Abstract
Poststroke hemiparesis, together with abnormal muscle tone, is a major cause of morbidity and disability. Although most hemiparetic patients are able to reach different ambulatory levels with rehabilitation efforts, upper and lower limb spasticity can impede activities of daily living, personal hygiene, ambulation and, in some cases, functional improvement. The goals of spasticity management include increasing mobility and range of motion, attaining better hygiene, improving splint wear and other functional activities. Conservative measures, such as positioning, stretching and exercise are essential in spasticity management, but alone often are inadequate to effectively control it. Oral antispastic medications often provide limited effects with short duration and frequent unwanted systemic side effects, such as weakness, sedation and dry mouth. Therefore, neuromuscular blockade by local injections have become the first choice for the treatment of focal spasticity, particularly in stroke patients. Botulinum toxin (BTX), being one of the most potent biological toxins, acts by blocking neuromuscular transmission via inhibiting acetylcholine release. Currently, focal spasticity is being treated successfully with BTX via injecting in the spastic muscles. Two antigenically distinct serotypes of BTX are available on the market as type A and B. Clinical studies of BTX used for spastic hemiplegic patients are reviewed in this article in two major categories, upper and lower limb applications. This review addresses efficacy in terms of outcome measures, such as muscle tone reduction and functional outcome, as well as safety issues. Application modifications of dose, dilutions, site of injections and combination therapies with BTX injections are also discussed.
Keywords: Botulinum toxin, Spasticity, Stroke
Spasticity is a well-known motor dysfunction arising from upper motor neuron lesions due to stroke, spinal cord injury, multiple sclerosis and traumatic brain injury. Clinically, it is diagnosed with the velocity-dependent resistance felt by passive examination of joint motion. In 1980, Lance1 defined spasticity as “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyper-excitability of the stretch reflex as one component of the upper motor neuron syndrome.” The pathophysiology of spasticity is not completely understood, but it is thought to be related to change in the balance of excitatory and inhibitory inputs to the motor neuron pool.
Poststroke hemiparesis, together with abnormal muscle tone, is a major cause of morbidity and disability. These patients often demonstrate recognizable antigravity postural patterns characterized by shoulder adduction, and elbow and wrist flexion in the upper limb, and hip adduction, knee extension and ankle plantar flexion in the lower limb. This “hemiplegic” posture, which is thought to result from increased motor neuron activity in antigravity muscles, significantly interferes with body image, balance and gait.2 Although most hemiparetic patients are able to reach different ambulatory levels with rehabilitation efforts, upper and lower limb spasticity can impede activities of daily living, personal hygiene, ambulation and, in some cases, functional improvement. Paresis and increased muscle tone can also cause joint stiffness leading to contractures.
Despite the ease of diagnosis, effective spasticity management is often challenging for the clinician. The goals of spasticity management include increasing mobility and range of motion, attaining better hygiene, improving body image and functional level, and facilitating splint wear. In addition to standard conservative measures such as positioning, stretching and exercise, treatment options also include physical agents, oral antispastic drugs, neuromuscular blockade by local injections of phenol and botulinum toxin (BTX), and intrathecal baclofen. Oral antispastic medications often provide limited effects with short duration and frequently lead to side effects such as weakness, drowsiness, confusion, dizziness, sedation and dry mouth.3,4 Neurolysis by phenol5 and alcohol6 injections reduce spasticity effectively in poststroke hemiparesis, but severe pain is considered to be a major limitation. Intrathecal baclofen, which can be administered by an implanted programmable pump, and used particularly in patients with intractable spinal spasticity, has been reported to be effective in a few studies in stroke patients.7,8 Although intrathecal baclofen therapy is an invasive treatment method that requires an implantation procedure and can lead to adverse reactions such as headache, nausea, vomiting, excessive weakness and transient urinary retention, highly significant reduction of muscle tone and some subsequent functional gain can bring the procedure to a more favorable status to be considered for use in intractable spastic hemiplegic patients.7,8 Surgical interventions like arthrodesis9 and tendon release10 can also be performed for severe spasticity, particularly in patients with contractures.
