Abstract
MoAbs against tumour-associated antigens (TAA) may be useful for the treatment of colorectal cancer. Since an increased expression of TAA may lead to enhanced antibody-dependent cellular cytotoxicity we examined whether the cytokines IL-2, IL-4, IL-6, IL-10, IL-12, interferon-alpha (IFN-α), IFN-γ, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor and tumour necrosis factor-alpha can influence EpCAM and LewisY expression on the surface of the colorectal carcinoma cell lines HT29, LoVo and SW480. We found that only IFN-α increased significantly whereas IL-4 decreased both EpCAM and LewisY expression. IFN-γ significantly increased LewisY expression only. When tumour cells were treated with MoAb, the LewisY-specific MoAb BR55-2 down-regulated LewisY antigen expression, whereas MoAb 17-1A, which binds to EpCAM, up-regulated this TAA after 3 days of culture. The cytokines IFN-α or IFN-γ combined with MoAb 17-1A enhanced further slightly the expression of EpCAM. In additional experiments with chemotherapeutic drugs commonly used for the treatment of colorectal cancer, we found that 5-fluorouracil, mitomycin-C and oxaliplatin up-regulated EpCAM and LewisY antigen expression. Raltitrexed enhanced LewisY and down-regulated EpCAM expression, whereas CPT-11 had no influence at all. The highest expression for EpCAM on HT29 cells was achieved by the combination of IFN-α, 5-fluorouracil and MoAb 17-1A. Our results may be useful for defining combinations of biological and chemotherapeutic drugs for the treatment of colorectal cancer. Further trials should evaluate to what extent these combinations enhance antibody-dependent cellular cytotoxicity.
Keywords: EpCAM, cytokines, flow cytometry, LewisY, monoclonal antibodies
Introduction
MoAbs which recognize tumour-associated antigens (TAA) are increasingly used for the treatment of cancer. Cytokines such as interferon-alpha (IFN-α) [1, 2], IFN-γ [2, 3], IL-2 [4, 5], IL-12 [6] and granulocyte-macrophage colony-stimulating factor (GM-CSF) [7, 8] can augment the antibody-dependent cellular cytotoxicity (ADCC) of MoAb. We recently developed a new flow cytometric cytotoxicity test, which can assess the long-term ADCC exerted by macrophages [9]. We could demonstrate with this assay that the cytokines IFN-α, IFN-γ, IL-2 and IL-12 significantly augment whereas the cytokines IL-6, macrophage colony-stimulating factor (M-CSF), GM-CSF and tumour necrosis factor-alpha (TNF-α) have no influence, and cytokine IL-4 even suppresses ADCC of MoAbs 17-1A and BR55-2 [10–12].
In this study, we examined whether these cytokines can influence the expression of the TAA EpCAM and LewisY blood group antigen [13], which are both expressed on the surface of epithelial cells of most gastrointestinal tissues, in particular on neoplastic colonic cells [14]. EpCAM was detected by the murine MoAb 17-1A [15], which was recently used with success for the adjuvant treatment of colorectal carcinoma [16]. For labelling of the LewisY antigen the murine MoAb BR55-2 was used [17]. Furthermore, we investigated the influence of five chemotherapeutic drugs, which are commonly used for the treatment of colorectal carcinoma, on EpCAM and LewisY antigen expression.
Materials and methods
Medium and cells
RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 200 μg/ml streptomycin, 200 U/ml penicillin and 300 μg/ml l-glutamine was used throughout. The colon carcinoma cell lines HT29, LoVo and SW480 (ATCC, Rockville, MD) were kept in exponential growth conditions in 12·5 ml medium in plastic 75-cm2 culture flasks (Greiner, Solingen, Germany). Cells were treated with 3 μg/ml mitomycin C for 2 h in order to suppress proliferation of tumour cells in the wells, washed three times and detached using a rubber policeman.
Then, 100 000 tumour cells were added in 96-well microtitre plates and incubated with 30 ng/ml final concentration IL-2, IL-4, IL-6, IL-10, IL-12, IFN-α, IFN-γ, GM-CSF, M-CSF and TNF-α, which was found in titration experiments to be effective in influencing tumour antigen-associated expression, or ADCC and MoAbs BR55-2 and 17-1A (50 μg/ml final concentration) in a volume of 200 μl per well of a microtitre plate for 3 days, which was found to be the optimal time point for tumour antigen-associated antigen expression. Each experiment was performed in triplicate. Thereafter, cells were detached by treatment with 50 μl warm EDTA 0·02%/trypsin 0·05% in PBS per well for 20 min and agitated on a plate shaker for 1 min. After washing twice indirect immunofluorescence was performed. In experiments with chemotherapeutic drugs, tumour cells were first treated for 2 h with 3 μg/ml final concentration of 5-fluorouracil (5-FU; Ribosepharm, Munich, Germany), mitomycin-C (Medac, Hamburg, Germany), oxaliplatin (Sanofi Winthrop, Munich, Germany), CPT-11 (Rhône-Poulenc Rorer, Antony Cedex, France) and raltitrexed (Zeneca, London, UK). Titration experiments were performed with all chemotherapeutic drugs at a concentration range of 0·78 μg/ml and 100 μg/ml. The concentration of 3 μg/ml for chemotherapeutic drugs was found optimal in means of TAA expression and remaining viable tumour cells in previous experiments. After treatment, tumour cells were washed twice and processed as mentioned below.
