Abstract
To investigate the pathogenic mechanisms of eosinophilic pleural effusion in patients with paragonimiasis, we measured the levels of IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon-gamma (IFN-γ) in pleural effusions. Samples were obtained from 11 patients with Paragonimus westermani infection. In addition, samples from 12 patients with pleural transudates, 16 with tuberculous pleurisy, seven with empyema and 20 with lung cancer were also examined. Eosinophilia was remarkable in peripheral blood (range 4–34%, median 23·4%) and pleural fluid (range 0–95%, median 71%) of paragonimiasis patients. IL-5 concentrations in pleural effusions of paragonimiasis were markedly higher than those in other groups. Although marked elevation of GM-CSF and IFN-γ levels was observed in pleural effusion of empyema and tuberculosis patients, it was marginal in the pleural effusion of paragonimiasis patients. In paragonimiasis patients, IL-5 levels in the pleural effusion correlated well with the percentage of eosinophils in peripheral blood and pleural fluid. Such a correlation was not observed between GM-CSF levels in pleural effusion and percentages of eosinophils in pleural fluid or peripheral blood. Our findings suggest that in paragonimiasis IL-5 in the local inflammatory site is particularly important in mediating eosinophilia in peripheral blood and pleural effusion.
Keywords: paragonimiasis westermani, pleural effusion, IL-5, granulocyte-macrophage colony-stimulating factor, interferon-gamma
Introduction
Eosinophilia is a characteristic feature of parasitic helminth infections. Paragonimiasis westermani is a parasitic disease typically associated with eosinophilia, and patients often have eosinophilic pleural effusions [1,2]. Recently, several cytokines (IL-5, IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF)) have been shown to stimulate eosinophilopoiesis in vitro [3–5]. While GM-CSF and IL-3 promote growth and differentiation of a broad range of bone marrow-derived cell types in vitro, IL-5, a representative cytokine produced by Th2 cells, appears to exert its effects primarily on the development and maturation of eosinophils [6]. Human IL-5 can also stimulate the function of mature eosinophils and acts as a potent and selective chemoattractant for eosinophils [7]. In addition, selective induction of IL-5 is known to be responsible for the development of eosinophilia in helminth-infected patients [8,9]. Thus, IL-5 appears to play a major role in parasite-induced eosinophilia, but only few case reports have examined the relationship between IL-5 and paragonimiasis [10–12]. In this study, to evaluate the role of these cytokines in eosinophil recruitment in paragonimiasis, we measured the concentrations of IL-5 and GM-CSF, and interferon-gamma (IFN-γ), a representative Th1 cytokine, in pleural effusions of patients with paragonimiasis westermani and some other diseases.
Patients and methods
Patients and diagnostic categories
Patients examined in this study were 11 cases of paragonimiasis westermani, and other pleural diseases including 12 cases of transudates, 16 tuberculous pleurisy, seven empyema, and 20 lung cancer. There were no baseline differences among the diagnostic groups with respect to age or sex. The clinical and laboratory data of paragonimiasis cases are shown in Table 1. The patients comprised one woman and 10 men, aged 51·4 ± 13·3 years. They were diagnosed as having paragonimiasis by immunodiagnosis using a multiple-dot ELISA test for screening of parasite diseases. The sensitivity and specificity of the test was > 95%. Binding inhibition ELISA and/or Ouchterlony's method were used for identification of the pathogen as Paragonimus westermani [13,14]. The percentage and absolute number of eosinophils in peripheral blood was 19·6 ± 8·2% (range 4–34%, median 23·4%) and 1724·7 ± 949·5/mm3 (range 288–3094/mm3, median 2430/mm3), respectively (Table 1). All but one (case 11) patients were effectively treated with praziquantel. Patient 11 required surgical decortication [15].
Table 1.