BTX, one of the most potent biologic toxins known to man,11 acts by blocking neuromuscular transmission via inhibiting acetylcholine release. Since its introduction by Scott12 to treat strabismus, it has been utilized in various neurologic and non-neurologic indications, such as dystonia, blepharospasm, tremor, hyperhydrosis and cosmetic applications. Focal spasticity, particularly resulting from cerebral disorders, is currently being treated successfully with BTX via injection in the spastic muscles, and BTX is now considered the pharmacological treatment of choice in focal spasticity.13 There are seven serologically distinct toxin types named A, B, C, D, E, F and G. They all inhibit acetylcholine release into the synaptic cleft by binding one or more of the transport protein chains with high specificity.14,15 These target proteins, on which the light chains of the toxin bind, vary among the different serotypes. Two antigenically distinct serotypes of BTX are available on the market as type A and B. BTX-A cleaves synaptosome-associated protein 25, whereas BTX-B cleaves vesicle-associated membrane protein. The specific site of action and the cleaved protein influence the duration of action of various BTX serotypes.16 BTX-A has been shown to have a longer duration of effect in cervical dystonia compared with BTX-B.17 The two serotypes differ from each other in their adverse effects. The anticholinergic unwanted effects such as dry mouth, dysphagia and voiding difficulties were found to be more common after BTX-B injections.18,19
Type A BTX formulations are available as Botox (Allergan, Inc., Irvine, CA) and Dysport (Ipsen Ltd., Berkshire, UK). Type B is available as Myobloc in the United States and NeuroBloc in Europe (Elan Pharmaceuticals, San Diego, CA).20 Although no direct comparison of these products has been made, approximate equivalence obtained from clinical experience and general survey of the literature is reported as follows:21
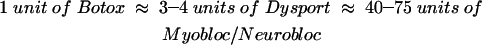 |
BTX doses are generally adjusted according to factors such as severity of spasticity, number of muscles involved, age, previous response to BTX therapy and adjunct therapy applications. Recommended BTX-A (Botox and Dysport) doses and dilutions for different muscle groups are well reviewed in the literature.22,23 Antibody formation against BTX proteins (including neurotoxin and non-toxin components24) is one of the reasons for therapy failure. In order to overcome therapy failure, injecting increased BTX doses,25 preventing short injection intervals and using different BTX serotypes26,27 are suggested. The reports of antibody formation are mostly from patients with cervical dystonia. One study evaluating antibody levels in patients with poststroke spastic upper extremity has reported that repeated treatment cycles of BTX-A were not associated with detectable levels of antibody and this result was partly attributed to a shorter treatment duration compared with the results of cervical dystonia studies.28
Another drawback for BTX therapy is high cost and the transient nature of the toxin. Although pharmacoeconomic evaluations of the available spasticity treatment alternatives are limited, BTX has been found to be cost-effective in poststroke spasticity when compared with oral anti-spastic drugs.29 Clinical studies of BTX used for spastic hemiplegic patients are reviewed in this article in two major categories, upper and lower limb applications. In addition to efficacy in terms of outcome measures such as muscle tone reduction and functional outcome, safety issues are addressed. Application modifications of dose, dilutions, site of injections and combination therapies with BTX injections are also discussed.
BTX for Upper Limb Spasticity
Functional recovery of the upper limb may be limited in stroke survivors. As arm and hand functions are crucial for many activities of daily living, rehabilitation efforts are focused on this aspect. Apart from loss of function due to paresis, spasticity may be a major contributing factor to disability. Therefore, even if there is no residual motor function in the upper limb muscles, management of spasticity should be strongly considered in an attempt to reduce disability in activities such as limb positioning, hygiene, dressing and walking.
Earlier case control studies suggest safe and effective use of BTX for upper limb spasticity.30–33 Several spastic upper limb muscles of chronic hemiparetic patients were injected in these studies. Tone reduction assessed by the Ashworth or Modified Ashworth Scale34 (0=normal muscle tone to 5=the effected part is rigid in flexion or extension) and improvement in functional outcome evaluated by the patients’ global assessment scores were reported. However, global functional assessment scores such as Barthel, FIM and SF-36 have generally failed to show significant improvement after BTX injections. Placebo-controlled trials have also provided further evidence for safe and effective use of BTX in the management of upper limb spasticity (table 1 ▶).35–44 As an extension to a 12-week double-blind study,41 111 patients were enrolled in a 42-week open label study.45 In this study, repeated BTX injections for upper limb spasticity have resulted in significant and sustained improvement in both Disability Assessment and Ashworth Scores. These results were consistent with a study that suggested sustained antispastic effects of BTX after repeated injections for up to at least three treatment cycles.46 Other uncontrolled trials with similar results have reported spasticity reduction in the hemiparetic upper limb without significant functional improvement in global assessment measures.47–49 Interestingly, an exploratory meta-analysis, including two randomized, controlled trials, has revealed that reduction in spasticity was associated with significant improvement in arm function.50
Table 1.
