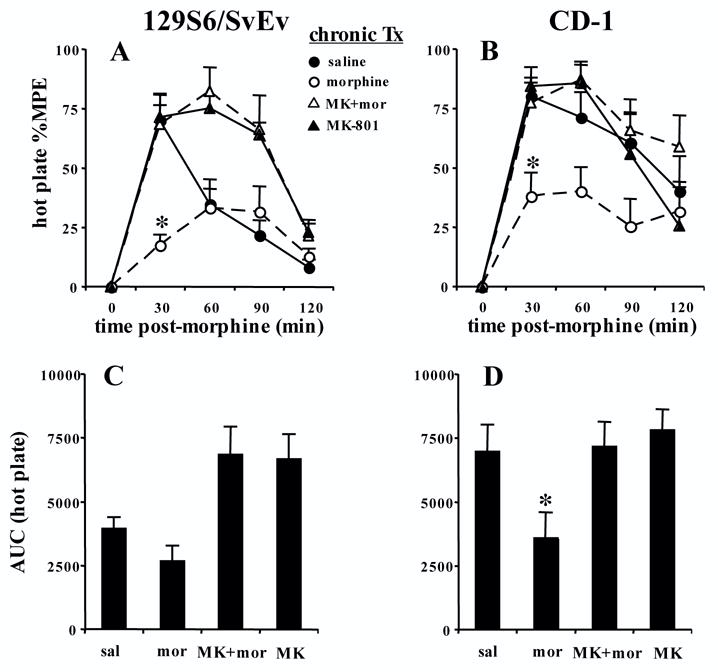
Male mice were treated once daily for 6 days with the non-competitive NMDA receptor antagonist MK-801 (1 mg/kg, i.p.) and a simultaneous dose of morphine (10–40 mg/kg, s.c.) followed 2 h later by an additional injection of antagonist (1 mg/kg, i.p.). On day 7, 30 min following measurement of baseline hot plate latencies, mice were then administered a morphine challenge (5 mg/kg, s.c. for 129S6/SvEv mice; N=8; 10 mg/kg, s.c. for CD-1 mice; N=12–14) and tested for analgesia every 30 min for 120 min. A and B). In both strains, mice previously receiving chronic administration of MK-801 plus morphine (“MK+mor”) showed significantly greater analgesia than mice previously receiving morphine alone (“*”; “morphine”) at 30 min, indicating an attenuation of tolerance. C and D). In analysis of AUC, significant tolerance developed in CD-1, but not 129S6/SvEv mice, which was attenuated by MK+mor treatment ("*"). Data are presented as the mean %MPE ± S.E.M. A p value of 0.05 was considered significant.
In examining the AUC, there was a main effect of treatment (F3,28=6.0; p<0.01). However, when considering the entire time course, tolerance was not significant (p>0.05; "sal" = "mor") (Figure 3C). 129S6/SvEv mice chronically treated with MK-801 and morphine ("MK+mor") and mice treated with MK-801 alone ("MK-801") showed significantly more analgesia than control mice, indicating a prolongation of morphine analgesia (Figure 3C).
With respect to CD-1 mice, in examining changes in baseline latencies, there was a main effect of treatment (F3,48=6.9; p<0.05). Mice receiving chronic MK-801 plus morphine ("MK+mor") or MK-801 alone ("MK-801") exhibited significantly higher baseline latencies than control mice receiving chronic saline (p<0.05) (Table 1). In examining modulation of tolerance, one-way ANOVA was conducted at the first 30 min time point to facilitate comparison with the 129S6/SvEv strain. There was a main effect of treatment (F3,48=6.46; p<0.001). Fisher’s PLSD indicated that morphine pretreated mice (“morphine”) showed significantly less analgesia than saline pretreated mice (“saline”), indicating the development of tolerance. Mice previously receiving MK-801 plus morphine (“MK+mor”) showed comparable analgesia to control mice (p>0.05) which was significantly greater than morphine-tolerant mice (p<0.05), indicating a complete blockade of morphine tolerance (Figure 3B).
In examining AUC, there was a main effect of treatment (F3,48=3.7; p<0.05). Significant tolerance developed as mice receiving chronic morphine administration showed less analgesia than mice receiving chronic saline ("mor" < "sal"; p<0.05). Co-administration of MK-801 with morphine ("MK+mor") completely blocked the development of morphine tolerance as these mice showed significantly greater analgesia than mice receiving morphine alone ("mor"; p<0.05) and did not differ from control mice receiving chronic saline ("sal"; p>0.05) (Figure 3D).
