Abstract
Thioester-containing proteins (TEPs) are a major component of the innate immune response of insects to invasion by bacteria and protozoa. TEPs form a distinct clade of a superfamily that includes the pan-protease inhibitors α2-macroglobulins and vertebrate complement factors. The essential feature of these proteins is a sequestered thioester bond that, after cleavage in a protease-sensitive region of the protein, is activated and covalently binds to its target. Recently, TEP1 from the malarial vector Anopheles gambiae was shown to mediate recognition and killing of ookinetes from the malarial parasite Plasmodium berghei, a model for the human malarial parasite Plasmodium falciparum. Here, we present the crystal structure of the TEP1 isoform TEP1r. Although the overall protein fold of TEP1r resembles that of complement factor C3, the TEP1r domains are repositioned to stabilize the inactive conformation of the molecule (containing an intact thioester) in the absence of the anaphylotoxin domain, a central component of complement factors. The structure of TEP1r provides a molecular basis for the differences between TEP1 alleles TEP1r and TEP1s, which correlate with resistance of A. gambiae to infection by P. berghei.
Keywords: Anopheles gambiae, crystal structure, innate immunity, thioester
Malaria is a major global health concern, with >300 million episodes per year (1). The increasing prevalence of malaria in tropical developing nations, the appearance of multidrug-resistant forms, and the lack of an effective vaccine have fueled interest in vector control strategies, including the vector's innate immune response to malarial infection (2). Thioester-containing proteins (TEPs) are a major component of the innate immune response of insects to invasion by bacteria and protozoa (3). TEPs form a distinct clade of a superfamily that includes the pan-protease inhibitors α2-macroglobulins and vertebrate complement factors (4). The essential feature of these proteins is a sequestered thioester bond that, after cleavage in a protease-sensitive region of the protein, is activated and covalently binds to its target. The vertebrate complement system is activated by multiple pathways and is an effector of acquired and innate immune responses, including opsonization and direct killing of pathogens by lysis (5).
The chief vector for the human malarial parasite Plasmodium falciparum is the mosquito Anopheles gambiae. The sequencing of the A. gambiae genome revealed 19 TEP ORFs, including 4 putative haplotypes (6). Two of these, TEP1 and TEP16, are alleles of a common gene, and were renamed TEP1s and TEP1r, respectively (7). TEP1 protein opsonizes bacteria in a thioester-dependent manner (8). TEP1 is up-regulated in the early stages of infection by P. berghei, a rodent model for P. falciparum (7), and furthermore, the two TEP1 alleles correlate with A. gambiae strains that are susceptible (S strain, TEP1s) or refractory (R strain, TEP1r), respectively, to infection by Plasmodium berghei. It is yet to be determined whether infection by P. falciparum produces a similar phenotype with respect to TEP1 (or any other TEP). TEP1s-expressing mosquitoes kill 80% of P. berghei ookinetes by lysis after they cross the midgut epithelium, but a substantial number successfully form oocysts and continue their life cycle. In contrast, TEP1r-expressing mosquitoes kill all ookinetes. TEP1r binds to parasites with faster kinetics and can facilitate clearance of dead parasites by melanization as well as by lysis.
The central component of the vertebrate complement pathway is complement factor C3. C3 is a 180-kDa glycoprotein that is proteolytically cleaved during secretion into two chains, the α-chain (992 aa including C terminus) and β-chain (645 aa including N terminus of the mature peptide). The published structures of human and bovine complement factor C3 (HumC3 and BovC3) in their native (inactive) state (9, 10) as well as structures of the activated form of human C3, C3b (11–14), and its major degradation product C3c (10, 13, 14), have provided a wealth of new information on the structure and mechanism of the complement system. In these structures, the β-chain forms a ring (the β-ring) comprised of six β-sheet domains termed macroglobulin (MG) domains. The sixth MG domain (MG6) is divided between the β-chain and the α-chain by an insert that includes a linker (LNK) at the end of the β-chain and the anaphylotoxin (ANA) domain at the start of the α-chain. Dissociation of the ANA domain after proteolysis at a site between it and the MG6 domain is required for complement activation. The remainder of the α-chain contains two MG domains that bracket a β-sheet domain (CUB) domain that is itself divided by insertion of the α-helical thioester-containing domain (TED). A short anchor region (ANK) lies between the final MG domain (MG8) and the C-terminal C345C domain.