Placebo controlled trials of stroke related spastic upper limb treated with botulinum toxin (BTX).
| Study (year) | No. of patients | Stroke duration (mean) | Injected muscles | Treatment groups compared to placebo | Result |
| Simpson35 (1996) | 39 | 37 months | BB, FCR, FCU | 75 U Botox 150 U Botox 300 U Botox |
|
| Hesse36 (1998) | 24 | 7.45 months | BB, Brachialis, FCR, FCU, FDP, FDS | 1,000 U Dysport+ES, 1,000 U Dysport, placebo+ES |
|
| Bakheit37 (2000) | 83 | NA | BB, FCU, FCR, FDP, FDS | 500 U Dysport 1,000 U Dysport 1,500 U Dysport |
|
| Smith38 (2000) | 21 (2 patients with head injury) | 36 months | Flexor muscles (names not given) | 500 U Dysport 1,000 U Dysport 1,500 U Dysport |
|
| Bhakta39 (2000) | 40 | 3.1 years | BB, Brachioradialis, FDS, FDP, FCU | 1,000 U Dysport |
|
| Bakheit40 (2001) | 59 | NA | BB, FDS, FDP FCR, FCU |
1,000 U Dysport |
|
| Brashea41 (2002) | 126 | 4.75 years | FCR, FCU, FDP, FDS in all subjects, 10 additionally FPL and 64 thumb muscles | 200–240 U Botox |
|
| Brashear42 (2004) | 15 | NA | BB, FCU, FCR, FDS, FDP | 10,000 U BTX-B (MyoBloc) |
|
| Childers43 (2004) | 91 | 25.8 months | BB, FCU, FCR, FDS, FDP | 90 U Botox 180 U Botox 360 U Botox |
|
| Suputtitada44 (2005) | 50 | 8.4 months | BB, FCU, FCR, FDS, FDP | 350 U Dysport 500 U Dysport 1,000 U Dysport |
|
BB, biceps brachii; FCU, flexor carpi ulnaris; FCR, flexor carpi radialis; FDP, flexor digitorum profundus; FDS, flexor digitorum superficialis; FPL, flexor pollicis longus; ROM, range of motion; NA, not applicable; ES, electrical stimulation.
Combined therapies were also studied in an attempt to enhance the effectiveness of BTX. In a randomized, double-blind, placebo-controlled study, Hesse et al36 suggested that BTX could be more effective when combined with electrical stimulation. Moreover, Carda and Molteni51 have demonstrated that muscle tone reduction was greater when adhesive taping was performed after BTX injections, compared to combined electrical stimulation and splinting after similar doses of BTX. In another attempt to increase the effectiveness of a given toxin dose, Francisco et al52 have compared two different dilutions of BTX and found no difference between high and low concentration treatment groups. Contrasting results have been reported in another study, suggesting that higher concentrations of BTX-A provides greater spasticity reduction.53
BTX for Lower Limb Spasticity
As previously stated in the context of hemiplegic posture, the lower limb of a hemiplegic patient tends to be in the position with adducted hip, extended knee, plantar flexed and inverted ankle. Although some patients experience gait abnormalities related to stiff knee pattern, extended position of the knee is generally considered to be useful for ambulation in patients who do not have sufficient quadricep strength to lock their knees while standing or walking. However, ankle plantar flexion and inversion are major drawbacks for ambulation in spastic hemiplegic patients causing problems like impaired foot contact, dragging of toes, reduced stance duration and stride length. Most clinical trials concerning BTX use in stroke patients with lower limb spasticity are designed in order to find an answer to the question whether BTX effectively alleviates ankle plantar flexor spasticity and provides functional improvement. Compared to the extensively studied upper limb applications in placebo-controlled trials with relatively large sample sizes, lower limb studies are relatively insufficient. In one of the earliest studies of drop foot treatment in poststroke patients, Dengler et al54 have reported improvement in 8 of 10 patients considering spasticity and facilitation of physiotherapy. Subsequently, Hesse et al55 injected 400 U of BTX (Botox) to the spastic calf muscles of 12 patients and has been able to show statistically significant improvement in gait analysis parameters, including velocity, stride length and stance symmetry. In another study with a similar number of patients and injected muscles, Hesse et al56 have investigated the effect of BTX on ankle muscle activity using dynamic electromyography. While correlation has been observed between muscle tone reduction and gait parameters, significant improvements have also been reported for walking speed, stride length and premature activity of soleus muscle after injection.
