Abstract
Annexin 1 (ANX-1) can reduce leucocyte migration in response to cytokines and chemokines in some rodent models of inflammation. However, its effectiveness against an inflammatory stimulus as strong as bacterial lipopolysaccharide (LPS) is unknown. Thus, we have examined whether ANX-1 can modulate LPS-induced neutrophil accumulation in the rat, as assessed by intravital microscopy and by myeloperoxidase (MPO) assay. The anti-inflammatory glucocorticoid, dexamethasone (DEX) was also studied for comparison. LPS superfusion induced adhesion of leucocytes to the endothelium and a subsequent increase in emigration from rat post-capillary venules over 2 h as assessed by intravital microscopy. Either ANX-1 or DEX was able to attenuate this adhesion and emigration of leucocytes. MPO activity in the lung, kidney and ileum was elevated after a 6-h exposure to LPS (intraperitoneal), indicating accumulation of neutrophils in these tissues. DEX attenuated the LPS-induced increase in MPO in the ileum but had no effect on MPO in the lungs or kidneys. This would suggest that the underlying mechanism by which neutrophils accumulate in the ileum, and more generally in the gastrointestinal compartment, is different from other vascular beds. ANX-1 had no effect on the LPS-induced increase in MPO activity in any of the tissues studied. Thus, from these data, ANX-1 appears to reduce leucocyte adhesion and emigration induced by a short-term (2 h), but not a longer (6 h) exposure to LPS.
Keywords: endotoxin, intravital microscopy, lipocortin 1
Introduction
In response to an inflammatory stimulus, leucocytes may infiltrate tissues as part of the host immune response. This process occurs in several stages. First, upon application of an inflammatory stimulus neutrophils roll on the endothelium of post-capillary venules, a process mediated by the selectins, CD62P, CD62E, CD62L [1]. The leucocytes may then become activated and adhere firmly via β2-integrins [2]. There are many compounds that can activate leucocytes, including chemoattractants, such as complement factor C5a [3], and chemokines, such as IL-8 [4]. Following adhesion, leucocytes undergo cytoskeletal reorganization before emigrating through junctions between endothelial cells into the extravascular space, a process called diapedesis.
Bacterial lipopolysaccharide (LPS), a potent stimulator of inflammation, can produce endotoxaemia and, in extremis, multiple organ failure. LPS releases an array of proinflammatory mediators, such as complement factor C5a [5], cytokines like tumour necrosis factor-alpha (TNF-α) [6], and chemokines like IL-8 [6] and CINC [7,8]. Furthermore, LPS can cause changes in adhesion molecules, such as shedding of l-selectin and expression of CD11/CD18 integrin [9,10]. These factors may combine to bring about neutrophil accumulation and infiltration into tissues that, in turn, may lead to tissue injury through the production of superoxide radicals.
Since neutrophils contribute to the tissue injury observed in endotoxaemia, it may be beneficial to prevent their accumulation. Glucocorticoids have long been used as anti-inflammatory agents and they have been shown to reduce leucocyte accumulation in many models [11,12], including LPS-induced leucocyte trafficking across rat mesenteric post-capillary venules [7]. However, glucocorticoids possess a wide range of side-effects. Thus, of increasing interest is the research into anti-inflammatory compounds that prevent neutrophil infiltration yet possessing less adverse effects than glucocorticoids.
Annexin 1 (ANX-1), also known as lipocortin 1, is an endogenous protein that is widely distributed among cells involved in the inflammatory response [13]. Since anti-ANX-1 antibodies can block some of the anti-inflammatory effects of dexamethasone [14] it has been suggested that ANX-1 mediates some, though by no means all [15], of the anti-inflammatory actions of glucocorticoids. Indeed, exogenous ANX-1 reduces neutrophil infiltration in several animal models and against various inflammatory stimuli including IL-1β and zymosan (for review see [16]). However, the effect of exogenous ANX-1 has yet to be investigated in a rat vascular bed or in a model of inflammation as severe as that produced by LPS.