Clinical and laboratory data of patients with paragonimiasis
| Peripheral blood | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eosinophils | |||||||||
| No. | Age/sex | Eating custom | Symptoms | Radiological findings | Leucocytes(mm3) | % | mm3 | IgE (U/ml) | Pleural effusions, eosinophils (%) |
| 1 | 55/M | Flesh of wild boars | Chest pain | Rt pleural effusion | 5900 | 13 | 767 | NT | 75 |
| 2 | 73/M | Flesh of wild boars | Chest pain, fever | Rt pleural effusion | 6100 | 12·3 | 750·3 | NT | 60 |
| 3 | 67/M | Flesh of wild boars | General fatigue | Bilateral pleural effusion | 10 340 | 23·5 | 2429·90 | 30 090 | 67 |
| 4 | 64/F | Unknown | Cough | Lt pleural effusion | 6100 | 24 | 1464 | 550 | 95 |
| 5 | 37/M | Flesh of wild boars and freshwater crabs | Haemoptysis | Lt pleural effusion | 11 000 | 23·4 | 2574 | 2707 | NT |
| 6 | 41/M | Flesh of wild boars | Cough, sputum | Lt pleural effusion | 6800 | 17·4 | 1183·20 | 365 | 50 |
| 7 | 48/M | Flesh of wild boars and freshwater crabs | Exertional dyspnoea | Rt pleural effusion | 9800 | 26·5 | 2597 | NT | 83 |
| 8 | 49/M | Flesh of wild boars | Cough, chest pain | Lt pleural effusion and pneumothorax | 12 000 | 21·2 | 2544 | 813 | 43·1 |
| 9 | 30/M | Unknown | (–) | Bilateral pleural effusion | 9100 | 34 | 3094 | 3725 | 95 |
| 10 | 44/M | Flesh of wild boars | Chest pain, dyspnoea | Rt pleural effusion | 8000 | 16 | 1280 | 2919 | 81 |
| 11 | 57/M | Flesh of wild boars | (–) | Lt pleural effusion | 7200 | 4 | 288 | 192 | 0 |
NT, Not tested.
Disease entities of non-parasitic diseases were as follows: patients with transudates were associated with congestive heart failure, nephrotic syndrome, and liver cirrhosis and were defined as those having pleural effusions with (i) pleural fluid protein to serum total protein ratio < 0·5, and (ii) pleural lactate dehydrogenase (LDH) to serum LDH ratio < 0·6. Patients with tuberculous pleural effusions were defined as those with (i) growth of Mycobacterium tuberculosis in cultures from pleural fluid or biopsy specimens, (ii) growth of M. tuberculosis from sputum, or (iii) recent conversion of tuberculin skin reactivity, granulomas on pleural biopsy specimens, or response to anti-tuberculosis therapy. Empyema patients were defined as having a neutrophilic effusion associated with (i) growth of bacteria on microbiological culture of pleural fluid, or (ii) organisms seen on Gram staining of pleural fluid. Patients having neoplastic effusions were defined as those having exudates associated with a diagnosis of lung cancer based on cytologic examination of pleural fluid or lung tissue.
Pleural fluid samples obtained from each patient by thoracocentesis were centrifuged at 500 g for 10 min and the supernatants stored at −20°C until analysis [16]. The degree of eosinophilia, levels of total protein and LDH in pleural effusions of these patients including paragonimiasis are summarized in Table 2. Malignant and transudative pleural samples were free from bacteria, mycobacteria, or fungi tested by cultures.
Table 2.
Eosinophilia (%), total protein, and lactate dehydrogenase (LDH) levels in pleural effusions of various diseases
| Eosinophils (%) | Total protein (g/dl) | LDH (U/l) | |
|---|---|---|---|
| Transudates | 0·4 ± 0·8 | 2·9 ± 1·6 | 185·6 ± 61·2 |
| Paragonimiasis | 64·9 ± 28·8 | 6·5 ± 1·0 | 2794·5 ± 2206·2 |
| Tuberculosis | 1·3 ± 2·1 | 4·8 ± 0·7 | 1368·8 ± 2197·1 |
| Empyema | 0·7 ± 1·6 | 4·3 ± 0·7 | 3584·0 ± 4565·2 |
| Neoplastic | 4·5 ± 11·7 | 4·3 ± 1·0 | 947·8 ± 592·7 |
Measurement of cytokines
IL-5 was measured using a sandwich ELISA. Briefly, 50 μl/well MoAb to human IL-5 (TRFK5; PharMingen, San Diego, CA) (4 μg/ml NaHCO3, pH 8·2) was bound to microtitre plates by incubating at 4°C overnight. The wells were washed twice with PBS containing 0·05% Tween 20. After blocking with 200 μl PBS/10% fetal calf serum (FCS) at room temperature for 2 h, 100 μl of pleural effusion or serum and recombinant human IL-5 (PharMingen) were added to each well and incubated at 4°C overnight. After washing twice with 0·05% Tween–PBS, 100 μl of secondary antibody (biotinylated anti-human IL-5 MoAb (TRFK4; PharMingen)) (4 μg/ml) were added to each well and incubated at room temperature for 1 h, followed by a further 1 h incubation with 100 μl of peroxidase-conjugated streptavidin (× 1000 diluted in PBS containing 10% FCS). Wells were subsequently washed five times with 0·05% Tween–PBS and incubated with 100 μl of 0·11 m sodium acetate buffer pH 5·5 containing tetramethylbenzidine at room temperature for 15 min or until a suitable colour developed. The reaction was stopped by adding 100 μl 1·8 m H2SO4 to each well. Absorbance of the plates was read at 450 nm in an ELISA reader. The average value from duplicate assays was obtained. IL-5 concentration in samples was calculated from the standard curve. The detection limit was 20·0 pg/ml. Concentrations below the lower limit of detection were assumed to be zero for the purpose of statistical analysis.