TEP1 is phylogenetically related to complement factor C3, with sequence identity between the two proteins of 31% within the TED and 25% overall. This excludes two domains, the ANA and the C345C domains, which are absent in TEP1. Unlike C3, TEP1 is secreted as a single-chain 150-kDa glycoprotein. Here, we present the crystal structure of TEP1r, describe its relationship to C3, and discuss the differences between TEP1r and TEP1s in light of the TEP1r structure.
Results and Discussion
Domain Arrangement.
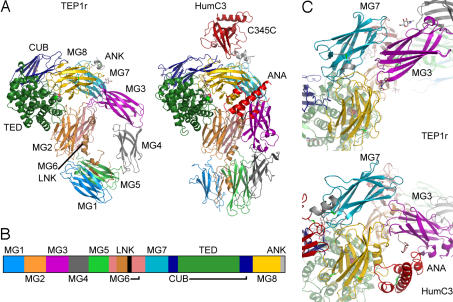
The structure of TEP1r was solved by using diffraction data to 2.7-Å resolution and phases determined by multiple isomorphous replacement with anomalous scattering (MIRAS) (see Materials and Methods and Table 1). The model comprises residues 1–1,318 of the mature peptide. Residues 36–43, 555–559, and 582–609 are disordered and could not be located within the electron density. The structure of TEP1r is similar to that of the mammalian complement factor C3 [Fig. 1A and supporting information (SI) Fig. 5]. TEP1r contains all of the C3 domains except the ANA and C345C domains as described above (Fig. 1B). Each domain aligns separately with the corresponding domains of HumC3, BovC3, and HumC3c (SI Table 3). The combined MG2, MG6, MG7, MG8, TED, and CUB domains form a core that aligns well between TEP1r and C3 (SI Table 4), as do the combined macroglobulin domains MG1–MG5 and, to a lesser extent, MG3–MG4. However, the arrangement of these combined domains is significantly altered in TEP1r compared with C3. MG3 and MG4 are rotated almost 90°, resulting in a parallel alignment of MG3 with MG7 (Fig. 1C). Conversely, the MG4 domain is rotated ≈50° away from the MG5 domain, making few contacts with either it or MG3. The combined MG3–MG4 domain swings about the hinge regions 198–201 and 395–400, which causes the combined MG1–MG5 domain to tilt, making a more acute angle with MG2–MG6. The MG4 domain contains significant sequence and structural differences in the outer β-sheet. Two β-strands (C and C′) are extended in TEP1r relative to C3, whereas the loop between the last two strands (the FG loop) is truncated (SI Fig. 5).
Table 1.
Data collection and refinement statistics
| Native (refinement) | Native (phasing) | MeHgOAc | EMTS | Xenon | |
|---|---|---|---|---|---|
| Data collection | |||||
| Beamline | APS 19BM | APS 19ID | APS 19ID | APS 19ID | APS 19ID |
| Wavelength, Å | 0.9791 | 0.9791 | 1.008 | 1.009 | 1.550 |
| Unit cell (P43212) | |||||
| a, b, Å | 150.515 | 150.781 | 150.806 | 150.541 | 151.420 |
| c, Å | 226.315 | 226.980 | 227.940 | 227.489 | 228.235 |
| Mosaicity, ° | 0.50 | 0.38 | 0.45 | 0.68 | 0.69 |
| Resolution, Å | 50–2.70 | 50–3.65 | 50–4.00 | 50–4.00 | 50–3.60 |
| (Final shell) | (2.75–2.70) | (3.71–3.65) | (4.07–4.00) | (4.07–4.00) | (3.66–3.60) |
| Observations | 563,996 | 251,521 | 180,036 | 178,917 | 182,332 |
| Unique reflections | 72,031 | 29,799 | 22,511 | 22,718 | 31,388 |
| Completeness, % | 99.9 (99.9) | 99.9 (98.1) | 98.2 (98.9) | 100.0 (100.0) | 98.7 (91.6) |
| Redundancy | 7.8 (7.5) | 8.4 (6.2) | 8.0 (8.1) | 7.9 (8.1) | 5.8 (4.8) |
| 〈I〉/〈σ〉 | 28.2 (2.1) | 14.0 (2.3) | 15.4 (3.7) | 20.6 (3.4) | 15.7 (4.9) |