High cost and transient nature of effect are among the major drawbacks of BTX treatment. Hesse et al57 have combined 2000 U of BTX (Dysport) with electrical stimulation of tibialis anterior and plantar flexor muscles in an attempt to enhance toxin effect. The combined treatment has been found to be superior with respect to the clinically assessed reduction of muscle tone, gait velocity, stride length, stance and swing symmetry.
Recently low dose (100 U Botox) BTX injections followed by short-term electrical stimulations were compared with high dose (400 U Botox) applications in poststroke spastic drop foot.58 Improvement has been recorded in both groups, while this preliminary single-blind study was unable to find a difference in terms of effectiveness between the two treatment groups. Randomized controlled trials investigating safety and efficacy of BTX comparing other techniques and combination therapies with BTX are presented in table 2 ▶.59–62 In general, the effect seems to have a shorter duration with lower doses. Longer spasticity duration is associated with poor outcome, and it is hard to demonstrate focal spasticity treatment efficacy by global functional assessment methods.
Table 2.
Randomized-controlled trials of stroke related spastic foot treated with botulinum toxin (BTX).
| Study (year) | No. of patients | Stroke duration (mean) | Injected muscles | Treatment groups | Result | |
| Burbaud59 (1996) | 23 | 23.5 months | Triceps surae, soleus, TP, FDL | 1000 U Dysport | Placebo |
|
| Reiter60 (1998) | 18 | 23.6 months | GM, GL, TP, FHL, injected according to limb posture and EMG activity in the first group) | 190–320 U | 100 U TP only plus ankle taping for 3 weeks |
|
| Kirazli60 (1998) | 20 | NA (<12 months) | Soleus, TP GL, GM | 400 U Botox to targeted muscles | 3 ml 5% phenol for tibial nerve block |
|
| Johnson62 (2004) | 21 | NA (<12 months) | GM, GL, TP | 800 U Dysport combined with FES | Exercise only |
|
| Bayram58 (2006) | 12 | 36.6 months | GM, GL, TP, soleus | 100 U Botox to TP with short term ES | 400 U Botox to all target muscles |
|
GM, gastrocnemius medialis; GL, gastrocnemius lateralis; TP, tibialis posterior; FHL, flexor hallucis longus; FDL, flexor digitorum longus; ES, Electrical stimulation; FES, functional electrical stimulation; ROM, range of motion; NA, not applicable.
The above-mentioned studies are inadequate to provide strong evidence due to their relatively small sample sizes. However, a recent pooled data analysis of 9 double-blind, placebo-controlled studies, including 792 patients following stroke (482 upper, 310 lower limb) has revealed an acceptable safety profile for BTX.63
Conclusion
The results of the reviewed studies of BTX treatment in poststroke upper and lower limb spasticity suggest that BTX injections safely and effectively decrease muscle tone and increase range of motion. Although some functional improvements may be seen after BTX injections, global functional assessment methods do not consistently reflect these changes. In light of the previous results and current evidence, we believe that future studies with large sample size, carefully selected patients and appropriate clinical outcome measures will provide more insight for clinical utilization of BTX.
References
- 1.Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, eds. Spasticity: disordered motor control. Chicago, NY: Yearbook Medical; 1980. 485–494.