GM-CSF and IFN-γ levels were measured using commercial ELISA kits (R&D systems, Minneapolis, MN) and the measurement followed to the manufacturer's instructions. The detection limits were 3 pg/ml and 0·03 U/ml for GM-CSF and IFN-γ, respectively.
Statistical analysis
All data are expressed as mean ± s.d. Statistical comparisons were performed by the Mann–Whitney U-test to examine differences between the means of unpaired samples. The Spearman's rank correlation was used to examine the relationship between various parameters. Significance was defined as P < 0·05.
Results
Patients with paragonimiasis exhibited eosinophilia in peripheral blood and pleural effusion (Table 1). Eosinophilia in pleural effusion was observed only in paragonimiasis patients (Table 2). Although the degree of eosinophilia in pleural effusions of paragonimiasis patients was proportional to that in peripheral blood (r = 0·74, P < 0·05), it was about three-fold higher in pleural fluid (Table 1).
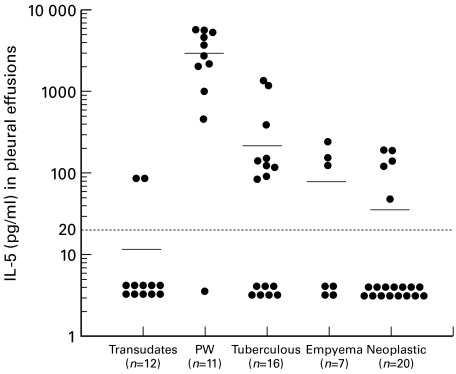
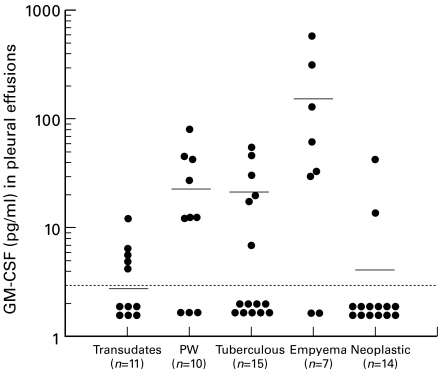
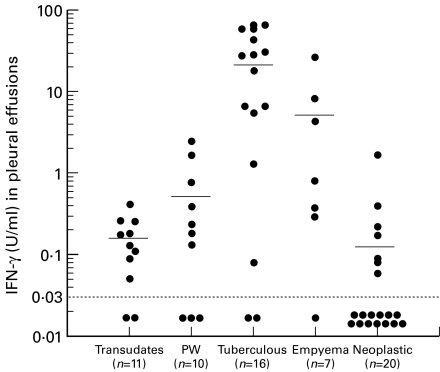
IL-5 concentrations in the pleural effusions of paragonimiasis patients (2902 ± 1987 pg/ml) were markedly higher than those in other disease groups (Fig. 1). IL-5 levels in the serum of paragonimiasis patients were measured in three cases; 38·8 pg/ml in one case and below the detection limit in two cases. In contrast to elevated IL-5 levels in pleural effusions of the paragonimiasis group, GM-CSF levels in pleural effusions were significantly higher in the empyema group (153 ± 216 pg/ml) and IFN-γ levels in pleural effusions were significantly higher in the tuberculosis group (25·4 ± 24·4 U/ml) compared with other diseases (Figs 2 and 3). Although slight increases in GM-CSF and IFN-γ levels were observed in paragonimiasis pleural effusions, their degree was almost comparable to those in transudates (Figs 2 and 3).