Cytokines and monoclonal antibodies
IFN-γ (10 × 106 U/mg), IL-2 (5 × 106 U/mg), IL-6 (1 × 107 U/mg), IL-10 (100 ng/ml), M-CSF (55 × 106 U/mg) and GM-CSF (10 × 106 U/mg) were all purchased from IC Chemikalien (Ismaning, Germany). IFN-α (200 × 106 U/mg) and IL-4 (1 × 108 U/mg) were obtained from Pharma Biotechnologie (Hannover, Germany). TNF-α (50 × 106 U/mg) was kindly provided by Fa. Bender (Vienna, Austria). IL-12 (2·4 × 108 U/mg) was kindly provided by Hoffmann La-Roche (Grenzach-Wyhlen, Germany). All cytokines were used at 30 ng/ml final concentration, which was found earlier to mediate reproducibly enhancement of ADCC, whereas concentrations of 0·3 ng/ml were ineffective in this respect [10, 11, 18]. The murine MoAb 17-1A of the IgG2a isotype was obtained from Glaxo Wellcome (Hamburg, Germany) and murine BR55-2 of the IgG3 isotype was kindly provided by H. Loibner (Novartis, Basel, Switzerland).
Indirect immunofluorescence
Indirect immunofluorescence was performed as described previously [19]. Briefly, tumour cells were incubated with primary MoAb for 30 min at room temperature and under continuous movement. The cells were then washed three times with PBS and 2·5% FCS, and incubated with an appropriate dilution of secondary, FITC-conjugated goat anti-mouse antibody in the dark for another 30 min. After washing the pellets were resuspended in PBS with 2·5% FCS, 1% propidium iodide (PI), and FITC mean fluorescence intensity (MFI) of living (PI−) cells was determined on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Statistical analysis
Significant differences between triplicates were calculated using Student's t-test; P < 0·05 was regarded as significant.
Results
First, we investigated the effect of the cytokines IL-2, IL-4, IL-6, IL-10, IL-12, IFN-α, IFN-γ, GM-CSF, M-CSF and TNF-α on EpCAM and LewisY antigen expression on three colorectal tumour cell lines. The cytokine IFN-α enhanced significantly the expression of EpCAM on HT29 tumour cells after 3 days of culture (P < 0·05), TNF-α and IL-2 did not essentially influence and the cytokine IL-4 suppressed it (Table 1). In contrast, IFN-α, IFN-γ and TNF-α enhanced, the cytokine IL-4 suppressed, whereas IL-2 did not influence the expression of LewisY on LoVo tumour cells. The cytokines IL-6, IL-10, IL-12, GM-CSF and M-CSF essentially did not influence expression of EpCAM and LewisY on any of the three tumour cell lines tested (data not shown).
Table 1.
Influence of selected cytokines on the expression of EpCAM and LewisY on colorectal tumour cell lines
| HT29, % | LoVo, % | SW480, % | |
|---|---|---|---|
| EpCAM | |||
| Control | 100 | 100 | 100 |
| IFN-α | 120 ± 12* | 121 ± 15 | 119 ± 12 |
| IFN-γ | 115 ± 19 | 109 ± 15 | 109 ± 11 |
| IL-2 | 103 ± 13 | 108 ± 16 | 118 ± 6 |
| IL-4 | 52 ± 18 | 65 ± 14 | 104 ± 16 |
| TNF-α | 109 ± 10 | 95 ± 12 | 95 ± 13 |
| LewisY | |||
| Control | 100 | 100 | 100 |
| IFN-α | 131 ± 15 | 132 ± 7 | 131 ± 5 |
| IFN-γ | 162 ± 17 | 160 ± 11 | 173 ± 17 |
| IL-2 | 96 ± 16 | 97 ± 11 | 109 ± 16 |
| IL-4 | 60 ± 14 | 76 ± 15 | 110 ± 12 |
| TNF-α | 121 ± 9 | 109 ± 16 | 137 ± 12 |
Relative percentage of mean fluorescence intensity (MFI) compared with control (without cytokine) demonstrating tumour-associated antigen (TAA) expression on three colorectal tumour cell lines (HT29, LoVo, and SW480) after 3 days of incubation with the indicated cytokines (mean and percent s.d. obtained from seven experiments). Indirect immunofluorescence for the TAA EpCAM and LewisY antigen was performed using the primary antibodies 17-1A for EpCAM and BR55-2 for LewisY expression and goat anti-mouse–FITC as secondary antibody. MFI was assessed by flow cytometry.The depicted relative percentage of MFI to control was calculated from the ratio MFI (probe)/MFI (control) × 100.Bold type indicates significant differences of cytokine treatment compared with control.