Morphine tolerance under a separate morphine regimen (Kest et al., 2002) following a within-subjects design and cumulative dosing or a between-subjects design and a single challenge dose
Following chronic morphine administration and a within-subjects design (Kest et al. 2002), C57BL/6J mice, but not 129P3/J mice, exhibited significant baseline hyperalgesia when comparing day 4 to day 1 [t(7)=2.4; p<0.05] (Table 1; "Kest" regimen). In 129P3/J mice, there was no significant change in ED50 values from day 1 to day 4 (day 1: ED50 = 16.0; 95% C.I.=4.6–55.2; day 4: ED50=14.3; 95% C.I.=6.4–31.9) (Figure 4A). ED50 estimates for days 1 and 4 were not comparable in the C57BL/6J strain because of the inability to achieve comparable maximum possible effects (Figure 4B).
FIGURE 4. Morphine tolerance under a separate morphine regimen (Kest et al., 2002) following a within-subjects design and cumulative dosing or a between-subjects design and a single challenge dose.
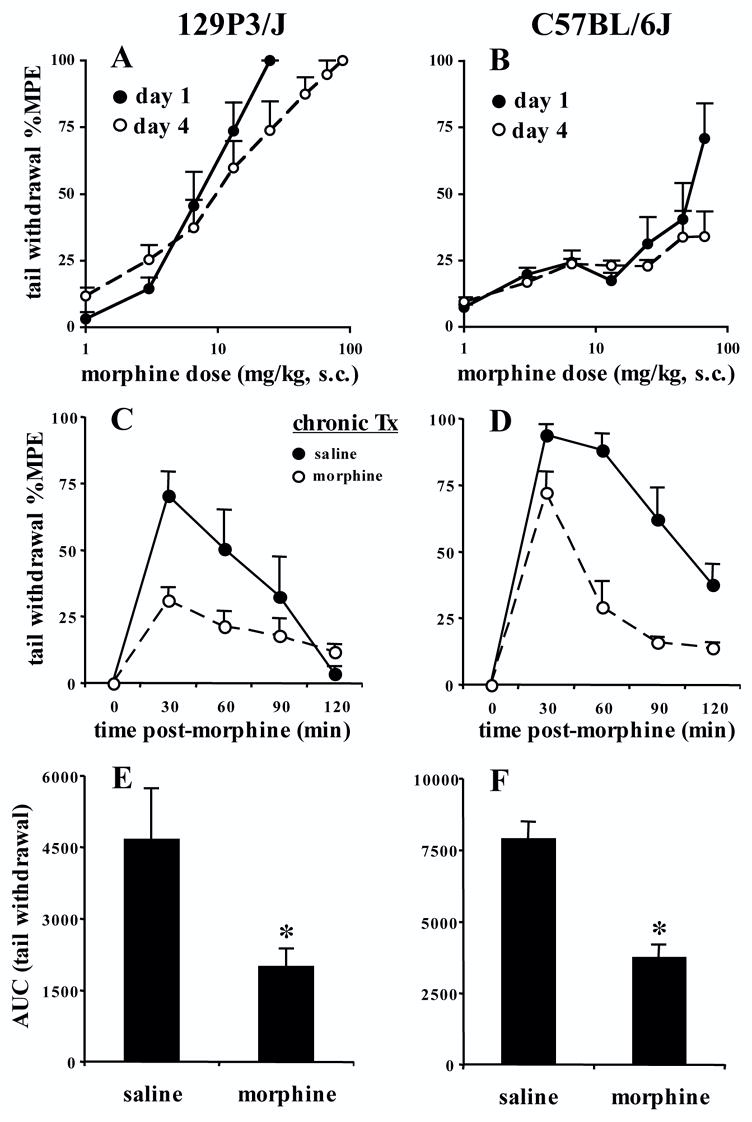
Mice (N=8) were assayed for baseline latencies on day 1, and immediately administered morphine (s.c.). Thirty min later, immediately following post-injection latency assessment mice, were administered a subsequent dose of morphine. This was repeated every 30 min. Doses of 1, 2, 3.6, 6.5, 11.7, and 21.0 mg/kg were administered. In the case of extra injections, 21.0 mg/kg was administered (Kest et al. 2002). All mice received the same number of injections and doses of morphine on day 1, although if they reached cut-off, they were not subjected to further pain testing (Kest et al. 2002). The x-axis represents the cumulative morphine dose. A and B). No tolerance developed in 129P3/J mice in the tail withdrawal assay as indicated by a lack of shift in the dose-response curve. Comparable ED50 values were obtained between days 1 and 4 (see Results). ED50 estimates for C57BL/6J mice were not possible, due to the lack of comparable maximum possible effects that could be reached, even with subsequent dosing on day 4 (data not shown). C and D). Tolerance developed in 129P3/J and C57BL/6J mice in the tail withdrawal assay following a between-subjects design, the regimen of Kest et al. (2002), and a single challenge dose of morphine on test day (129P3/J = 7.5 mg/kg, s.c.; C57BL/6J = 25 mg/kg, s.c.) . E and F). Tolerance as indicated by AUC in 129P3/J and C57BL/6J mice. Data are presented as the mean ± S.E.M. A p value of 0.05 was considered significant ("*").