Fig. 1.
Individual IL-5 levels in pleural fluid in various diseases. PW, Paragonimiasis westermani. IL-5 concentrations in PW were significantly higher than those in other disease groups (P < 0·0001). Means are indicated by horizontal bars. Detection limit of IL-5 is represented as a horizontal dotted line (< 20 pg/ml).
Fig. 2.
Individual granulocyte-macrophage colony-stimulating factor (GM-CSF) levels in pleural fluid in various diseases. PW, Paragonimiasis westermani. GM-CSF concentrations in empyema were significantly higher than those in transdates, tuberculous, neoplastic (P < 0·01), or in the PW group (P < 0·05). Means are indicated by horizontal bars. Detection limit of GM-CSF is represented as a horizontal dotted line (< 3 pg/ml).
Fig. 3.
Individual IFN-γ levels in pleural fluid in various diseases. Means are indicated by horizontal bars. PW, Paragonimiasis westermani. IFN-γ concentrations in the tuberculous group were significantly higher than those in transdates (P < 0·001), empyema (P < 0·05), neoplastic (P < 0·0001), or in PW (P < 0·001) groups. Detection limit of IFN-γ is represented by the horizontal dotted line (< 0·03 U/ml).
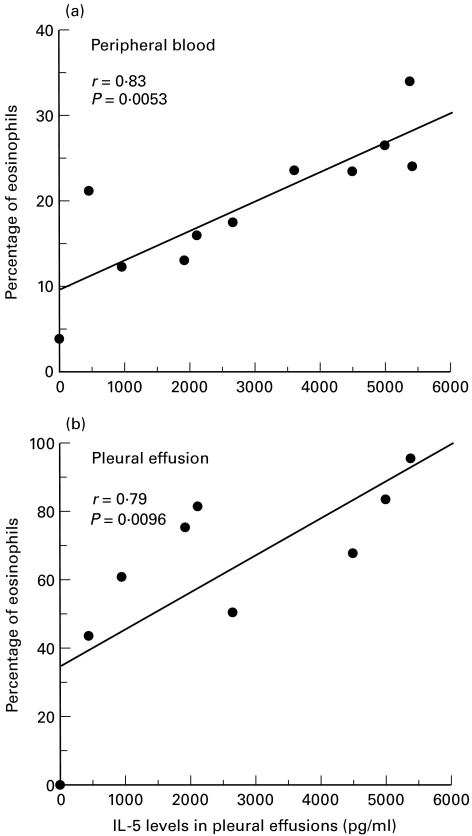
In paragonimiasis patients, IL-5 concentrations in pleural effusions significantly correlated with the percentage of eosinophils in peripheral blood (r = 0·83, P = 0·005, Fig. 4a) as well as that in pleural fluid (r = 0·79, P = 0·01, Fig. 4b). On the other hand, the level of GM-CSF in pleural effusion was not correlated with the degree of eosinophilia in pleural effusions (r = 0·33, P = 0·38) or peripheral blood (r = 0·50, P = 0·14) (data not shown as a figure).
Fig. 4.
(a) Relationship between percentages of eosinophils in peripheral blood and IL-5 concentrations in pleural fluid of patients with paragonimiasis westermani (n = 11). (b) Relationship between percentages of eosinophils and IL-5 concentrations in pleural fluid of patients with paragonimiasis westermani (n = 10).
Discussion
The major finding of the present study was the presence of high percentages of eosinophils and high concentrations of IL-5 in pleural effusions of patients with P. westermani infection, compared with the pleural effusions of other diseases. IL-5 levels in pleural effusions of paragonimiasis patients correlated significantly with the percentage of eosinophils in both pleural effusions and peripheral blood, though the degree of eosinophilia was higher in the pleural effusions.
Paragonimiasis westermani is a common parasitic zoonosis in Asia. The southern part of Kyusyu district, Japan, has long been known as one of the major endemic areas of this disease [13,14]. The infection is acquired from the traditional custom of eating freshwater crabs, Eriocheir japonicus, a second intermediate host, or the flesh of wild boars, Susscrofa leukomystax, a proven paratenic host [17,18]. Paragonimiasis is a disease typically associated with eosinophilia and a high incidence of eosinophilic pleural effusion [1,2].
