Abstract
Hypoxia is an important factor in the pathophysiology of vascular and inflammatory diseases. Leucocyte infiltration, as a consequence of adhesion molecule up-regulation and chemokine release, is a prominent feature of these diseases. The objective of our study was to investigate the potential role of resident fibroblasts in hypoxia-induced chemotactic responses. We show that MCP-1 and IL-8 mRNA are specifically induced by hypoxia in dermal fibroblasts. This response is paralleled by increased NF-κB p65/p50 binding activity, and it is inhibited by pretreatment with N-acetyl-L-cysteine. MCP-1 secreted by fibroblasts is chemotactic for monocytic cells and this activity is significantly increased by hypoxia. Chemotactic index correlates with MCP-1 protein levels and is significantly decreased by neutralizing anti-MCP-1 MoAb. These findings demonstrate the ability of resident fibroblasts to mediate chemotaxis of leucocytes through the release of chemokines in response to hypoxia. Our data point to MCP-1 as an important component in this response, and therefore it may be a potential target in inflammatory responses associated with hypoxia.
Keywords: fibroblasts, free radicals, chemokines, inflammation, chemotaxis
Introduction
Fibroblasts, a ubiquitous cell population, the most abundant cellular component of connective tissues, have traditionally been primarily considered for their important role in the synthesis of extracellular matrix (ECM) components necessary for tissue integrity and repair. Recent evidence suggests that fibroblasts may also have immunological functions that provide a first line of defence of connective tissue by initiating leucocyte recruitment and inflammatory responses (reviewed by Smith [1]). Upon stimulation with substances derived from infectious microorganisms or with early inflammatory mediators, such as tumour necrosis factor-alpha (TNF-α) or IL-1, fibroblasts are activated to produce cytokines and soluble growth factors that can drive inflammatory and reparative responses [2,3]. Moreover, fibroblasts are targets of autocrine or paracrine inflammatory mediators and may therefore play a pivotal role in the amplification and perpetuation of inflammatory processes.
Chemokines are a large family of small cytokines that induce migration of leucocytes to sites of inflammation [4]. In addition to their chemotactic effects, chemokines may induce cellular responses such as angiogenesis [5,6] or ECM synthesis [7,8] which are important factors in the pathogenesis of inflammatory and reparative processes. Chronic inflammatory disorders are also characterized by increased chemokine production and there is supporting evidence for the participation of chemokines in the pathogenesis of chronic inflammation [9–11]. The potential of fibroblasts to synthesize chemokines in response to a variety of cytokines and bacterial products has been demonstrated [2,3]. Nuclear factor-κB (NF-κB)/Rel factors are the best identified elements involved in the transcriptional activation of chemokine genes in most cell types [2,12,13]. However, important differences in the response to specific NF-κB/Rel members between fibroblasts and leucocytes have been demonstrated [14]. Fibroblasts are the cell type responsible for uncontrolled chemokine production and multiorgan leucocyte infiltration in RelB knockout mice [15].
Hypoxia and the generation of reactive oxygen intermediates (ROI) are important components in the pathogenesis of the inflammatory and scarring responses that occur during wound healing, and in vascular or inflammatory diseases [16–18]. Oxidative stress is a potent NF-κB activator and this intracellular pathway is an important mediator of the proinflammatory effects of ROI [19]. A heterogeneous group of chronic inflammatory autoimmune disorders such as systemic lupus erythematosus (SLE), dermatomyositis and systemic sclerosis (SSc) is characterized by early vascular alterations leading to chronic hypoxia, repeated cycles of hypoxia/reoxygenation (Raynaud's phenomenon), and ROI generation. These changes are followed by chronic inflammation of the skin and different organs [20]. Severe vascular changes and fibrosis are particularly prominent in SSc, although possible linkage between vascular lesions, inflammatory cell infiltration and fibrosis is unclear [21]. We here examine whether dermal fibroblasts can play a role in mediating leucocyte infiltration in conditions characterized by hypoxia. We describe the specific pattern of chemokines synthesized by normal and hypoxic fibroblasts and the participation of NF-κB activation in response to hypoxia. Our results underline the relevance of fibroblasts as a source of chemokines with chemotactic activity for monocytic cells and demonstrate that this activity increases in response to hypoxia.
Materials and methods
Fibroblast cultures
Human normal skin samples were obtained from healthy controls. All samples were taken from normal margins of benign cutaneous lesions treated with minor cosmetic surgery. Fibroblasts were obtained by outgrowth from minced skin explants in Dulbecco's modified Eagle's medium (DMEM) (BioWhittaker, Walkersville, MD) supplemented with antibiotics and 10% fetal calf serum (FCS; Gibco BRL, Karlsruhe, Germany). Fibroblasts were used between passages 2 and 9. As positive controls for the expression of chemokine mRNA in fibroblasts, confluent monolayers were exposed for 6 h to 10, 25 or 50 U/ml of human recombinant TNF-α (Genzyme, Cambridge, MA).