Following chronic morphine administration (Kest et al. 2002) and employing a between-subjects design, C57BL/6J, but not 129P3/J mice, exhibited baseline hyperalgesia [t(14)=3.3; p<0.01] (Table 1). In examining tolerance following a single challenge dose of morphine, in the 129P3/J strain, there was an interaction of treatment with time (F1,3=5.7; p<0.01) (Figure 4C). Significant tolerance developed in 129P3/J mice as indicated by AUC [t(14)=2.3; p<0.05] (Figure 4E). In C57BL/6J mice, there was a main effect of treatment (F1,14=28.1; p=0.0001) and an interaction of treatment with time (F1,3=4.4; p<0.01) (Figure 4D). Significant tolerance developed in C57BL/6J mice as indicated by AUC [t(14)=5.2; p=0.0001] (Figure 4F).
Increase in the frequency of the baseline nociceptive response following chronic morphine administration in C57BL/6J mice, but not 129P3/J mice
In Figure 5A, following chronic administration of morphine, C57BL/6J mice, but not 129P3/J mice exhibited a large increase in the frequency of tail flicks during the first second following the baseline nociceptive response. There was a main effect of treatment (F1,12=17.2; p=0.001), strain (F1,12=35.3; p<0.0001), and an interaction of treatment with strain (F1,1=6.3; p=0.01). C57BL/6J mice receiving chronic morphine administration, but not 129P3/J mice, showed a large, significant increase in the number of tail flicks during the first second following the first baseline nociceptive response [(t(6)=−5.0; p<0.01)] (Figure 5A) . Following a subsequent morphine challenge (Figure 5B), regardless of strain, tolerant mice did not exhibit any flicking during the 1 s following the first flick (data not shown). To confirm tolerance in both strains, we used a lower challenge dose of morphine in C57BL/6J mice than Figure 4D (15 mg/kg, s.c. instead of 25 mg/kg) which was equianalgesic with that used in the 129P3/J strain (7.5 mg/kg, s.c.). There was a main effect of treatment (F1,12=95.8; p<0.0001), but not strain, indicating a similar degree of tolerance between 129P3/J and C57BL/6J strains (Figure 5B).
FIGURE 5. Increase in the frequency of the baseline nociceptive response following chronic morphine administration in C57BL/6J mice, but not 129P3/J mice.