| 〈I〉/〈σ〉 >3, % | 73.5 (27.1) | 68.2 (31.1) | 76.6 (46.5) | 75.2 (42.4) | 79.6 (55.0) |
| Rsym, % | 8.0 (89.8) | 17.1 (70.9) | 14.3 (57.8) | 11.0 (63.6) | 13.0 (34.5) |
| MIRAS phasing* | |||||
| Resolution, Å | 50–2.70 | 50–4.0 | |||
| Rmerge, %† | 17.2 | 25.7 | 30.1 | ||
| No. of sites | 3‡ | 5 | 2 | ||
| Phasing power§ | 0.72/0.51 | 0.69/0.46 | 0.68/0.47 | ||
| RCullis (iso.)§ | 0.92/0.86 | 0.93/0.89 | 0.94/0.90 | ||
| RCullis (anom.) | 1.00¶ | 0.98 | 1.00 | ||
| FOM | 0.21 | 0.23 | 0.19 | ||
| Combined FOM | 0.38 | ||||
| RESOLVE FOM | 0.58 | ||||
| Refinement | |||||
| Resolution range | 45.88–2.70 (2.77–2.70) | ||||
| Reflections work set | 68,228 (4,901) | ||||
| Reflections test set | 3,631 (241) | ||||
| Rcryst | 0.239 (0.352) | ||||
| Rfree | 0.275 (0.398) | ||||
| Est. coord. error, Å | 0.422 | ||||
| rmsd. fom ideal | |||||
| Bond length, Å | 0.009 | ||||
| Bond angles, ° | 1.188 | ||||
| Atom type | Total no. of atoms | B-factors, Å2 | |||
| Protein atoms‖ | 10,333 | 63 | |||
| Heteroatoms | 73 | 71 | |||
| Waters | 140 | 48 |
EMTS, ethylmercurithiosalicylate.
*Refinement on isomorphous differences limited to reflections with FP, FPH > 3σ, |FPH − FP| -< 4.26σ based on SCALEIT analysis. No limits for refinement on anomalous differences.
†Scaling of data to Native (phasing).
‡These three sites are common to both Hg derivatives.
§Reported for acentric/centric reflections.
¶0.98 for resolution range 50–6.0 Å.
‖Includes 63 atoms from seven alternate residue conformations.
Fig. 1.
Overall structure of TEP1r. (A) Domain arrangements of TEP1r in comparison with human C3 (PDB ID 2A73). The mature protein commences with domain MG1 (medium blue) followed by MG2 (orange), MG3 (purple), MG4 (medium gray), MG5 (light green), MG6 (pink), LNK (light brown), MG7 (light blue), CUB (navy blue), TED (dark green), MG8 (yellow), and ANK (light gray). Additional domains in C3 are the ANA domain (red, between MG3 and MG8) and the C345C domain (dark red, top). (B) Sequence schematic of TEP1r, showing disposition of domains. (C) The relative position of MG3 to MG7-MG8 in TEP1r compared with human C3.
Four of 10 putative N-linked glycosylation sites in TEP1r display electron density consistent with at least one covalently attached N-acetylglucosamine residue: Asn 178 in MG2, Asn 221 and Asn 291 in MG3, and Asn 616 in MG6. The glycosylation at Asn 291 is located between MG3 and MG8, comprising 20% of the interface between these domains (Fig. 2 and SI Figs. 6 and 7b). A conserved glycosylation site within the CUB domain was modeled in the native HumC3 and BovC3 structures. This site is not conserved in TEP1r nor was density observed for a glycan in that region of our structure. Likewise, nonconserved are the 10 disulfide bridges that are a structural feature of complement factors and α2-macroglobulins. Three disulfide bonds occur in TEP1r, two of which are conserved between TEP1r and C3, those between MG8 β-strands B and E (residues 1,196 and 1,262 of TEP1r, 1,367 and 1,436 of HumC3), and within the anchor region (residues 1,308 and 1,313 of TEP1r, 1,484 and 1,489 of HumC3).
Fig. 2.
Stereo figure with refined density. 2Fo−Fc density (cyan) contoured at 1σ for the MG3/MG8 interface.
Mechanism of Activation.