- 2.Mayer NH. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve Suppl 1997;6:S1–S13. [PubMed] [Google Scholar]
- 3.Gallichio JE. Pharmacologic management of spasticity following stroke. Phys Ther 2004;84:973–981. [PubMed] [Google Scholar]
- 4.Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004;28:140–175. [DOI] [PubMed] [Google Scholar]
- 5.McCrea PH, Eng JJ, Willms R. Phenol reduces hypertonia and enhances strength: a longitudinal case study. Neurorehabil Neural Repair 2004;18:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang SH, Ahn SH, Park SM, Kim SH, Lee KH, Lee ZI. Alcohol neurolysis of tibial nerve motor branches to the gastrocnemius muscle to treat ankle spasticity in patients with hemiplegic stroke. Arch Phys Med Rehabil 2004;85:506–508. [DOI] [PubMed] [Google Scholar]
- 7.Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN. Intrathecal baclofen for spastic hypertonia from stroke. Stroke 2001;32:2099–2109. [DOI] [PubMed] [Google Scholar]
- 8.Francisco GE, Boake C. Improvement in walking speed in poststroke spastic hemiplegia after intrathecal baclofen therapy: a preliminary study. Arch Phys Med Rehabil 2003;84:1194–1199. [DOI] [PubMed] [Google Scholar]
- 9.Rayan GM, Young BT. Arthrodesis of the spastic wrist. J Hand Surg [Am] 1999;24:944–952. [DOI] [PubMed] [Google Scholar]
- 10.Keenan MA, Ure K, Smith CW, Jordan C. Hamstring release for knee flexion contracture in spastic adults. Clin Orthop Relat Res 1988;(236):221–226. [PubMed]
- 11.Jankovic J, Brin MF. Botulinum toxin: historical perspective and potential new indications. Muscle Nerve Suppl 1997;6:S129–S145. [PubMed] [Google Scholar]
- 12.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87:1044–1049. [DOI] [PubMed] [Google Scholar]
- 13.Ward AB, Aguilar M, De Beyl Z, Gedin S, Kanovsky P, Molteni F, Wissel J, Yakovleff A. Use of botulinum toxin type A in management of adult spasticity—a European consensus statement. J Rehabil Med 2003;35:98–99. [DOI] [PubMed] [Google Scholar]
- 14.Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol 2005;53:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Thakker MM, Rubin PA. Pharmacology and clinical applications of botulinum toxins A and B. Int Ophthalmol Clin 2004;44:147–163. [DOI] [PubMed] [Google Scholar]
- 16.Aoki KR. Pharmacology and immunology of botulinum toxin type A. Clin Dermatol 2003;21:476–480. [DOI] [PubMed] [Google Scholar]
- 17.Comella CL, Jankovic J, Shannon KM, Tsui J, Swenson M, Leurgans S, Fan W; Dystonia Study Group. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 2005;65:1423–1429. [DOI] [PubMed] [Google Scholar]
- 18.Dressler D, Eleopra R. Clinical use of non-A botulinum toxins: botulinum toxin type B. Neurotox Res 2006;9:121–125. [DOI] [PubMed] [Google Scholar]
- 19.Tintner R, Gross R, Winzer UF, Smalky KA, Jankovic J. Autonomic function after botulinum toxin type A or B: a double-blind, randomized trial. Neurology 2005;65:765–767. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien CF. Treatment of spasticity with botulinum toxin. Clin J Pain 2002;18:S182–S190. [DOI] [PubMed] [Google Scholar]
- 21.Pathak MS, Nguyen HT, Graham HK, Moore AP. Management of spasticity in adults: practical application of botulinum toxin. Eur J Neurol 2006;13:42–50. [DOI] [PubMed] [Google Scholar]
- 22.Francisco GE. Botulinum toxin: dosing and dilution. Am J Phys Med Rehabil 2004;83:S30–S37. [DOI] [PubMed] [Google Scholar]
- 23.Brin MF. Dosing, administration, and a treatment algorithm for use of botulinum toxin A for adult-onset spasticity. Spasticity Study Group. Muscle Nerve Suppl 1997;6:S208–S220. [DOI] [PubMed] [Google Scholar]
- 24.Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord 2004;19:S92–S100. [DOI] [PubMed] [Google Scholar]
- 25.Dressler D, Munchau A, Bhatia KP, Quinn NP, Bigalke H. Antibody-induced botulinum toxin therapy failure: can it be overcome by increased botulinum toxin doses? Eur Neurol 2002;47:118–121. [DOI] [PubMed] [Google Scholar]