Next, we examined the influence of MoAb 17-1A and BR55-2 on TAA expression. EpCAM expression was increased slightly during the 3-day culture (data not shown). Treatment of tumour cells with MoAb 17-1A at the beginning of the culture resulted in up-regulation of EpCAM (Table 2, MoAb on day 0), indicating expression of new EpCAM protein on the cell surface. Moreover, indirect immunofluorescence performed after addition of antibody at the end of the culture on day 3 resulted in even higher staining for EpCAM, indicating consumption of antibody added on day 0 (Table 2, MoAb on day 0 + 3). The addition of IFN-α or IFN-γ to MoAb 17-1A resulted in a slight increase in EpCAM compared with MoAb alone, which in all instances was not significant when compared with cultures without cytokines. In contrast, MoAb BR55-2 decreased the expression of LewisY (Table 2, MoAb on day 0), indicating that the LewisY antigen was modulated after MoAb binding. However, addition of antibody at the end of the culture on day 3 for indirect immunofluorescence resulted in equal staining for LewisY, indicating that expression of this TAA remained stable on cell surface, probably by production of new tetrasaccharide (Table 2, MoAb on day 0 + 3). Furthermore, only IFN-γ induced a pronounced significant up-regulation of LewisY on HT29 tumour cells, which was not enhanced by addition of MoAb BR55-2, whereas the combined addition of IFN-α and MoAb BR55-2 resulted in no significant changes compared with addition of antibody alone.
Table 2.
Modulation/induction of EpCAM and LewisY expression by MoAbs, IFN-α or IFN-γ
| MoAb added | Cytokine added | HT29, % | LoVo, % | SW480, % |
|---|---|---|---|---|
| EpCAM expression | ||||
| MoAb1 on day 3 | Ø | 100 | 100 | 100 |
| MoAb1 on day 0 | Ø | 134 ± 14† | 153 ± 10 | 103 ± 2 |
| MoAb1 on day 0 + 3 | Ø | 169 ± 11 | 190 ± 15 | 129 ± 18 |
| MoAb1 on day 3 | + IFN-α | 121 ± 7* | 116 ± 12 | 105 ± 21 |
| MoAb1 on day 0 | + IFN-α | 144 ± 17 | 162 ± 15 | 103 ± 2 |
| MoAb1 on day 0 + 3 | + IFN-α | 182 ± 13 | 207 ± 34 | 131 ± 27 |
| MoAb1 on day 3 | + IFN-γ | 111 ± 38 | 122 ± 15 | 99 ± 33 |
| MoAb1 on day 0 | + IFN-γ | 144 ± 22 | 162 ± 11 | 108 ± 3 |
| MoAb1 on day 0 + 3 | + IFN-γ | 175 ± 34 | 228 ± 2 | 153 ± 29 |
| LewisYexpression | ||||
| MoAb2 on day 3 | Ø | 100 | 100 | ND |
| MoAb2 on day 0 | Ø | 30 ± 18 | 47 ± 18 | ND |
| MoAb2 on day 0 + 3 | Ø | 98 ± 6 | 89 ± 17 | ND |
| MoAb2 on day 3 | + IFN-α | 124 ± 1* | 149 ± 5* | ND |
| MoAb2 on day 0 | + IFN-α | 29 ± 12 | 78 ± 16 | ND |
| MoAb2 on day 0 + 3 | + IFN-α | 115 ± 17 | 96 ± 18 | ND |
| MoAb2 on day 3 | + IFN-γ | 248 ± 6* | 152 ± 9* | ND |
| MoAb2 on day 0 | + IFN-γ | 61 ± 4* | 89 ± 16 | ND |
| MoAb2 on day 0 + 3 | + IFN-γ | 265 ± 26* | 109 ± 20 | ND |
Relative percentage of mean fluorescence intensity (MFI) compared with control (MoAb on day 3 without cytokine) demonstrating tumour-associated antigen (TAA) expression on three colorectal tumour cell lines (HT29, LoVo and SW480) after 3 days of incubation (mean and percent s.d. obtained from four experiments). Murine MoAbs 17-1A (MoAb1) and BR55-2 (MoAb2) and the cytokines IFN-α and IFN-γ were added to tumour cells as indicated and the cells were incubated for 3 days. Then, indirect immunofluorescence was performed using the primary antibody MoAbs 17-1A for EpCAM and BR55-2 for LewisY expression and goat anti-mouse–FITC as secondary antibody. ‘MoAb on day 3’ indicates that primary MoAb was added only on day 3 for assessment of TAA expression, ‘MoAb on day 0’ indicates addition of primary MoAb on day 0 for modulation of the respective antigen and secondary MoAb on day 3 for immunofluorescence, and ‘MoAb on day 0 + 3’ represents modulation of TAA by primary MoAb and assessment of newly synthesized TAA by immunofluorescence on day 3.
MFI was assessed by flow cytometry and the depicted relative percentage of MFI to control was calculated from the ratio MFI (probe)/MFI (control) × 100.
Bold type indicates significant differences of MoAb treatment on day 0 compared with control (MoAb on day 3).
Significant differences of cytokine treatment compared with control.
ND, Not done.
In further experiments, we evaluated whether five different chemotherapeutic drugs, i.e. 5-FU, mitomycin-C, oxaliplatin, CPT-11 and raltitrexed can influence TAA expression. Tumour cells were treated for 2 h with 3 μg/ml final concentration of these chemotherapeutic drugs and after 3 days the expression of TAA was assessed by flow cytometry. The expression of EpCAM was significantly (P < 0·05) increased by 5-FU in both tumour cell lines tested, whereas mitomycin C and oxaliplatin augmented its expression only on HT29 tumour cells (Table 3). In contrast, raltitrexed decreased the expression of EpCAM on both cell lines tested. Interestingly, 5-FU, mitomycin C and oxaliplatin induced a marked increase of LewisY on both HT29 and LoVo tumour cells. In contrast to EpCAM, raltitrexed induced a marked increase of LewisY expression on both tumour cell lines tested.