It is now well known that in helminth-infected patients, an expansion of a Th2-like population results in increased production of IL-5 in vitro [7,8]. In addition, anti-IL-5 antibody treatment greatly reduces the development of eosinophilia in parasitic helminth-infected mice [7,9]. IL-5 thus appears to play a major role in mediation of eosinophilia in paragonimiasis like other helminth infections. However, there are only few case reports that have examined the relationship between IL-5 and paragonimiasis [10–12]. Kan et al. [10] reported a case with extraordinarily high peripheral eosinophilia and elevated serum IL-5 associated with P. westermani infection. In the present study, IL-5 levels were extraordinarily high in pleural effusions of paragonimiasis patients, while serum IL-5 levels were low. Based on this finding, we postulate that IL-5 produced in the local inflammatory site may play a key role in inducing eosinophilia in paragonimiasis. In fact, the degree of eosinophilia in pleural effusions was higher than that in peripheral blood. Related to this, Hatano and colleagues [11] demonstrated that peripheral blood mononuclear cells (PBMC) freshly isolated from a cutaneous paragonimiasis patient expressed high levels of IL-4, IL-5, IL-10 and IL-13 mRNAs. Matsumoto et al. [12] also demonstrated that PBMC from a paragonimiasis patient incubated with mitogen released IL-5. PBMC may also contribute to eosinophilia in paragonimiasis in a manner different from those in the local inflammatory site.
GM-CSF is also known to stimulate eosinophilopoiesis in vitro [5]. In the present study, however, GM-CSF levels were not significantly elevated in pleural effusions of paragonimiasis (Fig. 2). This finding is consistent with the previous results of Limaye et al. [8] that although IL-3 and GM-CSF production upon mitogen stimulation of PBMC were comparable between normal individuals and eosinophilic patients with filarial helminth infections, IL-5 production was far greater in eosinophilic patients. These findings also support our notion that IL-5 is a crucial cytokine involved in induction of eosinophilia in parasitic infections. The effect of GM-CSF on bone marrow cell growth and differentiation extends to cells of the neutrophil lineage in addition to eosinophils, and in concordance with this, GM-CSF levels in pleural effusions were significantly increased in empyema (Fig. 2). Although IL-8 and G-CSF are known to be important cytokines in empyema [16,19], GM-CSF may also play an important role in this neutrophilic inflammation.
In the present study, we also evaluated the levels of IFN-γ, a representative cytokine produced by Th1 cells, in pleural effusion of various diseases. IFN-γ levels were not elevated in paragonimiasis (Fig. 3), suggesting that Th1 cells are probably not mainly involved in P. westermani infection. In contrast, extraordinarily high IFN-γ levels were noted in tuberculous pleurisy (Fig. 3), a finding consistent with previous reports [20–22]. According to Ogawa et al. [21], IFN-γ levels in pleural effusions of > 5 U/ml is a useful diagnostic tool for tuberculous pleurisy. In the present study, IFN-γ levels in 12 of 16 tuberculosis patients (75%) were > 5 U/ml, while only two (4·2%) of the remaining 48 effusion samples of various diseases had IFN-γ levels of > 5 U/ml. The latter two samples were from the patients with empyema.
In conclusion, the present study demonstrates that IL-5 produced in the local inflammatory site may play an important role in mediating eosinophilia in paragonimiasis. Measurement of Th1/Th2 cytokines in pleural effusions may have some diagnostic value for patients with pleurisy.
Acknowledgments
The authors thank Drs K. Takatsu and Y. Kikuchi for technical advice regarding the IL-5 ELISA.