Thus, we have investigated the anti-inflammatory effects of dexamethasone (DEX) and ANX-1 by their ability to modulate (i) LPS-induced leucocyte adhesion and infiltration, assessed by intravital microscopy of rodent mesenteric post-capillary venules, and (ii) neutrophil accumulation in various tissues, assessed by the myeloperoxidase (MPO) assay, following a longer exposure to LPS.
Materials and methods
Intravital microscopy
The rat mesenteric preparation was performed as described previously [12,17]. Male Sprague–Dawley rats (170–220 g supplied by Tuck, UK) were fasted 24 h prior to use. Animals were anaesthetized (Inactin; 120 mg/kg, i.p.) and the trachea cannulated to facilitate spontaneous breathing. The jugular vein was also cannulated for administration of drugs. The abdominal area was shaved, a midline laparotomy performed and the mesenteric vascular bed exteriorized and placed on a Plexiglas viewing stage. This was mounted on a light microscope (Zeiss Axioskop FS, eyepiece magnification × 10) with a water immersion objective lens (× 40 magnification) and then transluminated with a 100-W halogen light source. Images were acquired by a colour camera (Hitachi CCD, model KPC571; Tokyo, Japan), displayed on a colour video monitor (Sony Trinitron, model PVM 1440QM) and then recorded on a VHS video recorder (Sony, model SVO-9500 MDP) for subsequent off-line analysis. A video time-date generator (FOR.A video timer, model VTG-33; Tokyo, Japan) projected the time, date and stopwatch function on to the monitor. Erythrocyte velocity was measured using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A & M University, College Station, TX). The mesentery was superfused with warmed (37°C), gassed (5% CO2/95% N2) bicarbonate-buffered solution (g/l: NaCl 7·71; KCl 0·35; MgSO4·7H2O 0·145; NaHCO3 1·51; CaCl2 0·22; pH 7·4) at a rate of 2 ml/min. Rat body temperature was maintained at 37°C via the use of a heating lamp.
Protocol for intravital microscopy
A ‘real time’ protocol was used. Briefly, after a suitable mesenteric venule (25–40 μm diameter and less than eight adherent cells) was found the preparation was superfused with buffer and allowed to stabilize for 20 min before a basal recording of 10 min was made. The mesentery was then superfused (2 ml/min) with LPS (serotype 0127:B8, 1 μg/ml) in the bicarbonate buffer or without LPS in control animals. To investigate the effects of ANX-1 on leucocyte adhesion and emigration induced by LPS, animals were treated with ANX-1 (100 μg/kg, i.v.) or vehicle (PBS) 20 min after the LPS superfusion was started. Alternatively, to investigate the effect of DEX on adhesion and emigration induced by LPS, DEX (1 mg/kg s.c.) was given 1 h prior to the LPS superfusion. DEX was given as a pre-treatment in order that its genomic effects would have time to work, while ANX-1 was given therapeutically, i.e. after the inflammatory stimulus, as it produces its effects rapidly [18]. The doses chosen have been validated in previous studies [12,18]. The mesenteric post-capillary venule was then recorded onto video cassette for 2 h and the 10-min periods at 10, 30, 60 and 120 min after the LPS were analysed. The following parameters were monitored: (i) rolling velocity (Vwbc), calculated from 20 randomly selected rolling leucocytes; (ii) cell adhesion (the number of cells that adhere to the endothelium for > 30 s; (iii) cell emigration (the number of cells outside the vessel but within the field of view (50 μm); and (iv) blood velocity. From the mean blood velocity (Vmean = centreline blood velocity/1·6) and the vessel diameter the wall shear could be calculated assuming a cylindrical geometry. Wall shear rate was calculated by the Newtonian definition: SR = 8000 × (Vmean/diameter) [19].