Table 3.
Influence of selected chemotherapeutic drugs on the expression of EpCAM and LewisY on colorectal tumour cell lines
| HT29 | LoVo | HT29 | LoVo | |
|---|---|---|---|---|
| EpCAM, % | LewisY, % | |||
| Control | 100 | 100 | 100 | 100 |
| 5-fluorouracil | 127 ± 16* | 137 ± 15 | 209 ± 42 | 138 ± 21 |
| Mitomycin-C | 135 ± 19 | 104 ± 13 | 210 ± 20 | 199 ± 43 |
| Oxaliplatin | 129 ± 11 | 114 ± 39 | 230 ± 6 | 176 ± 18 |
| CPT-11 | 98 ± 15 | 104 ± 17 | 98 ± 7 | 106 ± 17 |
| Raltitrexed | 51 ± 17 | 40 ± 16 | 216 ± 20 | 155 ± 27 |
Relative percentage of mean fluorescence intensity (MFI) compared with control (without chemotherapeutic drug) demonstrating tumour-associated antigen (TAA) expression of EpCAM and LewisY antigen on the colorectal tumour cell lines HT29 and LoVo after 3 days of incubation with the indicated chemotherapeutic drugs (mean and percent s.d. obtained from five experiments). Indirect immunofluorescence was performed as described in Table 1.
Bold type indicates significant differences of chemotherapeutic drug treatment compared with control.
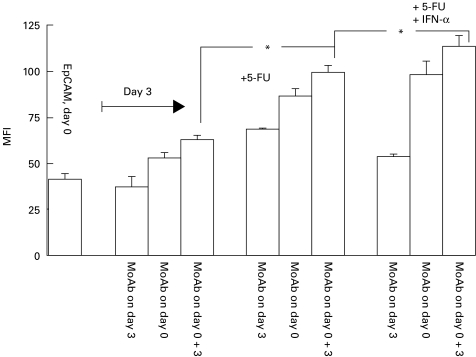
Finally, we examined the influence of the combination IFN-α and 5-FU on EpCAM expression. As presented in Fig. 1, both substances combined did not induce a higher expression of EpCAM on HT29 tumour cells (compare bars ‘MoAb on day 3’ in Fig. 1). However, the highest EpCAM expression was observed when MoAb 17-1A was combined with IFN-α and 5-FU (P < 0·05).
Fig. 1.
Influence of 5-fluorouracil (5-FU) and IFN-a on EpCAM expression. HT29 tumour cells (100 000) were incubated with 3 μg/ml 5-FU and/or 30 ng/ml IFN-α for 3 days. MoAb 17-1A was added as indicated and explained in Table 2. The mean fluorescence intensity (MFI) of EpCAM was measured by indirect immunofluorescence on day 3 as described in Table 2 and in Materials and Methods. *Significant difference (one representative of three experiments performed).
Discussion
In this study, we systematically investigated the influence of the cytokines IL-2, IL-4, IL-6, IL-10, IL-12, IFN-α, IFN-γ, GM-CSF, M-CSF and TNF-α on EpCAM and LewisY expression in three colorectal carcinoma cell lines. We could demonstrate that only IFN-α reproducibly enhanced the expression of both TAA on all cell lines tested. This is in agreement with earlier results concerning the expression of two other TAA, namely carcinoembyonic antigen (CEA) and sialyl-Tn in vitro [20, 21] in an animal model [22] and after in vivo treatment with IFN-α on primary tumours [23]. In contrast, IFN-γ significantly increased only the expression of LewisY antigen in all three cell lines tested, which is in agreement with experiments with prostate and pancreatic cancer cell lines [24]. EpCAM was not influenced by IFN-γ, which is compatible with experiments with human ovarian cancer cells [25] and also prostate and pancreatic cancer cell lines [24]. A heterogeneous pattern was observed with TNF-α, which increased LewisY antigen expression only in two out of three cell lines tested. IL-2 increased EpCAM expression on SW480 tumour cells. Since solid tumours may also express functional IL-2 receptors [26–28] we speculate that this might apply to SW480 tumour cells.
The other tested cytokines including the colony stimulating factors GM-CSF and M-CSF did not significantly influence TAA expression. However, it has been reported that IL-6 can augment expression of CEA on colorectal tumour cells [29, 30]. Since this enhancement was abrogated by specific neutralizing antibodies for type I interferons, indirect evidence was obtained that IFN-β may be responsible for the observed effects [31]. In our study we could not detect a essential impact of IL-6 with either TAA tested.
An interesting finding arising from our study is that IL-4 reproducibly leads to a marked down-regulation of EpCAM and LewisY expression in two out of three tested colorectal carcinoma cell lines. It is already known that functional IL-4 receptors are expressed on colorectal tumour cells [32], which can mediate growth inhibition and enhancement of differentiation [33, 34]. Therefore, we suppose that SW480 tumour cells, which were refractory to the effects of IL-4, do not express functional IL-4 receptors on their surface. Since we have already demonstrated that IL-4 suppresses ADCC and cytokine-induced ADCC by IFN-α, IL-2 or IL-12 [11, 12], we conclude that a combination of IL-4 with MoAbs specific for TAA for treatment of colorectal cancer is not reasonable.