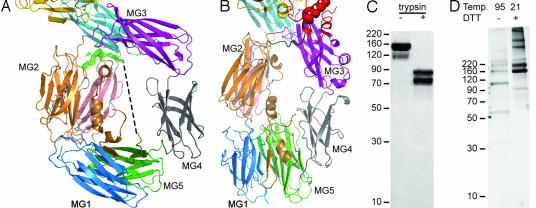
The domain rearrangements in TEP1r described above create a wide central cavity in the β-ring that frames the LNK domain and protease-sensitive region (Fig. 3A). Although the first 40 residues of the TEP1r LNK domain are structurally similar to C3, the peptide chain between Ser 582 and Gln 609 is disordered in our crystal structure. Limited proteolysis of purified TEP1r by trypsin defines this region as ending at residue 601 (Fig. 3C). TEP1r crystals contain full-length protein and an intact thioester, even after 6 months of storage at room temperature (Fig. 3D). Weak electron density is observed before residue 609, passing beneath the MG2/MG3 linker and probably accounts for residues 604–608. The direct distance remaining to be spanned by residues 583–603 is 38 Å (dashed line in Fig. 3A), in comparison, this is the distance spanned by 12 residues of the F and G β-strands in the MG3 domain. Hence, the protease-sensitive region may partially fill the cavity in the β-ring but probably adopts multiple extended conformations within and outside this space. The enlarged cavity in TEP1r suggests that the protease-sensitive region is more accessible in TEP1r than in C3, so that TEP1r may not require specific convertases as for C3 but, rather, can be activated by a range of proteases, a feature analogous to α2-macroglobulins. The sequence 580–601 of TEP1r contains multiple predicted cleavage sites for diverse proteases including trypsin, thermolysin, pepsin LysC/N, clostripain, chymotrypsin, and AspN.
Fig. 3.
The protease-sensitive region. (A) View of the cavity surrounding the protease-sensitive region of TEP1r. Positive Fo−Fc density contoured at 2σ (green) for the end of the protease-sensitive region between domains MG3 and MG6. Dashed line represents line-of-sight path for residues 583–603. (B) Similar view for HumC3. (C) Coomassie-stained SDS/PAGE from limited proteolysis of purified TEP1r (160 kDa) shows two fragments identified as the N terminus (75 kDa) and the result of cleavage at position 601 (85 kDa) by N-terminal sequencing. (D) Silver-stained SDS/PAGE of dissolved crystals (>6 months old) displays autocatalytic cleavage at Gln 841 (105- and 55-kDa bands) upon heating in the absence of DTT, whereas unheated sample contains full-length TEP1r (160 kDa).
In published structures of C3, C3b, and C3c, the β-ring, comprising domains MG1–6 and LNK (Fig. 3B) forms a stable core, whereas the α chain undergoes dramatic domain rearrangements (12). This does not seem to hold true for the structure of TEP1r. Although the individual domains of the β-ring are conserved structural entities, their arrangement varies. The six domains are distinguished as three pairs, MG1–MG5, MG2–MG6 and MG3–MG4, between TEP1r and C3. The conserved core domain between TEP and C3 comprises MG2, MG6, MG7, MG8, CUB, and TED. This may represent the essential structural unit required for an intact thioester bond and its exposure upon activation as observed in C3, C3b, and C3c. The arrangement of the other domains however, can influence the mechanism of activation itself, i.e., the requirement of specific convertases versus general proteolysis.
A striking distinction between TEP1r and C3 is the lack of the ANA domain. In C3, the ANA domain acts as a wedge between MG3 and MG8, stabilizing the interface between MG8 and the TED that protects the thioester bond from inadvertent hydrolysis. How can full-length TEP1r exist in a stable, inactive state without this domain? We observe that the rotation of the MG3 domain produces a triangular arrangement of MG3, MG7, and MG8, with more extensive interfaces that effectively compensate for the lost interface with ANA (Table 2).
Table 2.