MPO study
In initial experiments, male Sprague–Dawley rats (170–220 g) received LPS (1 mg/kg i.p., serotype 0127:B8), or sterile saline (i.p.) for controls, and 6 h or 18 h later the animals were exsanguinated and 50 ml of sterile saline perfused through the animal via the left ventricle to flush blood from the organs. Tissues (kidney, ileum, lung and liver) were then harvested, snap-frozen in liquid nitrogen and kept at −80°C until ready for the MPO assay.
In subsequent experiments, DEX (0·1–1 mg/kg, s.c.) or sterile PBS was given 1 h prior to i.p. administration of LPS (1 mg/kg) or sterile saline. Alternatively, rats received ANX-1 (100 μg/kg, s.c.) concomitantly with the LPS. Tissues were collected 6 h later as described earlier.
For the MPO assay 50 mg of tissue were homogenized on ice in 1 ml of a 0·5% hexa-decyl-trimethyl ammonium bromide (HTAB) in MOPS (10 mm, pH 7) buffer. After homogenization, samples were centrifuged (4000 g) for 20 min and then the supernatant was removed and aliquoted for the assay. The MPO assay was performed as previously described [20,21]. Briefly, the sample supernatant was allowed to react with a solution of tetra-methyl benzidine (TMB; 1·6 mm) and 0·1 mm H2O2. The change in absorbance was measured with a spectrophotometer at 650 nm and compared with a standard curve of MPO (0·03125–1 U/ml).
Statistical analysis
Data points were compared with an unpaired t-test or a Mann–Witney U-test as appropriate.
Materials
LPS (serotype 0127:B8), NaCl, KCl, MOPS, HTAB, TMB, H2O2 and MPO for the standard curve were all obtained from Sigma Chemical Co. (Poole, UK). NaHCO3 and CaCl2 were obtained from BDH (Lutterworth, UK). MgSO4·7H2O was obtained from FSA Lab Supplies (Loughborough, UK). Dexamethasone was obtained from David Bull Labs (distributed by Central Laboratories Ltd, Dublin, Eire). Inactin was obtained from Research Biochemicals Int. (Natick, MA). Highly purified human recombinant ANX-1 was supplied by Dr E. Solito (INSERM U332, Institute Cochin de Génétique Moleculaire, Paris, France) and endotoxin contamination was < 20 pg/ml as measured by the Limulus amoebocyte chromogenic assay [18]. ANX-1 was kept at a stock solution of 0·4 mg/ml (in 20 mm 2-N-morpholinoethanesulphonic acid, pH 6). Subsequent dilutions were in PBS pH 7·4 and were made on the day of the experiment.
Results
Intravital microscopy
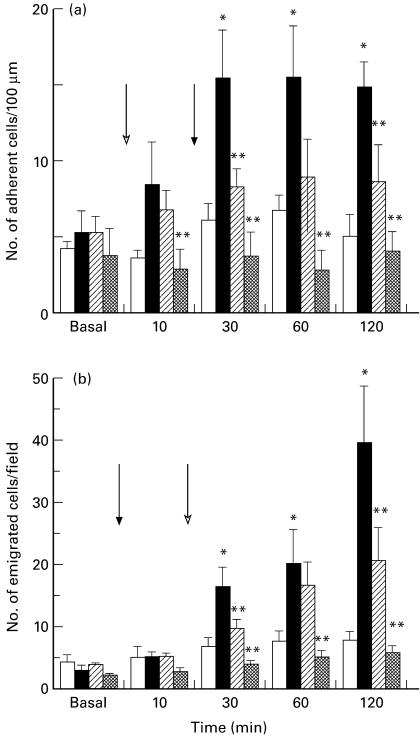
Intravital microscopy of the rat mesentery was utilized initially. LPS (1 μg/ml superfusion) induced adhesion of leucocytes to the endothelium that had reached a maximum within 30 min (Fig. 1a). This increase in adhesion was followed by a subsequent increase in emigration that was still increasing by 120 min (Fig. 1b). LPS superfusion had no significant effect on leucocyte rolling velocity (Fig. 2a), blood velocity (Fig. 2b) or shear rate (Table 1) in mesenteric post-capillary venules. ANX-1 (100 μg/kg, i.v.) was able to attenuate the LPS-induced adhesion and emigration of leucocytes (Fig. 1a,b) without altering rolling velocity (Fig. 2a), blood velocity (Fig. 2b) or shear rate (Table 1). DEX (1 mg/kg s.c.) given 1 h prior to an LPS superfusion blocked the increase in leucocyte adhesion and emigration. Leucocyte rolling velocity was significantly greater in DEX-treated animals compared with control (at 60 min) or LPS-treated (at 30 and 60 min) animals. DEX pre-treatment had no significant effect on blood flow or shear rate throughout the experiment.