- 26.Barnes MP, Best D, Kidd L, Roberts B, Stark S, Weeks P, Whitaker J. The use of botulinum toxin type-B in the reatment of patients who have become unresponsive to botulinum toxin type-A — initial experiences. Eur J Neurol 2005;12:947–955. [DOI] [PubMed] [Google Scholar]
- 27.Factor SA, Molho ES, Evans S, Feustel PJ. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov Disord 2005;20:1152–1160. [DOI] [PubMed] [Google Scholar]
- 28.Bakheit AM, Fedorova NV, Skoromets AA, Timerbaeva SL, Bhakta BB, Coxon L. The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 2004;75:1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward A, Roberts G, Warner J, Gillard S. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med 2005;37:252–257. [DOI] [PubMed] [Google Scholar]
- 30.Hesse S, Friedrich H, Domasch C, Mauritz KH. Botulinum toxin therapy for upper limb flexor spasticity: preliminary results. J Rehab Sci 1992;5:98–101. [Google Scholar]
- 31.Reiter F, Danni M, Ceravolo MG, Provinciali L. Disability changes after treatment of upper limb spasticity with botulinum toxin. J Neurol Rehab 1996;10:47–52. [Google Scholar]
- 32.Bhakta BB, Cozens JA, Bamford JM, Chamberlain MA. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J Neurol Neurosurg Psychiatry 1996;61:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampaio C, Ferreira JJ, Pinto AA, Crespo M, Ferro JM, Castro-Caldas A. Botulinum toxin type A for the treatment of arm and hand spasticity in stroke patients. Clin Rehabil 1997;11:3–7. [DOI] [PubMed] [Google Scholar]
- 34.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–207. [DOI] [PubMed] [Google Scholar]
- 35.Simpson DM, Alexander DN, O’Brien CF, Tagliati M, Aswad AS, Leon JM, Gibson J, Mordaunt JM, Monaghan EP. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology 1996;46:1306–1310. [DOI] [PubMed] [Google Scholar]
- 36.Hesse S, Reiter F, Konrad M, Jahnke MT. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Clin Rehabil 1998;12:381–388. [DOI] [PubMed] [Google Scholar]
- 37.Bakheit AM, Thilmann AF, Ward AB, Poewe W, Wissel J, Muller J, Benecke R, Collin C, Muller F, Ward CD, Neumann C. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 2000;31:2402–2406. [DOI] [PubMed] [Google Scholar]
- 38.Smith SJ, Ellis E, White S, Moore AP. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil 2000;14:5–13. [DOI] [PubMed] [Google Scholar]
- 39.Bhakta BB, Cozens JA, Chamberlain MA, Bamford JM. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry 2000;69:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakheit AM, Pittock S, Moore AP, Wurker M, Otto S, Erbguth F, Coxon L. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol 2001;8:559–565. [DOI] [PubMed] [Google Scholar]
- 41.Brashear A, Gordon MF, Elovic E, Kassicieh VD, Marciniak C, Do M, Lee CH, Jenkins S, Turkel C; Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med 2002;347:395–400. [DOI] [PubMed] [Google Scholar]
- 42.Brashear A, McAfee AL, Kuhn ER, Fyffe J. Botulinum toxin type B in upper-limb poststroke spasticity: a double-blind, placebo-controlled trial. Arch Phys Med Rehabil 2004;85:705–709. [DOI] [PubMed] [Google Scholar]
- 43.Childers MK, Brashear A, Jozefczyk P, Reding M, Alexander D, Good D, Walcott JM, Jenkins SW, Turkel C, Molloy PT. Dose-dependent response to intramuscular botulinum toxin type A for upper-limb spasticity in patients after a stroke. Arch Phys Med Rehabil 2004;85:1063–1069. [DOI] [PubMed] [Google Scholar]
- 44.Suputtitada A, Suwanwela NC. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil 2005;27:176–184. [DOI] [PubMed] [Google Scholar]
- 45.Gordon MF, Brashear A, Elovic E, Kassicieh D, Marciniak C, Liu J, Turkel C; BOTOX Poststroke Spasticity Study Group. Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology 2004;63:1971–1973. [DOI] [PubMed] [Google Scholar]