MG8 solvent-accessible surface (Å2) buried by neighboring domains
| Domain interface | TEP1r | HumC3 | BovC3 |
|---|---|---|---|
| MG3/MG7 | 389 | 186 | 200 |
| MG3/MG8 | 415 | 191 | 206 |
| MG7/MG8 | 785 | 711 | 693 |
| ANA/MG8 | 605 | 574 | |
| Combined | 1,589 | 1,693 | 1,673 |
| TED/MG8 | 898 | 950 | 1,170 |
| Total | 2,487 | 2,643 | 2,843 |
Proteolytic dissociation of the ANA domain from C3 leads to dramatic rearrangements of the MG7, CUB, TED, and MG8 domains, and exposes the thioester to produce the active form of C3, C3b, which binds to pathogen surfaces. The published structures of C3 and C3b permit the simple interpretation that dissociation of the ANA domain destabilizes the position of MG8, leading to its rotation away from the TED, the exposure of the thioester bond and large-scale domain motions to generate C3b. Assuming that proteolytic cleavage in the region 580–601 triggers the activation of TEP1r, by what mechanism does this occur in the absence of the ANA domain? We propose that the passage of the (disordered) protease-sensitive region between MG2–MG6 and MG3 prevents a rotation of the combined MG3–MG4 and MG1–MG5 domains to the positions adopted in the β-ring of C3, which would remove the triangular interface among MG3, MG7, and MG8. The β-ring of TEP1r is as the eye of a needle threaded by the region 580–601; when this thread is cut, the ends fall on either side of the cavity that is now free to close (see SI Movie 1). This hypothesis predicts that the major N-terminal cleavage product of TEP1r should contain a β-ring with a conformation similar to C3c.
Comparison of TEP1r and TEP1s.
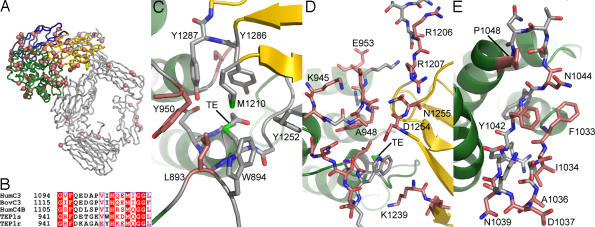
The two alleles of the TEP1 gene in A. gambiae, TEP1r and TEP1s, code for proteins with 93% identity and 96% similarity and no insertions or deletions, yet they display a significantly different phenotype in the binding to P. berghei ookinetes in the mosquito midgut. Both TEP1r and TEP1s proteins effectively bind to ookinetes, resulting in the killing of the majority of parasites and their clearance by lysis. In addition, TEP1r is demonstrated to bind to ookinetes faster and also to trigger the clearance of dead parasites by both lysis and melanization (7). The substitutions between TEP1r and TEP1s are distributed unevenly. The majority occur within the TED domain (Fig. 4A and SI Fig. 6), including three clusters localized in the loop between α-helices 5 and 6 containing the catalytic histidine (the catalytic loop, Fig. 4B), the loop before α-helix 4 (the pre-α4 loop), and the loop between α-helices 9 and 10. The structure of TEP1r provides not only the molecular detail of these three sites but also reveals additional important substitutions that complement these hot spots.
Fig. 4.
TEP1r and TEP1s substitutions. (A) Mapping of TEP1r/TEP1s substitutions onto TEP1r structure, peptide chain in gray, substitutions are pink spheres. The CUB, TED, and MG8 domains are colored according to Fig. 1 (B) alignment of TEP1r and TEP1s catalytic loop with HumC3, BovC3, and HumC4B. (C) The thioester (TE) chamber and surrounding aromatic residues. S1087 (unlabeled) is in the background between Y1286 and Y1287. (D) TED/MG8 interface. Residues K945 to E953 of catalytic loop and the pre-α4 loop (unlabeled) are to the left, MG8 loops interacting with the TED are to the right. (E) The β-hairpin motif between TED helices α9 and α10. In C–E, conserved residues have gray carbon atoms, and residues substituted in TEP1s have pink carbon atoms.
The thioester is in a watertight chamber between the TED and MG8 domains (Fig. 4C), surrounded by seven hydrophobic or aromatic residues, three from the TED and four from the MG8 domain, that are strongly conserved with other thioester-containing proteins. One of these, Tyr 950, is also part of the catalytic loop (941–958 in TEP1r, 1,094–1,011 in HumC3, Fig. 4B) containing a histidine (His 951 in TEP1r, 1,104 in HumC3) that, upon activation, converts the thioester to an acyl intermediate and thiolate ion favoring covalent attachment to hydroxyl groups over amines (15, 16). The conformation of the catalytic loop that keeps Tyr 950 of the TED domain and Tyr 1287 of the MG8 domain between His 951 and the thioester is stabilized by the interface between the TED and MG8 domains. The TEP1s substitutions L893V in the pre-α4 loop and Y950W in the catalytic loop directly affect the protective ring about the thioester. The structure reveals an additional substitution S1087R that is likely to be highly significant, because it would interact directly with the strictly conserved Tyr 1286 and Tyr 1287 of the MG8 domain in TEP1s.