Fig. 1.
Effect of annexin 1 (ANX-1; 100 μg/kg, i.v., hatched columns) or dexamethasone (DEX; 1 mg/kg s.c., cross-hatched columns) on the changes in leucocyte adhesion (a) and emigration (b) induced by a lipopolysaccharide (LPS) superfusion (1 μg/ml, ▪) in mesenteric post-capillary venules as assessed by intravital microscopy. □, Time-matched controls superfused with normal bicarbonate buffer. The open arrow indicates the onset of the LPS superfusion while the closed arrow indicates the time of the ANX-1 injection. Data are the mean and s.e.m. mean of n = 5–6 rats. *P < 0·05 versus controls; **P < 0·05 versus LPS-treated rats.
Fig. 2.
Effect of annexin 1 (ANX-1; 100 μg/kg, i.v., hatched columns) or dexamethasone (DEX; 1 mg/kg s.c., cross-hatched columns) on the changes in leucocyte rolling velocity (a) and blood flow (b) induced by a lipopolysaccharide (LPS) superfusion (1 μg/ml, ▪) in mesenteric post-capillary venules as assessed by intravital microscopy. Time-matched controls (□) were superfused with normal bicarbonate buffer. The open arrow indicates the onset of the LPS superfusion, while the closed arrow indicates the time of the ANX-1 injection. Data are the mean and s.e.m. mean of n = 5–6 rats. *P < 0·05 versus controls; **P < 0·05 versus LPS-treated rats.
Table 1.
Effect of dexamethasone (DEX; 1 mg/kg, s.c.), and annexin 1 (ANX-1; 100 μg/kg, i.v.) on changes in shear stress (s−1) induced by a lipopolysaccharide (LPS) superfusion (1 μg/ml) on rat mesenteric post-capillary venules
| 0 min | 30 min | 60 min | 120 min | |
|---|---|---|---|---|
| Control | 451·5 ± 54·5 | 427·7 ± 57·4 | 416·3 ± 51·0 | 403·8 ± 55·5 |
| LPS (1 μg/ml) | 435·8 ± 48·3 | 450·8 ± 61·3 | 436·7 ± 47·7 | 367·1 ± 62·9 |
| LPS + ANX-1 | 343·4 ± 34·2 | 347·4 ± 45·5 | 354·2 ± 47·2 | 328·3 ± 37·5 |
| LPS + DEX | 384·5 ± 35·5 | 418·9 ± 56·0 | 370·1 ± 52·8 | 241·8 ± 59·6 |
Data are the mean ± s.e.m. mean of n = 5–6 experiments.