Whether combinations of cytokines can enhance TAA expression was not tested in this study. However, recent data demonstrate that combination of interferons with IL-6 further increases CEA expression on tumour cells [35]. We were also able recently to demonstrate that combinations of cytokines effectively can enhance ADCC mediated by MoAbs specific for the TAA EpCAM and LewisY [11, 12]. Therefore, this issue should be pursued in subsequent studies.
MoAbs after binding on the cell surface are internalized, gradually degraded and released from the cell over a 2–3-day period, while only a small fraction appears to dissociate intact [36, 37]. Both, LewisY-specific MoAbs like BR96 [38] and the EpCAM-specific MoAb 17-1A can be internalized. For radiolabelled MoAb 17-1A the degree of internalization was quantitatively measured and found to increase over time to 49% after a 48-h incubation period [39]. In our experiments, we could demonstrate that the LewisY-specific MoAb BR55-2 gradually disappeared from the cell surface, indicating antigen modulation. IFN-α and IFN-γ could not prevent this modulation in either tested cell line. However, IFN-γ together with MoAb BR55-2 probably induced the expression of new LewisY on HT29 tumour cells only, since significant LewisY expression was observed with this combination (Table 2). MoAb 17-1A induced EpCAM on HT29 and LoVo tumour cells and this was moderately enhanced by the addition of IFN-α or IFN-γ. Since the addition of MoAb 17-1A on day 0 of incubation resulted in a marked increase of EpCAM, one can speculate that new protein appears on the tumour cell surface. To our knowledge an induction of TAA by a specific MoAb has not been described yet, demanding investigations of the mRNA level and analysis of this issue with other tumour-specific antibodies as well.
Investigations concerning the effect of chemotherapeutic drugs are scarce. 5-FU alone [40,41] in combination with IFN-γ [42] or IFN-α [43] are found to increase the expression of CEA on the surface of colorectal tumour cell lines. Combined treatment with interferons induced a strong inhibition of proliferation of tumour cells in vitro [43, 44] but inconsistent results were provided from human clinical trials in colorectal cancer [45–47]. We first investigated the influence on EpCAM and LewisY expression of five chemotherapeutic drugs, which are commonly used for the treatment of colorectal cancer. We incubated tumour cells for 2 h with chemotherapeutic drugs, which may reflect more appropriately the physiological situation since chemotherapeutic drugs reach peak concentrations after infusion and concentrations decline thereafter. The concentration used of 3 μg/ml is clinically relevant, as already shown in pharmacokinetic analyses [48–50]. Similar concentrations for chemotherapeutic drugs and treatment schedules were used in vitro by other authors, e.g. for 5-FU [51], mitomycin C [52] or oxaliplatin [53]. Interestingly, we found that 5-FU, mitomycin-C and oxaliplatin at the concentrations tested up-regulated distinctly EpCAM and LewisY antigen expression. Particularly, mitomycin-C and oxaliplatin induced a pronounced expression of LewisY. In contrast, raltitrexed enhanced LewisY and down-regulated EpCAM expression.
Since the combination IFN-α and MoAb 17-1A induced the highest expression of EpCAM, we next investigated whether 5-FU might further enhance EpCAM expression. Indeed, the triple combination of IFN-α, MoAb 17-1A and 5-FU induced the highest expression of EpCAM on HT29 cells. In ongoing investigations with our flow cytometric cytotoxicity assay [9], we now examine whether chemotherapeutic drugs can enhance the ADCC mediated by MoAb 17-1A. Since the cytokine concentrations used in this study cannot be achieved by systemic treatment one should consider using in vivo combinations of low concentrations of cytokines as already suggested by us [11, 18] or employ cytokines locoregionally [54].
The results of our study may have relevance in view of the current plethora of biological response modifiers and chemotherapeutic drugs as a preclinical model, which may aid in finding optimal combinations of cytokines, MoAbs and chemotherapeutic drugs for treatment of cancer.
Acknowledgments
Supported by Deutsche Krebshilfe (10-09281-Fl1, Bonn, Germany), Megapharm (Sankt Augustin, Germany) and H.W. & J. Hector Stiftung (Mannheim, Germany). MoAb BR55-2 was kindly provided by H. Loibner (Novartis, Basel, Switzerland). This work is part of the doctoral thesis of A.S.H.