Confluent fibroblasts were maintained in DMEM with 0·1% FCS for at least 24 h before exposure to hypoxia (3% O2) for 6 h in an anaerobic cell incubator under 5% CO2/92% N2 atmosphere. Following hypoxia, cells were reoxygenated for 1 h or 2 h under normoxic (21% O2) atmosphere. To study the role of ROI-induced NF-κB activation in chemokine gene expression, cells were pretreated with 25 mm N-acetyl-L-cysteine (NAC; Sigma, St Louis, MO), for 1 h prior to exposure to hypoxia and reoxygenation. To study whether chemokine gene expression was independent of protein synthesis, cells were pretreated with 10 μg/ml cycloheximide (CHX; Sigma).
RNase protection assay
A multiprobe DNA template including a set of different chemokines was used for in vitro transcription of α32P-UTP (Amersham Pharmacia Biotech, Aylesbury, UK)-labelled riboprobes with T7 polymerase (RiboQuant; PharMingen, San Diego, CA). Riboprobes for chemokines lymphotactin (Ltn), regulated upon activation normal T cell-expressed and secreted (RANTES), interferon-gamma (IFN-γ)-inducible protein (IP-10), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, monocyte chemoattractant protein‐1 (MCP-1), IL-8, I-309, and for the housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal controls, were included.
Total RNA (15 μg) obtained from fibroblasts was hybridized overnight with α32P-UTP-labelled riboprobes and eventually digested with RNase A + T1. Protected riboprobes were run on PAGE, and autoradiographed.
MCP-1 protein determination
To quantify secreted MCP-1 protein in fibroblast culture supernatants, we used a specific solid-phase ELISA following the manufacturer's protocol (Quantikine Human MCP-1 Immunoassay, cat. no. DCP00; R&D Systems, Minneapolis, MN).
Chemotaxis assay
We performed a chemotaxis assay with the monocytic cell line, MonoMac1 (DSM ACC252; German Micro-organism and Cell Culture Collection, Braunschweig, Germany), that has been previously shown to migrate in response to MCP-1 via CCR2 receptor activation [22]. Conditioned media from normoxic or hypoxic fibroblasts were diluted 1:1 with 0·1% FCS DMEM and placed in the bottom chamber of 6·5-mm Transwell tissue culture inserts with polyester membranes of 5 μm pore diameter (Corning-Costar Corp., Cambridge, MA). MonoMac1 monocytic cells were diluted at 5 × 106/ml in RPMI (BioWhittaker) media and 100-μl aliquots were added to the top chamber. Plates were incubated for 2 h at 37°C to allow transmigration. Cells that had transmigrated to the lower chamber were counted by flow cytometry. As a negative control, 0·1% FCS DMEM was used. Migration index was calculated as the x-fold increase in migration observed over the negative control. Chemotaxis inhibition studies were performed by preincubating fibroblast supernatants with 1 μg/ml neutralizing anti-MCP-1 MoAb (R&D Systems) or mouse isotype-matched IgG1 control (Sigma).
NF-κB electromobility shift assay
Activation of NF-κB on fibroblasts exposed to hypoxia and reoxygenation was studied by analysing the DNA-binding activity of fibroblast nuclear protein extracts in an electromobility shift assay (EMSA) as previously described [23]. Briefly, fibroblasts were collected by centrifugation and lysed in 1 ml of lysis buffer (10 mm HEPES pH 8, 50 mm NaCl, 0·5 m sucrose, 1 mm EDTA, 0·5 mm espermidine, 0·15 mm espermine, 0·5% Triton X-100, 1 mm phenylmethylsulphonyl fluoride (PMSF), 0·5 μg/ml leupeptin, 0·5 μg/ml pepstatin, 0·2 U/ml aprotinin and 7 mm β-mercaptoethanol (β-ME)) for 5 min. After removal of the supernatant, nuclear pellets were resuspended and washed in lysis buffer without Triton X-100 and centrifuged at 4000 g for 3 min. After careful removal of the supernatant, nuclear pellets were resuspended and maintained in permanent agitation for 30 min at 4°C in 100 μl of 10 mm HEPES pH 8·0, 350 mm NaCl, 0·1 mm EDTA, 0·5 mm espermidine, 0·15 mm espermine, 1 mm PMSF, 0·5 μg/ml leupeptin, 0·5 μg/ml pepstatin, 0·2 U/ml aprotinin and 7 mm β-ME. Nuclear fractions were recovered after centrifugation at 4000 g for 15 min.
Nuclear protein extracts (3 μg) were analysed as described [23] using a α32P-dCTP-labelled double-stranded synthetic wild-type HIV enhancer oligonucleotide 5′-AGCTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGA-3′ for NF-κB. Binding competition was performed by preincubating extracts with a 40-fold excess of unlabelled oligonucleotide and specific antibodies against p50 (a kind gift from Dr R. T. Hay, Southampton, UK), c-Rel (a kind gift from Dr N. Rice, Frederick, MD), and p65 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
To compare levels of MCP-1 protein and migration indices in different samples we analysed means by Student's t-test for paired samples. To evaluate the correlation between MCP-1 protein concentration and migration index, Pearson's test was used.