Systemic endotoxaemia
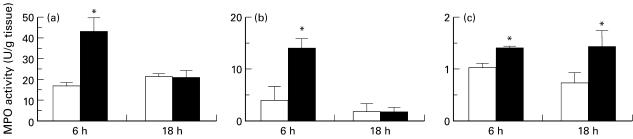
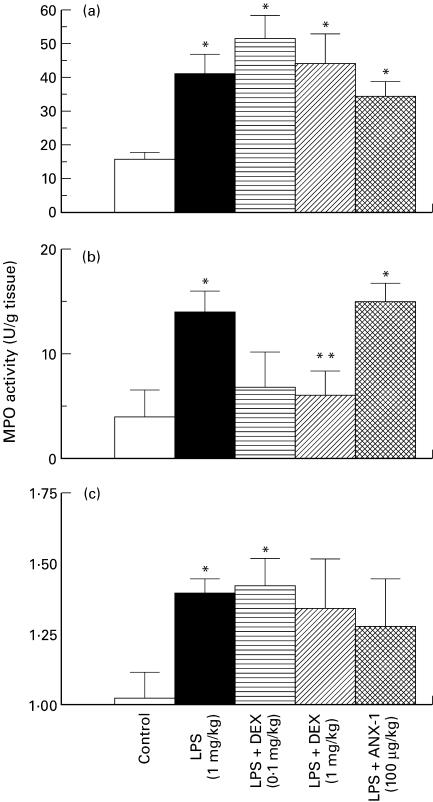
When rats were treated with LPS (1 mg/kg, i.p.) for 6 h, the MPO activity in the lung, kidney and ileum (Fig. 3), but not the liver (control 8·29 ± 2·36 versus LPS 9·63 ± 2·73 U/mg tissue, n = 5) was elevated over levels found in control rats. Since the increase in MPO activity induced by LPS had waned by 18 h in all tissues studied except the kidney (Fig. 3), the 6 h time point was used to investigate the effects of DEX or ANX-1. ANX-1 (100 μg/kg s.c.) had no effect on the increased MPO activity in any of the tissues induced by LPS for 6 h (Fig. 4). DEX (1 mg/kg, s.c.) pre-treatment attenuated the increase in MPO in the ileum at 6 h but had no affect on MPO in the lungs or kidneys (Fig. 4). The lower dose of DEX administered (0·1 mg/kg s.c.) did not significantly affect the LPS-induced increase in MPO activity in any of the tissues collected.
Fig. 3.
Effect of lipopolysaccharide (LPS; 1 mg/kg i.p., ▪) or sterile saline (control, □) on myeloperoxidase (MPO) activity in the lung (a), ileum (b) and kidney (c) at 6 h and 18 h post-injection. Data are the mean and s.e.m. mean of n = 5–12 samples. *P < 0·05 compared with controls.
Fig. 4.
Effect of dexamethasone (DEX; 0·1 mg/kg, s.c., horizontally hatched; 1 mg/kg, s.c., diagonally hatched columns), or annexin 1 (ANX-1; 100 μg/kg, s.c., cross-hatched columns) on the lipopolysaccharide (LPS; 1 mg/kg i.p., ▪)-induced increase in myeloperoxidase (MPO) activity in the lung (a), ileum (b) and kidney (c) at 6 h. □, Control injection of sterile saline. Data are the mean and s.e.m. mean of n = 6–12 samples. *P < 0·05 compared with controls; **P < 0·05 compared with LPS-treated rats.
Discussion
Bacterial LPS is a potent stimulus of neutrophil adhesion and infiltration. LPS induced an increase in leucocyte adhesion and emigration in our rat intravital microscopy model, in keeping with previous studies [7,22]. When injected intraperitoneally, LPS increases in the circulation, until it peaks after 30 min [23]. This then increases the systemic levels of proinflammatory cytokines, such as IL-1 and TNF-α [6,24] and chemokines such as CINC [7,8], as well as activating the complement cascade [5]. Furthermore, LPS can affect the expression of adhesion molecules, with L- and P-selectin [22,25] and the integrins CD11/CD18 [9] all being important in LPS-induced leucocyte adhesion to post-capillary venules.