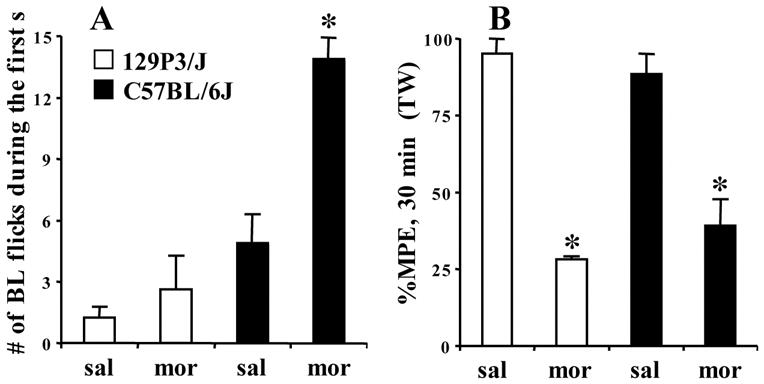
Using a between-subjects design, mice (N=4) were administered escalating doses of morphine over 3 days (Kest et al. 2002). On day 4, following the first baseline tail flick, the tails were left in the waterbath for 1 s and the total number of flicks was recorded on a digital video. A). Morphine-tolerant C57BL/6J mice, but not morphine-tolerant 129P3/J mice, exhibited a large, significant increase in the number of baseline flicks following the first nociceptive response. Following a subsequent morphine challenge, regardless of strain, tolerant mice did not exhibit any flicking during the 1 s following the first flick (data not shown). B). To confirm tolerance in both strains, we used a lower challenge dose of morphine in C57BL/6J mice (15 mg/kg, s.c.) as compared to Figure 4 (25 mg/kg, s.c.) and the same challenge dose in 129P3/J mice (7.5 mg/kg, s.c.). Comparable acute analgesia and tolerance was observed in both strains at 30 min post-injection. "sal" = chronic saline treatment. "mor" = chronic morphine treatment. "BL" = baseline. "TW" = tail withdrawal. Data are presented as the mean ± S.E.M. A p value of 0.05 was considered significant.
DISCUSSION
Using a between-subjects design and a single morphine challenge dose, 129S6/SvEv and 129P3/J strains exhibited analgesic tolerance to morphine in the hot plate and tail withdrawal assays following two separate regimens of chronic morphine administration. These results were surprising, given three reports utilizing three different methodologies and indicating no tolerance in the 129 strains (Crain & Shen 2000; Kest et al. 2002; Kolesnikov et al. 1998). Because there is a reduced intensity of the tail withdrawal response under morphine, we thought that we might be measuring a weaker response than previous investigators and this might account for the discrepant result. As such, we used a lower challenge dose of morphine and measured both the first and second tail flick response following a morphine challenge; however tolerance was observed in both instances. It is possible that tolerance might not have been observed in 129S6/SvEv mice had we employed the same temperature (55ºC) and exact methodology as previous investigators with this particular strain (Crain & Shen 2000). Indeed, when employing the same chronic regimen and method of tolerance assessment as one of the three studies, no tolerance was observed in the 129P3/J strain (Kest et al. 2002). Last, we report for the first time that following chronic morphine administration, there was a dramatic increase in the number of tail flicks during the first second following the baseline nociceptive response in C57BL/6J, but not 129P3 mice. This strain-specific rapid tail fibrillation represents an additional adaptive behavioral response that can occur following chronic morphine and which may be heritable.
Although we replicated the lack of morphine tolerance in 129P3/J mice following a within-subjects design and cumulative dosing (Kest et al. 2002), we were unable to reproduce the robust shift to the right in the dose-response curve of C57BL/6J mice that was previously reported, in part because on day 4, the mice did not reach comparable analgesia to day 1, even up to a cumulative dose of 108.8 mg/kg (data not shown), and thus, ED50 calculations between days 1 and 4 were not comparable. This may be due to the fact that in our hands, morphine was considerably less sensitive in this strain compared to the previous study (Kest et al. 2002) and thus, perhaps maniuplating the starting dose or increment dose would have affected the degree of tolerance detected (Duttaroy et al. 1997). Nevertheless, in C57BL/6J mice, we only saw a significant change in analgesic efficacy (i.e., tolerance) at the higher morphine doses, beginning at 66.8 mg/kg (Figure 4B). Given our effort to conduct the experiment exactly as previously described, the source of discrepancy is not clear. However, in the past, using C57BL/6J mice, we have constructed cumulative dose-response curves following a number of different chronic morphine regimens and cumulative dosing procedures and have never observed large shifts in the dose-response curve or ED50 values. Although using separate mice for each dose might alleviate the problem, due to the exponential costs that this would require, we frequently report a difference in analgesic efficacy following a single morphine challenge dose (Bryant et al. 2006; Bryant 2005; Eitan et al. 2003).