REFERENCES
- 1.Nawa Y. Recent trends of Paragonimiasis westermani in Miyazaki Prefecture, Japan. Southeast Asian J Trop Med Public Health. 1991;22:342–4. [PubMed] [Google Scholar]
- 2.Johnson JR, Falk A, Iber C, et al. Paragonimiasis in the United States. A report of nine cases in Hmong immigrants. Chest. 1982;82:168–71. doi: 10.1378/chest.82.2.168. [DOI] [PubMed] [Google Scholar]
- 3.Clutterbuck EJ, Sanderson CJ. Regulation of human eosinophil precursor production by cytokines: a comparison of recombinant human interleukin-1 (rhIL-1), rhIL-3, rhIL-5, rhIL-6, and rh-granulocyte-macrophage colony-stimulating factor. Blood. 1990;75:1774–9. [PubMed] [Google Scholar]
- 4.Lopez AF, To LB, Yang Y-C, et al. Stimulation of proliferation, differentiation and function of human cells by primate interleukin 3. Proc Natl Acad Sci USA. 1987;84:2761–5. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark SC, Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987;236:1229–37. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 6.Kern P, Horstmann RD, Dietrich M. Eosinophil production in human bone-marrow cultures induced by 80–85 kDa serum component(s) of patients with eosinophilia. Br J Haematol. 1987;66:165–72. doi: 10.1111/j.1365-2141.1987.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 7.Takatsu K, Takaki S, Hitoshi Y. Interleukin-5 and its receptor system: implications in immune system and inflammation. Adv Immunol. 1994;57:145–90. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- 8.Limaye AP, Abrams JS, Silver JE, et al. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990;172:399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–9. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 10.Kan H, Ogata T, Taniyama A, et al. Extraordinarily high eosinophilia and elevated serum interleukin-5 level observed in a patient infected with Paragonimus westermani. Pediatrics. 1995;96:351–4. [PubMed] [Google Scholar]
- 11.Hatano Y, Katagiri K, Ise T, et al. Expression of Th1 and Th2 cytokine mRNAs in freshly isolated peripheral blood mononuclear cells of a patient with cutaneous paragonimiasis. J Dermatol Sci. 1999;19:144–7. doi: 10.1016/s0923-1811(98)00057-7. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Kimura S, Yamauchi M, et al. Soluble CD23 and IL-5 levels in the serum and culture supernatants of peripheral blood mononuclear cells in a girl with cutaneous paragonimiasis: case report. Annu Trop Paediatr. 1998;18:49–53. doi: 10.1080/02724936.1998.11747926. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama H, Noda S, Nawa Y. Emerging problems of parasitic diseases in southern Kyusyu. Japan Jpn J Parasitol. 1996;45:192–200. [Google Scholar]
- 14.Uchiyama F, Morimoto Y, Nawa Y. Re-emergence of paragonimiasis in Kyusyu, Japan. Southeast Asian J Trop Med Public Health. 1999;30:686–91. [PubMed] [Google Scholar]
- 15.Tomita M, Ichinari H, Matsuzaki Y, et al. A case of chronic pleural empyema by Paragonimus westermani infection resistant to chemotherapy and cured by surgical decortication. Jpn J Parasitol. 1996;45:242–6. [Google Scholar]
- 16.Ashitani J, Mukae H, Nakazato M, et al. Elevated pleural fluid levels of defensins in patients with empyema. Chest. 1998;113:788–94. doi: 10.1378/chest.113.3.788. [DOI] [PubMed] [Google Scholar]
- 17.Yokogawa M. Paragonimus and paragonimiasis. Adv Parasitol. 1965;3:99–158. doi: 10.1016/s0065-308x(08)60364-4. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki I, Hirose H. Immature lung flukes first found in the muscle of the wild boar in Japan. J Parasitol. 1976;62:836–7. [PubMed] [Google Scholar]
- 19.Broaddus VC, Hebert CA, Vitangcol RV, et al. Interleukin-8 is a major neutrophil chemotactic factor in pleural liquid of patients with empyema. Am Rev Respir Dis. 1992;146:825–30. doi: 10.1164/ajrccm/146.4.825. [DOI] [PubMed] [Google Scholar]
- 20.Valdes L, Jose ES, Alvarez D, et al. Diagnosis of tuberculous pleurisy using the biologic parameters adenosine deaminase, lysozyme, and interferon gamma. Chest. 1993;103:458–65. doi: 10.1378/chest.103.2.458. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa K, Koga H, Hirakata Y, et al. Differential diagnosis of tuberculous pleurisy by measurement of cytokine concentrations in pleural effusion. Tuber Lung Dis. 1997;78:29–34. doi: 10.1016/s0962-8479(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 22.Wongtim S, Silachamroon U, Ruxrungtham K, et al. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax. 1999;54:921–4. doi: 10.1136/thx.54.10.921. [DOI] [PMC free article] [PubMed] [Google Scholar]