Leucocyte migration in many models of inflammation, including those caused by IL-1 [26] and zymosan [27], can be reduced by ANX-1. Indeed, it is not just exogenous ANX-1 that reduces neutrophil accumulation, as experiments with anti-ANX-1 antibodies show that endogenous ANX-1 contributes to the anti-migratory effects of glucocorticoids [14]. In static conditions, endogenous ANX-1 does not appear to modulate adhesion of leucocytes to endothelial cell monolayers [28]. However, in murine post-capillary venules in vivo, exogenous ANX-1 can reduce adhesion to a significant extent [18], an observation that is in agreement with the findings presented here. Reduced leucocyte adhesion does not appear to be the main mechanism by which ANX-1 attenuates leucocyte trafficking. Instead, ANX-1 seems to promote leucocyte detachment and to prolong the process of diapedesis [14,18]. The exact mechanisms by which ANX-1 achieves this are unknown, although it does first require that endogenous leucocytic ANX-1 be externalized [28].
Although ANX-1 was able to inhibit emigration of leucocytes caused by LPS over 2 h in mesenteric post-capillary venules, no effect was observed on neutrophil accumulation in the ileum after 6 h. Why there is a difference between 2 h and 6 h is unknown. It may be that the difference observed is methodological in origin, since the route of administration differed and subcutaneous ANX-1 may not be absorbed and distributed into the circulation. This route of administration was chosen for the MPO work since it was a more convenient method of dosing conscious animals and it has been shown to be effective for ANX-1-derived peptides [27]. An alternative explanation is that ANX-1 may have been degraded over the 6-h period of the experiment so that it was no longer present at a pharmacologically effective concentration in the plasma. A more likely suggestion may be that there are differences in the regional blood flow at 6 h. For instance, following LPS, the blood pressure falls and nitric oxide synthase is induced [29], an occurrence that typically takes place within 4–6 h. Peripheral vasoconstriction and a fall in blood pressure are hallmarks of LPS-induced shock and may lead to poor organ perfusion. Thus, at 6 h, blood flow and shear rates in the tissues may fall so that leucocytes become adherent regardless of ANX-1 treatment.
For comparison against ANX-1, DEX was also utilized as an anti-inflammatory agent in our experiments. DEX greatly reduced leucocyte adhesion and emigration induced by LPS in mesenteric post-capillary venules. These findings were somewhat unsurprising, since DEX has been previously shown to reduce leucocyte rolling and adhesion to IL-1β [12] and LPS [7] and glucocorticoids can down-regulate some cytokines and chemokines (for review see [30]). DEX was also able to raise significantly leucocyte rolling velocity compared with both LPS and control treatments. This is probably associated with an interference with the selectins, since both L- and P-selectin mediate leucocyte rolling induced by LPS [22]. Furthermore, DEX has been shown to alter LPS-induced changes in L-selectin expression in the rat [7], and has been shown to attenuate the increase in P- and L-selectin expression induced by LPS in the mouse [31]. That DEX can increase leucocyte rolling velocity compared with control rats may indicate that there is a small amount of activation in our intravital model that seems to affect leucocyte rolling velocity, but not adhesion or emigration. Indeed, previous studies have shown that activation and increased leucocyte rolling is a consequence of disturbing the microcirculation [32]. We found that DEX was also able to reduce MPO levels in the ileum of LPS-treated rats, which is in keeping with our data from intravital microscopy experiments in the mesentery. Furthermore, this may add credence to the theory that blood flow changes are responsible for the lack of effect of ANX-1 at 6 h. For instance, DEX can produce genomic effects, such as reducing nitric oxide production by decreasing nitric oxide synthase (NOS) induction [33,34], that may result in improved organ perfusion at these later times.
DEX was unable to affect the increase in neutrophil accumulation induced by LPS in the lung and kidney at 6 h. Similar findings where DEX was ineffective at preventing an increase in MPO in the lungs of mice induced by i.p. LPS have been reported [35]. Histological sections of rat [23] or mouse [35] lung following i.p. treatment with LPS show an increase in neutrophils in the pulmonary vessels but that these neutrophils had not extravasated. Thus, neutrophil accumulation in the lung does not seem to be due to emigration and may be due to trapping in small vessels. This indicates a clear difference in how neutrophils accumulate in lungs and the ileum/mesentery in response to i.p. LPS, and may explain why DEX is not effective attenuating neutrophil gathering in the lung. It may be that a similar mechanism is in operation to cause renal neutrophil accumulation. An additional factor which may account for the tissue differences in neutrophil accumulation is that, whilst LPS increases in the circulation following i.p. injection, it may still be present locally in the ileum, thus triggering the release of local chemotactic agents.