A likely explanation for the lack of tolerance in the lower cumulative doses is that acute tolerance develops rapidly and progressively in both strains on both days 1 and 4, and thus, there is no difference in analgesia at the initial doses. This hypothesis is supported by the observation that a cumulative dose of 24.8 mg/kg produces approximately 30% MPE in C57BL/6J mice (Figure 4B), whereas roughly the same acute dose (25 mg/kg) produces almost 100% MPE in naive C57BL/6J mice (Figure 4D). Although not as drastic of a difference, this holds true for 129P3/J mice such that a cumulative dose of 6.6 mg/kg produces approximately 45%MPE (Figure 4A) whereas a similar acute dose (7.5 mg/kg) in naive mice produces approximately 75–95%MPE (Figure 4C, 5B). An alternative explanation to acute tolerance is that repeated testing induces tissue inflammation in the tail which results in hyperalgesia on top of morphine analgesia and as a result, an apparent reduction in morphine analgesia.
We replicated the strain-specific spontaneous hyperalgesia resulting from chronic morphine that occurs in the tail withdrawal assay in C57BL/6J, but not 129P3/J mice (Bryant et al. 2006; Bryant 2005; Kest et al. 2002) (Table 1). Additionally, C57BL/6J, but not 129P3/J mice, exhibited a large increase in the frequency of tail flicks during the first second following the first baseline nociceptive response (Figure 5A). To our knowledge, this sharp increase in the number of flicks is the first piece of evidence to suggest a change in the intensity of the nociceptive response following chronic morphine. Following a subsequent morphine challenge (Figure 5B), we did not observe any flicking during the second following the first flick in either strain. However, given the spontaneous hyperalgesia and the accompanied rapid flicking in C57BL/6J mice receiving chronic morphine, it is possible that there is a strain-specific increase in intensity of the response under morphine. In support, there is a strong relationship between the intensity of the nociceptive stimulus and the intensity of the electromyogram response of the extensor caudae medialis muscle of the tail (Tsuruoka et al. 1988). Similar to an increase in stimulus intensity, chronic morphine also shortens the latency to the nociceptive response (Table 1). Interestingly, strain differences in the firing rate of cutaneous nociceptors to thermal stimulation have recently been reported with C57BL/6 mice exhibiting the highest frequency, in particular, at 49ºC (Lawson 2006). Future studies using electrophysiological and electromyogram techniques will determine if there are strain-dependent changes in the frequency and intensity of the nociceptive response and whether these changes correlate with spontaneous morphine-induced hyperalgesia and tolerance.
In the hot plate assay, the development of analgesic tolerance to morphine was blocked in 129S6/SvEv and CD-1 mice by chronic co-administration with the non-competitive NMDA receptor antagonist MK-801 (Figure 3), as previously reported with CD-1 mice in the tail flick assay (Elliott et al. 1994; McLemore et al. 1997) and with C57BL/6J mice using the same regimen and hot plate assay (Bryant et al. 2006). Data from a previous study suggested that 129S6/SvEv mice had an NMDA receptor defect based on the observation that exogenous NMDA administration facilitated the development of morphine tolerance only in the outbred CD-1 strain (Kolesnikov et al. 1998). An important difference between the previous study and the present one is that we used the hot plate assay and they used the tail flick assay. One drastic example of how these two pain assays can differ in terms of modulation of morphine tolerance comes from our recent observation that under the same chronic regimen (but in male C57BL/6J mice), MK-801 attenuated the development of morphine tolerance in the hot plate assay, while in the tail withdrawal assay, it actually facilitated it (Bryant et al. 2006). Thus, that an NMDA receptor antagonist attenuated morphine tolerance in the hot plate assay does not eliminate the possibility that 129S6/SvEv mice have dysfunctional NMDA receptors. However, because of the mere fact that these mice exhibit tolerance in the tail withdrawal assay (Figure 1E, G), if this strain has a defect in NMDA receptor function, it is not relevant to morphine tolerance under these conditions.
Chronic administration of MK-801 with morphine produced a significant increase in baseline latency in both 129S6/SvEv and CD-1 strains (Table 1), as previously reported in male C57BL/6J mice (Bryant et al. 2006). Furthermore, chronic MK-801 per se prolonged the subsequent analgesic effect of a challenge morphine dose in 129S6/SvEV mice (Figure 3A, C), while having no effect in CD-1 mice (Figure 3B, D). One or both of these effects could contribute to the modulation of morphine tolerance in 129S6/SvEv mice. Enhanced morphine analgesia following chronic NMDA receptor antagonism has been reported previously (Dunbar & Yaksh 1996). The strain dependency of the prolongation of morphine analgesia following chronic NMDA receptor antagonism suggests that the effect depends on the genotype. A complete strain survey examining the modulation of acute morphine analgesia and tolerance by chronic NMDA receptor antagonism will be necessary to determine the heritability of these traits. Possible mechanisms could include an upregulation of functional opioid receptors or enhanced binding of morphine as occurs with the mu opioid receptor-selective peptide agonist DAMGO following co-administration with an NMDA receptor antagonist (Wong et al. 1996).