REFERENCES
- 1.Herberman RB, Ortaldo JR, Mantovani A, Hobbs DS, Kung HF, Pestka S. Effect of human recombinant interferon on cytotoxic activity of natural killer (NK) cells and monocytes. Cell Immunol. 1982;67:160–7. doi: 10.1016/0008-8749(82)90208-8. [DOI] [PubMed] [Google Scholar]
- 2.Ralph P, Nakoinz I, Rennick D. Role of interleukin 2, interleukin 4, and alpha, beta, and gamma interferon in stimulating macrophage antibody-dependent tumoricidal activity. J Exp Med. 1988;167:712–7. doi: 10.1084/jem.167.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama Y, Lubeck MD, Steplewski Z, Koprowski H. Induction of mouse IgG2a- and IgG3-dependent cellular cytotoxicity in human monocytic cells (U937) by immune interferon. Cancer Res. 1984;44:5127–31. [PubMed] [Google Scholar]
- 4.Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47:6600–5. [PubMed] [Google Scholar]
- 5.Ortaldo JR, Woodhouse C, Morgan AC, Herberman RB, Cheresh DA, Reisfeld R. Analysis of effector cells in human antibody-dependent cellular cytotoxicity with murine monoclonal antibodies. J Immunol. 1987;138:3566–72. [PubMed] [Google Scholar]
- 6.Nguyen QH, Roberts RL, Ank BJ, Lin SJ, Lau CK, Stiehm ER. Enhancement of antibody-dependent cellular cytotoxicity of neonatal cells by interleukin-2 (IL-2) and IL-12. Clin Diagn Lab Immunol. 1998;5:98–104. doi: 10.1128/cdli.5.1.98-104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liesveld JL, Frediani KE, Winslow JM, Duerst RE, Abboud CN. Cytokine effects and role of adhesive proteins and Fc receptors in human macrophage-mediated antibody dependent cellular cytotoxicity. J Cell Biochem. 1991;45:381–90. doi: 10.1002/jcb.240450412. [DOI] [PubMed] [Google Scholar]
- 8.Masucci G, Wersall P, Ragnhammar P, Mellstedt H. Granulocyte-monocyte-colony-stimulating factor augments the cytotoxic capacity of lymphocytes and monocytes in antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 1989;29:288–92. doi: 10.1007/BF00199217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flieger D, Gruber R, Schlimok G, Reiter C, Pantel K, Riethmüller G. A novel non-radioactive cellular cytotoxicity test based on the differential assessment of living and killed target and effector cells. J Immunol Methods. 1995;180:1–13. doi: 10.1016/0022-1759(94)00293-6. [DOI] [PubMed] [Google Scholar]
- 10.Bungard S, Flieger D, Schweitzer S, Sauerbruch T, Spengler U. The combination of interleukin-2 and interferon-α effectively augments the antibody dependent cellular cytotoxicity of monoclonal antibodies 17-1A and BR55-2 against the colorectal carcinoma cell line HT29. Cancer Immunol Immunother. 1998;46:213–20. doi: 10.1007/s002620050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flieger D, Spengler U, Beier I, Sauerbruch T, Schmidt-Wolf I. Combinations of the cytokines IL-12, IL-2 and IFN-alpha significantly augment whereas the cytokine IL-4 suppresses the cytokine-induced antibody-dependent cellular cytotoxicity of monoclonal antibodies 17-1A and BR55-2. Cytokine. 2000;12:756–61. doi: 10.1006/cyto.1999.0610. [DOI] [PubMed] [Google Scholar]
- 12.Flieger D, Spengler U, Beier I, Kleinschmidt R, Hoff A, Varvenne M, Sauerbruch T, Schmidt-Wolf I. Enhancement of antibody dependent cellular cytotoxicity (ADCC) by combination of cytokines. Hybridoma. 1999;18:63–68. doi: 10.1089/hyb.1999.18.63. [DOI] [PubMed] [Google Scholar]
- 13.Blaszczyk-Thurin M, Thurin J, Hindsgaul O, Karlsson KA, Steplewski Z, Koprowski H. Y and blood group B type 2 glycolipid antigens accumulate in a human gastric carcinoma cell line as detected by monoclonal antibody. Isolation and characterization by mass spectrometry and NMR spectroscopy. J Biol Chem. 1987;262:372–9. [PubMed] [Google Scholar]
- 14.Göttlinger HG, Funke I, Johnson JP, Gokel JM, Riethmüller G. The epithelial cell surface antigen 17-1A, a target for antibody-mediated tumor therapy: its biochemical nature, tissue distribution and recognition by different monoclonal antibodies. Int J Cancer. 1986;38:47–53. doi: 10.1002/ijc.2910380109. [DOI] [PubMed] [Google Scholar]
- 15.Herlyn M, Steplewski Z, Herlyn D, Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci USA. 1979;76:1438–52. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riethmüller G, Holz E, Schlimok G, et al. Monoclonal antibody therapy for resected Dukes' C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–94. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 17.Shetye J, Christensson B, Rubio C, Rodensjo M, Biberfeld P, Mellstedt H. The tumor-associated antigens BR55-2, GA73-3 and GICA 19-9 in normal and corresponding neoplastic human tissues, especially gastrointestinal tissues. Anticancer Res. 1989;9:395–404. [PubMed] [Google Scholar]