Other substitutions in the TED domain affect the TED/MG8 interface (Fig. 4D). Five additional TEP1s substitutions occur in the catalytic loop itself. In particular, the substitution A948K brings a positive charge and a significantly larger side chain directly to the TED/MG8 interface. Moreover, the structure of TEP1r reveals three pairs of complementary substitutions in the MG8 domain (R1206S/R1207Q, K1239N/T1240M, D1254Y/N1255K) that participate in the interface with the TED and are predicted to interact with the catalytic loop to maintain the integrity of the thioester chamber in TEP1s.
Variation in the catalytic loop is expected not only to influence the integrity of the TED/MG8 interface in intact TEP1, but also to affect the reactivity and specificity of the activated protein (17). This is also the case for the pre-α4 loop, which contains an additional four consecutive substitutions between TEP1r and TEP1s. These residues do not interact directly with the thioester or MG8 domain (Fig. 4D). However, in the “open” conformation observed in the structure of C3d (18) and recent published structures of C3b (11–13), this loop occupies a position closer to the TE loop and projects directly out from the concave face of the TED, where it can interact with potential substrates for the activated thioester.
The third cluster of substitutions between TEP1r and TEP1s occur in the stretch of residues between α-helices 9 and 10 (Fig. 4E). This region (residues 1,033–1,048 of TEP1r, 1,193–1,207 of HumC3), is a variable region in the TEP superfamily (24-aa insert in HumC4B, 15-aa insert in HumC5 and α2-MG), and is part of a putative binding region for factor H to C3b (19). Half the residues in this short segment are mutated between TEP1r and TEP1s. Surprisingly, although the number of residues in this loop is similar (1-aa difference) between TEP1r and C3, the structure is distinct. In TEP1r, residues 1,033–1,042 form a β-hairpin with a tight turn between residues 1,036–1,039, whereas in C3 there is only a tight turn between residues 1,193–1,196. After residue 1,044 in TEP1r has a second tight turn ending with Pro 1048, whereas in C3, the corresponding tight turn is 1,200–1,203 and juts out from the surface because of an extra turn at the N-terminal end of helix α10. Also, residue Asn 1044 is a putative glycosylation site in TEP1r and is removed by substitution with glycine in TEP1s.
Conclusion
Can the amino acid substitutions described above explain the phenotypic difference observed between the R and S strains of A. gambiae with respect to infection by P. berghei? Analysis of the TEP1r structure and mapping of substitutions between TEP1r and TEP1s allows us to draw the following inferences. First, the two proteins are most likely identical in their susceptibility to proteolytic activation, because the protease-sensitive region, the MG3/MG7/MG8 interface and virtually the entire β-ring are identical. However, differences observed at the TED/MG8 interface may affect the rate of dissociation of the domains upon proteolytic activation, thereby affecting the general reactivity of the molecule. Furthermore, those substitutions on the concave face of the TED, especially the pre-α4 and catalytic loops, are expected to affect the reactivity and selectivity of TEP1r and TEP1s for available sites on the surface of the Plasmodium ookinete. Variation of surface-exposed residues, such as those in the β-hairpin motif, could affect direct binding of downstream effectors to the TED after its attachment to the pathogen surface. Alternatively, a different specificity between TEP1r and TEP1s for sites on the ookinete could indirectly affect the interactions with downstream effectors that bind independently to the parasite. Differences in other domains of TEP, such as the CUB and MG8 domains, may represent cryptic binding sites that are exposed after activation. Clarification of these different mechanisms must await structures of activated and cleaved forms of TEP1r, structure–function analysis of the critical substitutions identified, and discovery of the other molecules that cooperate with TEP1 to affect the killing of invading pathogens in A. gambiae.
Materials and Methods
Protein Purification.