In conclusion, here we show for the first time that ANX-1 itself reduces neutrophil trafficking induced by LPS. Furthermore, this is the first study that reports the ability of ANX-1 to interfere directly with neutrophil extravasation in the rat, since previous studies had been performed with ANX-1-derived N-terminal peptides [21]. Our observations of rat mesenteric post-capillary venules point to a cellular mechanism for the anti-inflammatory action exerted by ANX-1 in several rat models including carrageenin paw oedema [36] and zymosan pleurisy [37]. ANX-1 however, was ineffective at preventing neutrophil accumulation in tissues after a more prolonged exposure to LPS. Finally, our data also suggest that DEX may modulate neutrophil accumulation by distinct mechanisms in different tissues.
Acknowledgments
This work was funded by a grant from the British Heart Foundation (PG/97131). R.J.F. is a Principal Research Fellow of the Wellcome Trust and M.P. is supported by the Arthritis Research Campaign.
REFERENCES
- 1.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 2.Newton RA, Thiel M, Hogg N. Signaling mechanisms and the activation of the leukocyte integrins. J Leuk Biol. 1997;61:422–6. doi: 10.1002/jlb.61.4.422. [DOI] [PubMed] [Google Scholar]
- 3.Collins PD, Jose PJ, Williams TJ. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil activating protein 1. J Immunol. 1991;146:677–84. [PubMed] [Google Scholar]
- 4.Yoshimura T, Matsushima K, Oppenheim JJ, et al. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–93. [PubMed] [Google Scholar]
- 5.Smedegard G, Cui L, Hugli TE. Endotoxin-induced shock in rat: a role for C5a. Am J Pathol. 1989;135:489–97. [PMC free article] [PubMed] [Google Scholar]
- 6.Martich GD, Danner RL, Ceska M, et al. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antinflammatory agents. J Exp Med. 1991;173:1021–4. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenpeck KL, Zagorski J, Schleimer RP, et al. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J Immunol. 1998;161:6861–70. [PubMed] [Google Scholar]
- 8.Ohira H, Ueno T, Torimura T, et al. Leukocyte adhesion molecules in the liver and plasma cytokine levels in endotoxin-induced rat liver injury. Scand J Gastroenterol. 1995;30:1027–35. doi: 10.3109/00365529509096349. [DOI] [PubMed] [Google Scholar]
- 9.Harris NR, Russell JM, Granger DN. Mediators of endotoxin-induced leukocyte adhesion in mesenteric postcapillary venules. Circ Shock. 1994;43:155–60. [PubMed] [Google Scholar]
- 10.Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J Leuk Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Mori Y, Tsurufuji S. The characteristic feature of glucocorticoids after local application with reference to leukocyte migration and protein exudation. Eur J Pharmacol. 1969;7:201–5. doi: 10.1016/0014-2999(69)90011-9. [DOI] [PubMed] [Google Scholar]
- 12.Tailor A, Flower RJ, Perretti M. Dexamethasone inhibits leukocyte emigration in rat mesenteric post-capillary venules: an intravital microscopy study. J Leuk Biol. 1997;62:301–8. doi: 10.1002/jlb.62.3.301. [DOI] [PubMed] [Google Scholar]
- 13.Flower RJ. Lipocortin and the mechanism of action of glucocorticoids. Br J Pharmacol. 1988;94:987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso F, Flower RJ, Perretti M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule: involvement of endogenous lipocortin 1. J Immunol. 1995;155:377–86. [PubMed] [Google Scholar]
- 15.Goulding NJ, Ogbourn S, Pipitone N, et al. The inhibitory effect of dexamethasone on lymphocyte adhesion molecule expression and intercellular aggregation is not mediated by lipocortin 1. Clin Exp Immunol. 1999;118:376–83. doi: 10.1046/j.1365-2249.1999.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perretti M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen Pharmacol. 1998;31:545–52. doi: 10.1016/s0306-3623(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 17.Kubes P, Suzuki M, Granger DN. Modulation of PAF-induced leukocyte adherence and increased microvascular permeability. Am J Physiol. 1990;259:G859–64. doi: 10.1152/ajpgi.1990.259.5.G859. [DOI] [PubMed] [Google Scholar]