- 18.Flieger D, Spengler U, Beier I, Kleinschmidt R, Sauerbruch T, Schmidt-Wolf IG. Augmentation of 17-1A-induced antibody-dependent cellular cytotoxicity by the triple cytokine combination of interferon-alpha, interleukin-2, and interleukin-12. J Immunother. 2000;23:480–6. doi: 10.1097/00002371-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Flieger D, Ziegler-Heitbrock HW. Abnormal blood monocytes in chronic lymphocytic leukemia. Cancer Res. 1988;48:4812–6. [PubMed] [Google Scholar]
- 20.Guadagni F, Schlom J, Johnston WW, et al. Selective interferon-induced enhancement of tumor-associated antigens on a spectrum of freshly isolated human adenocarcinoma cells. J Natl Cancer Inst. 1989;81:502–12. doi: 10.1093/jnci/81.7.502. [DOI] [PubMed] [Google Scholar]
- 21.Attallah AM, Needy CF, Noguchi PD, Elisberg BL. Enhancement of carcinoembryonic antigen expression by interferon. Int J Cancer. 1979;24:49–52. doi: 10.1002/ijc.2910240109. [DOI] [PubMed] [Google Scholar]
- 22.Guadagni F, Schlom J, Greiner JW. In vitro and in vivo regulation of tumor antigen expression by human recombinant interferons. Int J Rad Appl Instrum. 1991;18:409–12. doi: 10.1016/0883-2897(91)90068-v. [DOI] [PubMed] [Google Scholar]
- 23.Mahvi DM, Madsen JA, Witt PL, Sondel PM. Interferon alpha enhances expression of TAG-72 and carcinoembryonic antigen in patients with primary colorectal cancer. Cancer Immunol Immunother. 1995;40:311–4. doi: 10.1007/BF01519631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivinski CL, Lindner DJ, Borden EC, Tempero MA. Modulation of tumor-associated antigen expression on human pancreatic and prostate carcinoma cells in vitro by alpha- and gamma-interferons. J Immunother Emphasis Tumor Immunol. 1995;18:156–65. doi: 10.1097/00002371-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kievit E, Pinedo HM, Schluper HM, Haisma HJ, Boven E. Determination of tumor-related factors of influence on the uptake of the monoclonal antibody 323/A3 in experimental human ovarian cancer. Int J Cancer. 1997;71:237–45. doi: 10.1002/(sici)1097-0215(19970410)71:2<237::aid-ijc19>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Kayser K, Ernst M, Bubenzer J. Expression of transferrin- and interleukin-2-receptors, and HLA-DR in human lung carcinoma. Exp Pathol. 1991;41:37–43. doi: 10.1016/s0232-1513(11)80044-7. [DOI] [PubMed] [Google Scholar]
- 27.Katano M, Matsuo T, Morisaki T, et al. Increased proliferation of a human breast carcinoma cell line by recombinant interleukin-2. Cancer Immunol Immunother. 1994;39:161–6. doi: 10.1007/BF01533381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plaisance S, Rubinstein E, Alileche A, et al. Human melanoma cells express a functional interleukin-2 receptor. Int J Cancer. 1993;55:164–70. doi: 10.1002/ijc.2910550129. [DOI] [PubMed] [Google Scholar]
- 29.Ullmann CD, Schlom J, Greiner JW. Interleukin-6 increases carcinoembryonic antigen and histocompatibility leukocyte antigen expression on the surface of human colorectal carcinoma cells. J Immunother. 1992;12:231–41. doi: 10.1097/00002371-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Tsang KY, Kashmiri SV, Qi CF, et al. Transfer of the IL-6 gene into a human colorectal carcinoma cell line and consequent enhancement of tumor antigen expression. Immunol Letters. 1993;36:179–85. doi: 10.1016/0165-2478(93)90050-c. [DOI] [PubMed] [Google Scholar]
- 31.Dansky-Ullmann C, Salgaller M, Adams S, Schlom J, Greiner JW. Synergistic effects of IL-6 and IFN-gamma on carcinoembryonic antigen (CEA) and HLA expression by human colorectal carcinoma cells: role for endogenous IFN-beta. Cytokine. 1995;7:118–29. doi: 10.1006/cyto.1995.1016. [DOI] [PubMed] [Google Scholar]
- 32.Kaklamanis L, Gatter KC, Mortensen N, Harris AL. Interleukin-4 receptor and epidermal growth factor receptor expression in colorectal cancer. Br J Cancer. 1992;66:712–6. doi: 10.1038/bjc.1992.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahm H, Schnyder B, Wyniger J, et al. Growth inhibition of human colorectal-carcinoma cells by interleukin-4 and expression of functional interleukin-4 receptors. Int J Cancer. 1994;59:440–7. doi: 10.1002/ijc.2910590325. [DOI] [PubMed] [Google Scholar]
- 34.Al Tubuly AA, Spijker R, Pignatelli M, Kirkland SC, Ritter MA. Inhibition of growth and enhancement of differentiation of colorectal carcinoma cell lines by MAb MR6 and IL-4. Int J Cancer. 1997;71:605–11. doi: 10.1002/(sici)1097-0215(19970516)71:4<605::aid-ijc16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Verhaar MJ, Damen CA, Zonnenberg BA, Blijham GH. In vitro upregulation of carcinoembryonic antigen expression by combinations of cytokines. Cancer Letters. 1999;139:67–73. doi: 10.1016/s0304-3835(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 36.Kusano A, Ohta S, Shitara K, Hanai N. Immunocytochemical study on internalization of anti-carbohydrate monoclonal antibodies. Anticancer Res. 1993;13:2207–12. [PubMed] [Google Scholar]