TEP1r was produced by using the bacculovirus expression system. TEP1r was cloned into pFastBac1 (Invitrogen, Carlsbad, CA) by using RsrII/SpeI restriction sites, including the native signal peptide and a C-terminal 6XHis-tag. Protein was expressed in High Five cells (Invitrogen) grown to a density of 1.2 million/ml in Excel-405 medium (JRH Biosciences, Andover, Hampshire, U.K.) and infected with virus [multiplicity of infection (moi) ≈ 1.0]. Protein was expressed for 80–90 h at 27°C. The cells were spun down at 3,300 × g, and the supernatant was concentrated to 1/10 the starting volume by using a tangential concentrator (Millipore, Bedford, MA). Concentrated medium was dialyzed in PBS (pH 7.4) for 12–16 h at 4°C. The dialyzed medium was loaded onto Talon resin (Clontech, Palo Alto, CA), washed with 10 CV (100 ml) wash buffer [20 mM Tris (pH 7.8)/250 mM NaCl) and eluted by a step gradient [250 mM imidazole (pH 7.8)]. The eluate was immediately desalted on a HiTrap 26/10 column (GE Healthcare, Piscataway, NJ) equilibrated with Q buffer [20 mM Tris (pH 8.0)/100 mM NaCl). Desalted protein was loaded onto a MonoQ column (GE Healthcare) and eluted with a linear gradient from 100–600 mM NaCl. Further purification was achieved by gel filtration (Superdex 200; GE Healthcare) equilibrated with S buffer [20 mM Hepes (pH 7.5)/100 mM NaCl), followed by cation exchange on a MonoS column (GE Healthcare). Pure TEP1r protein eluted at ≈200 mM NaCl.
Crystallization.
TEP1r was crystallized by hanging-drop vapor diffusion. Protein was concentrated to 3.5 mg/ml in S buffer as eluted from the MonoS column [20 mM Hepes (pH 7.5)/≈0.2 M NaCl). The reservoir contained 2 M 1:1 NaH2/K2HPO4, 0.2 M NaCl, 0.1 M imidazole (pH 8.0), and 50 mM Na/K tartrate. Crystals grew in 3–4 days, diffraction-quality crystals were obtained by variation of total phosphate concentration (1.8–2.3 M), NaH2/K2HPO4 ratio (1.2:0.8–0.8:1.2) and protein/reservoir ratio (1:2–2:1 (vol/vol)) around the initial condition. Once grown, crystals were transferred to a soak buffer of 2.2 M 1:1 NaH2/K2HPO4 for heavy-atom derivatization. Native and derivative crystals were transferred to a cryobuffer containing 25% sucrose and frozen in liquid nitrogen before data collection. Derivatives with MeHgOAc were obtained by 24-h soak (1 mM concentration), with EMTS by cocrystallization in the absence of Na/K tartrate (2 mM concentration), and with xenon by 10-min equilibration with 400 MPa Xe in a xenon chamber (Hampton Research, Aliso Viejo, CA).
Data Collection and Phasing.
Diffraction data were collected at SBC-CAT, sector 19, Advanced Photo Source, Argonne National Laboratory. Data sets were processed by using HKL2000 (20) and imported into CCP4 (21). Five sites were found for the EMTS dataset by SAD phasing with SHELXD (22) to 6 Å, and refined to 4 Å in MLPHARE.¶ By using phases from a partial molecular replacement to generate isomorphous and anomalous difference maps, a MeHgOAc data set was identified as well as a data set that was sufficiently isomorphous with both Hg data sets to allow for MIRAS refinement of heavy atom positions in MLPHARE. Density modification with 70% solvent content and phase extension in RESOLVE (23) produced an interpretable map and ≈400 autobuilt residues. A Xe data set was identified during refinement that led to an improved experimental map.
Refinement.
The model was constructed in seven rounds of manual building in COOT (24), and maximum likelihood (ML) refinement against experimental phases (w = 0.01) in REFMAC5 (25). During the last round of building (95% complete model) simulated annealing (SA) and the calculation of SA-omit maps in CNS was used to build remaining loop regions (26). Two additional cycles of translation, libration, and screw (TLS) refinement (27) and ML refinement against experimental phases were performed before the addition of waters and the final rounds of refinement by using model phases. Structural alignments with complement proteins were performed by using LSQMAN (28). All protein model figures were generated with PyMOL (W. L. Delano, www.pymol.org), and sequence alignments were generated with ESPRIPT (29).