- 46.Bakheit AM, Fedorova NV, Skoromets AA, Timerbaeva SL, Bhakta BB, Coxon L. The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 2004;75:1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HC, Hsieh LF, Chi WC, Lou SM. Effect of intramuscular botulinum toxin injection on upper limb spasticity in stroke patients. Am J Phys Med Rehabil 2002;81:272–278. [DOI] [PubMed] [Google Scholar]
- 48.Rousseaux M, Kozlowski O, Froger J. Efficacy of botulinum toxin A in upper limb function of hemiplegic patients. J Neurol 2002;249:76–84. [DOI] [PubMed] [Google Scholar]
- 49.Pandyan AD, Vuadens P, van Wijck FM, Stark S, Johnson GR, Barnes MP. Are we underestimating the clinical efficacy of botulinum toxin (type A)? Quantifying changes in spasticity, strength and upper limb function after injections of Botox to the elbow flexors in a unilateral stroke population. Clin Rehabil 2002;16:654–660. [DOI] [PubMed] [Google Scholar]
- 50.Francis HP, Wade DT, Turner-Stokes L, Kingswell RS, Dott CS, Coxon EA. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry 2004;75:1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carda S, Molteni F. Taping versus electrical stimulation after botulinum toxin type A injection for wrist and finger spasticity. A case-control study. Clin Rehabil 2005;19:621–626. [DOI] [PubMed] [Google Scholar]
- 52.Francisco GE, Boake C, Vaughn A. Botulinum toxin in upper limb spasticity after acquired brain injury: a randomized trial comparing dilution techniques. Am J Phys Med Rehabil 2002;81:355–363. [DOI] [PubMed] [Google Scholar]
- 53.Gracies JM, Weisz DJ, Yang BY, Flanagan S, Simpson DM. Impact of botulinum toxin type A (BTX-A) dilution and endplate targeting technique in upper limb spasticity. Ann Neurol 2002;52:S87. [Google Scholar]
- 54.Dengler R, Neyer U, Wohlfarth K, Bettig U, Janzik HH. Local botulinum toxin in the treatment of spastic drop foot. J Neurol 1992;239:375–378. [DOI] [PubMed] [Google Scholar]
- 55.Hesse S, Lucke D, Malezic M, Bertelt C, Friedrich H, Gregoric M, Mauritz KH. Botulinum toxin treatment for lower limb extensor spasticity in chronic hemiparetic patients. J Neurol Neurosurg Psychiatry 1994;57:1321–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hesse S, Krajnik J, Luecke D, Jahnke MT, Gregoric M, Mauritz KH. Ankle muscle activity before and after botulinum toxin therapy for lower limb extensor spasticity in chronic hemiparetic patients. Stroke 1996;27:455–460. [DOI] [PubMed] [Google Scholar]
- 57.Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett 1995;201:37–40. [DOI] [PubMed] [Google Scholar]
- 58.Bayram S, Sivrioglu K, Karli N, Ozcan O. Low-dose botulinum toxin with short-term electrical stimulation in poststroke spastic drop foot: a preliminary study. Am J Phys Med Rehabil 2006;85:75–81. [DOI] [PubMed] [Google Scholar]
- 59.Burbaud P, Wiart L, Dubos JL, Gaujard E, Debelleix X, Joseph PA, Mazaux JM, Bioulac B, Barat M, Lagueny A. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J Neurol Neurosurg Psychiatry 1996;61:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiter F, Danni M, Lagalla G, Ceravolo G, Provinciali L. Low-dose botulinum toxin with ankle taping for the treatment of spastic equinovarus foot after stroke. Arch Phys Med Rehabil 1998;79:532–535. [DOI] [PubMed] [Google Scholar]
- 61.Kirazli Y, On AY, Kismali B, Aksit R. Comparison of phenol block and botulinus toxin type A in the treatment of spastic foot after stroke: a randomized, double-blind trial. Am J Phys Med Rehabil 1998;77:510–515. [DOI] [PubMed] [Google Scholar]
- 62.Johnson CA, Burridge JH, Strike PW, Wood DE, Swain ID. The effect of combined use of botulinum toxin type A and functional electric stimulation in the treatment of spastic drop foot after stroke: a preliminary investigation. Arch Phys Med Rehabil 2004;85:902–909. [DOI] [PubMed] [Google Scholar]
- 63.Turkel CC, Bowen B, Liu J, Brin MF. Pooled analysis of the safety of botulinum toxin type A in the treatment of poststroke spasticity. Arch Phys Med Rehabil 2006;87:786–792. [DOI] [PubMed] [Google Scholar]