- 37.Kyriakos RJ, Shih LB, Ong GL, Patel K, Goldenberg DM, Mattes MJ. The fate of antibodies bound to the surface of tumor cells in vitro. Cancer Res. 1992;52:835–42. [PubMed] [Google Scholar]
- 38.Garrigues J, Anderson J, Hellstrom KE, Hellstrom I. Anti-tumor antibody BR96 blocks cell migration and binds to a lysosomal membrane glycoprotein on cell surface microspikes and ruffled membranes. J Cell Biol. 1994;125:129–42. doi: 10.1083/jcb.125.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo DV, Li D, Mattis JA, Steplewski Z. Selective chromosomal damage and cytotoxicity of 125I-labeled monoclonal antibody 17-1a in human cancer cells. Cancer Res. 1989;49:2952–8. [PubMed] [Google Scholar]
- 40.Prete SP, Aquino A, Masci G, et al. Drug-induced changes of carcinoembryonic antigen expression in human cancer cells: effect of 5-fluorouracil. J Pharmacol Exp Ther. 1996;279:1574–81. [PubMed] [Google Scholar]
- 41.Maas IW, Boven E, Pinedo HM, Schluper HM, Haisma HJ. The effects of gamma-interferon combined with 5-fluorouracil or 5-fluoro-2′-deoxyuridine on proliferation and antigen expression in a panel of human colorectal cancer cell lines. Int J Cancer. 1991;48:749–56. doi: 10.1002/ijc.2910480520. [DOI] [PubMed] [Google Scholar]
- 42.Aquino A, Prete SP, Greiner JW, et al. Effect of the combined treatment with 5-fluorouracil, gamma-interferon or folinic acid on carcinoembryonic antigen expression in colon cancer cells. Clin Cancer Res. 1998;4:2473–81. [PubMed] [Google Scholar]
- 43.De Filippi R, Prete SP, Giuliani A, et al. Differential effects of recombinant interferon-alpha and 5-fluorouracil against colon cancer cells or against peripheral blood mononuclear cells. Anticancer Res. 1994;14:1767–73. [PubMed] [Google Scholar]
- 44.Kubota T, Kase S, Otani Y, Watanabe M, Teramoto T, Kitajima M. Interferons alpha-2a and beta increase the antitumor activity, detected by MTT assay, of 5-fluorouracil against experimental and clinical human gastrointestinal carcinomas. Anticancer Res. 1997;17:725–8. [PubMed] [Google Scholar]
- 45.Wolmark N, Bryant J, Smith R, et al. Adjuvant 5-fluorouracil and leucovorin with or without interferon alfa-2a in colon carcinoma: National Surgical Adjuvant Breast and Bowel Project protocol C-05. J Natl Cancer Inst. 1998;90:1810–6. doi: 10.1093/jnci/90.23.1810. [DOI] [PubMed] [Google Scholar]
- 46.Wadler S, Schwartz EL, Goldman M, et al. Fluorouracil and recombinant alfa-2a-interferon: an active regimen against advanced colorectal carcinoma. J Clin Oncol. 1989;7:1769–75. doi: 10.1200/JCO.1989.7.12.1769. [DOI] [PubMed] [Google Scholar]
- 47.Hill M, Norman A, Cunningham D, et al. Royal Marsden phase III trial of fluorouracil with or without interferon alfa-2b in advanced colorectal cancer. J Clin Oncol. 1995;13:1297–302. doi: 10.1200/JCO.1995.13.6.1297. [DOI] [PubMed] [Google Scholar]
- 48.Gamelin E, Bouil AL, Boisdron-Celle M, et al. Cumulative pharmacokinetic study of oxaliplatin, administered every three weeks, combined with 5-fluorouracil in colorectal cancer patients. Clin Cancer Res. 1997;3:891–9. [PubMed] [Google Scholar]
- 49.Bocci G, Danesi R, Di Paolo AD, et al. Comparative pharmacokinetic analysis of 5-fluorouracil and its major metabolite 5-fluoro-5,6-dihydrouracil after conventional and reduced test dose in cancer patients. Clin Cancer Res. 2000;6:3032–7. [PubMed] [Google Scholar]
- 50.Verweij J, Stuurman M, de Vries J, Pinedo HM. The difference in pharmacokinetics of mitomycin C, given either as a single agent or as a part of combination chemotherapy. J Cancer Res Clin Oncol. 1986;112:283–4. doi: 10.1007/BF00395925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fountzilas G, Gratzner H, Lim LO, Yunis AA. Comparative effects of selected drug combinations on the growth of a human pancreatic carcinoma cell line (MIA PaCa-2) J Natl Cancer Inst. 1986;76:37–43. [PubMed] [Google Scholar]
- 52.Matsuno S, Hisano H, Kobari M, Akaishi S. Growth-inhibitory effect of combination chemotherapy for human pancreatic cancer cell lines. Cancer. 1990;66:2369–74. doi: 10.1002/1097-0142(19901201)66:11<2369::aid-cncr2820661120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 53.Faivre S, Raymond E, Woynarowski JM, Cvitkovic E. Supraadditive effect of 2′,2′-difluorodeoxycytidine (gemcitabine) in combination with oxaliplatin in human cancer cell lines. Cancer Chemother Pharmacol. 1999;44:117–23. doi: 10.1007/s002800050955. [DOI] [PubMed] [Google Scholar]
- 54.Flieger D, Kleinschmidt R, Schild HH, Fischer HP, Sauerbruch T, Schmidt-Wolf I. Immunoembolization of the liver with IFN-a/IL-2 and intravenous treatment with monoclonal antibody 17-1A in patients suffering from colorectal liver metastasis. Proc Am Soc Clin Oncol. 2000;19(Suppl.):476a. [Google Scholar]