Supplementary Material
Acknowledgments
We thank Dr. Kentaro Ihara (Photon Factory, Ibaraki, Japan), Dr. Norma Duke, Dr. Frank Rotella, and Jack Lazarz (Advanced Photon Source, Argonne, IL); Dr. Dominika Borek [University of Texas Southwestern (UTSW)] for assistance in data collection; Dr. Borek and Drs. Mischa Machius and Diana Tomchick of the UTSW Structural Biology Laboratory and Drs. Christopher Colbert, Yihua Huang, and Hyock Kwon of the J.D. laboratory for many helpful discussions. J.D. is an investigator in the Howard Hughes Medical Institute. E.A.L. is an International Research Scholar of the Howard Hughes Medical Institute. This work was supported by a grant from the Welch Foundation (to J.D.). Results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the U.S. Department of Energy Office of Biological and Environmental Research under contract DE-AC02-06CH11357.
Abbreviations
- ANA
anaphylotoxin
- MG
macroglobulin
- TED
thioester-containing domain
- TEP
thioester-containing protein.
Footnotes
The authors declare no conflict of interest.
Data Deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2PN5).
This article contains supporting information online at www.pnas.org/cgi/content/full/0704967104/DC1.
Otwinowski Z (1991) in Proceedings of the CCP4 Study Weekend: Isomorphous Replacement and Anomalous Scattering, eds Wolf W, Evans PR, Leslie AGW (Daresbury Laboratory, Warrington, UK), pp 80–86.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christophides GK. Cell Microbiol. 2005;7:325–333. doi: 10.1111/j.1462-5822.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 3.Cherry S, Silverman N. Nat Immunol. 2006;7:911–917. doi: 10.1038/ni1388. [DOI] [PubMed] [Google Scholar]
- 4.Blandin S, Levashina EA. Mol Immunol. 2004;40:903–908. doi: 10.1016/j.molimm.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Dodds AW. Immunobiology. 2002;205:340–354. doi: 10.1078/0171-2985-00137. [DOI] [PubMed] [Google Scholar]
- 6.Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, et al. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 7.Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 8.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 9.Fredslund F, Jenner L, Husted LB, Nyborg J, Andersen GR, Sottrup-Jensen L. J Mol Biol. 2006;361:115–127. doi: 10.1016/j.jmb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Janssen BJ, Huizinga EG, Raaijmakers HC, Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B, Gros P. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 11.Abdul Ajees A, Gunasekaran K, Volanakis JE, Narayana SV, Kotwal GJ, Murthy HMK. Nature. 2006;444:221–225. doi: 10.1038/nature05258. [DOI] [PubMed] [Google Scholar]
- 12.Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Nature. 2006;444:213–216. doi: 10.1038/nature05172. [DOI] [PubMed] [Google Scholar]
- 13.Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ, McCallum SA, Embuscado L, DeForge L, Hass PE, et al. Nature. 2006;444:217–220. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- 14.Nishida N, Walz T, Springer TA. Proc Natl Acad Sci USA. 2006;103:19737–19742. doi: 10.1073/pnas.0609791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodds AW, Ren XD, Willis AC, Law SKA. Nature. 1996;379:177–179. doi: 10.1038/379177a0. [DOI] [PubMed] [Google Scholar]
- 16.Law SKA, Dodds AW. Protein Sci. 1997;6:263–274. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodds AW, Law SKA. Immunol Rev. 1998;166:15–26. doi: 10.1111/j.1600-065x.1998.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagar B, Jones RG, Diefenbach RJ, Isenman DE, Rini JM. Science. 1998;280:1277–1281. doi: 10.1126/science.280.5367.1277. [DOI] [PubMed] [Google Scholar]
- 19.Herbert AP, Uhrin D, Lyon M, Pangburn MK, Barlow PN. J Biol Chem. 2006;281:16512–16520. doi: 10.1074/jbc.M513611200. [DOI] [PubMed] [Google Scholar]
- 20.Otwinowski Z, Minor W. In: Macromolecular Crystallography, Part A. Carter CW, Sweet RM, editors. Vol 276. New York: Academic; 1997. pp. 307–326. [Google Scholar]
- 21.CCP4. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 22.Schneider TR, Sheldrick GM. Acta Crystallogr D. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- 23.Terwilliger TC. Acta Crystallogr D. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 25.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 26.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 27.Winn MD, Isupov MN, Murshudov GN. Acta Crystallogr D. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 28.Kleywegt GJ, Jones TA. CCP4/ESF-EACBM Newslett Protein Crystallogr. 1994;31:9–14. [Google Scholar]
- 29.Gouet P, Robert X, Courcelle E. Nucleic Acids Res. 2003;31:3320–3323. doi: 10.1093/nar/gkg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.