We conclude that the detection of morphine analgesic tolerance in 129 strains depends on the method of tolerance assessment. Utilizing a between-subjects design and a single challenge dose of morphine, comparable tolerance was observed between 129P3/J and C57BL/6J mice. This suggests that the degree and pattern of heritability of morphine tolerance across mouse strains previously reported (Kest et al. 2002) may look completely different when employing this type of design and this may have implications for gene mapping studies where previous attempts to identify quantitative trait loci with morphine tolerance have been unsuccessful (Kest et al. 2004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Bassat J, Peretz E, Sulman FG. Analgesimetry and ranking of analgesic drugs by the receptacle method. Arch Int Pharmacodyn Ther. 1959;122:434–47. [PubMed] [Google Scholar]
- Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA Receptor Antagonism Disrupts the Development of Morphine Analgesic Tolerance in Male, but not Female C57BL/6J Mice. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zaki PA, Carroll FI, Evans CJ. Opioids and Addiction: Emerging pharmaceutical strategies for reducing reward and opponent processes. Clinical Neuroscience Research. 2005;5:103–115. [Google Scholar]
- Crain SM, Shen K. Enhanced analgesic potency and reduced tolerance of morphine in 129/SvEv mice: evidence for a deficiency in GM1 ganglioside-regulated excitatory opioid receptor functions. Brain Res. 2000;856:227–35. doi: 10.1016/s0006-8993(99)02446-4. [DOI] [PubMed] [Google Scholar]
- Dunbar S, Yaksh TL. Concurrent spinal infusion of MK801 blocks spinal tolerance and dependence induced by chronic intrathecal morphine in the rat. Anesthesiology. 1996;84:1177–88. doi: 10.1097/00000542-199605000-00020. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Kirtman R, Farrell F, Phillips M, Philippe J, Monderson T, Yoburn BC. The effect of cumulative dosing on the analgesic potency of morphine in mice. Pharmacol Biochem Behav. 1997;58:67–71. doi: 10.1016/s0091-3057(96)00463-7. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–9. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain. 1994;56:69–75. doi: 10.1016/0304-3959(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Foley KM. Current and emerging issues in cancer pain: research and practice. Raven Press; New York: 1993. pp. 331–50. [Google Scholar]
- Hoffmann O, Plesan A, Wiesenfeld-Hallin Z. Genetic differences in morphine sensitivity, tolerance and withdrawal in rats. Brain Res. 1998;806:232–7. doi: 10.1016/s0006-8993(98)00768-9. [DOI] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–8. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–7. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–9. [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Anderson CE, Koerber HR. Properties of cutaneous nociceptors in different mouse strains as assessed by comprehensive phenotyping. Society for Neuroscience Abstract. 2006;441.3 [Google Scholar]
- Lewis JW, Sherman JE, Liebeskind JC. Opioid and non-opioid stress analgesia: assessment of tolerance and cross-tolerance with morphine. J Neurosci. 1981;1:358–63. doi: 10.1523/JNEUROSCI.01-04-00358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–40. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Mas M, Sabater E, Olaso MJ, Horga JF, Faura CC. Genetic variability in morphine sensitivity and tolerance between different strains of rats. Brain Res. 2000;866:109–15. doi: 10.1016/s0006-8993(00)02255-1. [DOI] [PubMed] [Google Scholar]
- McLemore GL, Kest B, Inturrisi CE. The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res. 1997;778:120–6. doi: 10.1016/s0006-8993(97)00985-2. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. The neurobiology of opiate tolerance, dependence and sensitization: mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotox Res. 2002;4:373–91. doi: 10.1080/10298420290023954. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Matsui A, Matsui Y. Quantitative relationship between the stimulus intensity and the response magnitude in the tail flick reflex. Physiol Behav. 1988;43:79–83. doi: 10.1016/0031-9384(88)90101-1. [DOI] [PubMed] [Google Scholar]
- Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS. Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors. Eur J Pharmacol. 1996;297:27–33. doi: 10.1016/0014-2999(95)00728-8. [DOI] [PubMed] [Google Scholar]