- 18.Lim LHK, Solito E, Russo-Marie F, et al. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci USA. 1998;95:14535–9. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bienvenu K, Granger DN. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am J Physiol. 1993;264:H1504–8. doi: 10.1152/ajpheart.1993.264.5.H1504. [DOI] [PubMed] [Google Scholar]
- 20.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Cuzzocrea S, Tailor A, Zingarelli B, et al. Lipocortin 1 protects against splanchnic artery occlusion and reperfusion injury by affecting neutrophil migration. J Immunol. 1997;159:5089–97. [PubMed] [Google Scholar]
- 22.Davenpeck KL, Steeber DA, Tedder TF, et al. P- and L-selectin mediate distinct but overlapping functions in endotoxin-induced leukocyte–endothelial interactions in the rat mesenteric circulation. J Immunol. 1997;159:1977–86. [PubMed] [Google Scholar]
- 23.Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996;270:L836–45. doi: 10.1152/ajplung.1996.270.5.L836. [DOI] [PubMed] [Google Scholar]
- 24.Cybulsky MI, McComb DJ, Movat HZ. Neutrophil leukocyte emigration induced by endotoxin: mediator roles of interleukin 1 and tumor necrosis factor α. J Immunol. 1988;140:3144–9. [PubMed] [Google Scholar]
- 25.Johnston B, Walter UM, Issekutz AC, et al. Differential roles of selectins and the α4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J Immunol. 1997;159:4514–23. [PubMed] [Google Scholar]
- 26.Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–9. [PubMed] [Google Scholar]
- 27.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–82. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perretti M, Croxtall JD, Wheller SK, et al. Mobilizing lipocortin 1 in adherent human leukocytes down-regulates their transmigration. Nature Med. 1996;2:1259–62. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 29.Wu C-C, Croxtall JD, Perretti M, et al. Lipocortin 1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc Natl Acad Sci USA. 1995;92:3473–7. doi: 10.1073/pnas.92.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–41. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 31.Mori N, Horie Y, Gerritsen ME, et al. Anti-inflammatory drugs and endothelial cell adhesion molecule expression in murine vascular beds. Gut. 1999;44:186–95. doi: 10.1136/gut.44.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaki K, Lindbom L, Thorlacius H, et al. An approach for studies of mediator-induced leukocyte rolling in the undisturbed microcirculation of the rat mesentery. Br J Pharmacol. 1998;123:381–9. doi: 10.1038/sj.bjp.0701617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees DD, Cellek S, Palmer RM, et al. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990;173:541–7. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 34.Wright CE, Rees DD, Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992;26:48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- 35.Lefort J, Singer M, Leduc D, et al. Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-α formation and neutrophil sequestration into the murine lungs. J Immunol. 1998;161:474–80. [PubMed] [Google Scholar]
- 36.Cirino G, Peers SH, Flower RJ, et al. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA. 1989;86:3428–32. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becherucci C, Perretti M, Solito E, et al. Conceivable difference in the anti-inflammatory mechanisms of lipocortins 1 and 5. Med Inflammation. 1993;2:109–13. doi: 10.1155/S0962935193000158. [DOI] [PMC free article] [PubMed] [Google Scholar]