Results
Hypoxia induces MCP-1 and IL-8 expression in dermal fibroblasts
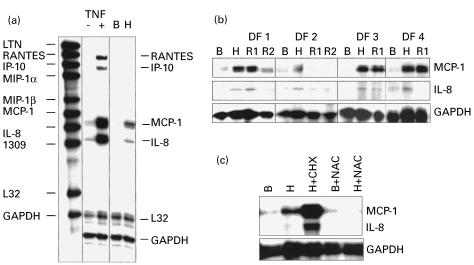
We first studied the pattern of expression of chemokines in cultured dermal fibroblasts under basal conditions or exposed to human TNF-α, which has been demonstrated to be a potent inducer of chemokine gene expression in different cell types, including fibroblasts [2,3]. Cells were maintained under low FCS concentrations (0·1%) to avoid the influence of growth factors present in FCS on chemokine gene expression. In most fibroblasts lines, a low constitutive expression level of MCP-1 and IL-8 mRNA was detected. Exposure of fibroblasts to TNF-α at doses of 10, 25 or 50 U/ml induced a dose-dependent increase in the levels of RANTES, IP-10, MCP-1, and IL-8 mRNA (Fig. 1a). In contrast, Ltn, MIP-1α, MIP-1β, and I-309 were not detectable after TNF-α treatment.
Fig. 1.
Chemokine mRNA expression in cultured dermal fibroblasts exposed to tumour necrosis factor-alpha (TNF-α) or to hypoxia. (a) Riboprobes for multiple chemokine genes (left column) were hybridized to dermal fibroblasts mRNA pretreated (+) or not (–) with TNF-α, or under basal normoxic (B) or hypoxic (H) conditions. (b) Protected bands for chemokines MCP-1, IL-8, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in dermal fibroblast lines DF1–DF4, under basal conditions (B), exposed to hypoxia for 6 h (H), or reoxygenated for 1 h (R1) or 2 h (R2). (c) Effect of preincubating cells with cycloheximide (CHX) or N-acetyl-L-cysteine (NAC) prior to hypoxia.
Exposure of dermal fibroblasts to hypoxia for 6 h induced a strong increase in the level of MCP-1 mRNA and, to a lesser extent, that of IL-8 (Fig. 1a,b). The maximal response was observed after hypoxia. After 1 h of reoxygenation, MCP-1 and IL-8 levels did not further increase and, after 2 h, MCP-1 and IL-8 levels returned to basal levels. Pretreatment with NAC reduced the response of MCP-1 and IL-8 mRNA to hypoxia (Fig. 1c). Pretreatment with 10 μg/ml CHX superinduced MCP-1 and IL-8 mRNA in hypoxic fibroblasts (Fig. 1c).
Hypoxia and reoxygenation induce activation of NF-κB in dermal fibroblasts
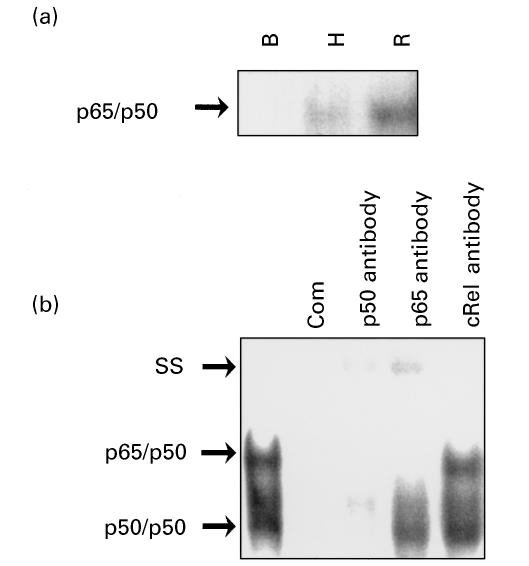
To study the activation of transcription factor NF-κB in parallel to the observed increase in MCP-1 and IL-8 expression, we studied the DNA-binding activity of nuclear protein extracts from cultured fibroblasts exposed to hypoxia and hypoxia reoxygenation (Fig. 2a). Shifted bands migrating mainly as p65/p50 complexes were detected in fibroblasts exposed to hypoxia and reoxygenation. Reoxygenated fibroblasts displayed higher shifting activity than hypoxia-exposed fibroblasts.
Fig. 2.
NF-κB activation by electromobility shift assay (EMSA) in nuclear protein extracts from fibroblasts exposed to hypoxia and reoxygenation. (a) p50/p65 complex in nuclear protein extracts from fibroblasts under basal (B), hypoxic (H) and reoxygenated (R) conditions. (b) Identification of NF-κB complexes in hypoxic fibroblast nuclear protein extracts by competition with unlabelled oligonucleotides (Com) and antibodies to p50, p65 and c-Rel. SS, Supershifted complexes. Autoradiographs were exposed longer in (b) to detect all complexes better.
To confirm the identity of the activated NF-κB complexes, competition with antibodies against p50, p65, and cRel was performed. Supershifted bands and a decrease in the intensity of original bands were only observed with anti-p50 and anti-p65 antibodies (Fig. 2b).
Hypoxia induces MCP-1 protein secretion by dermal fibroblasts
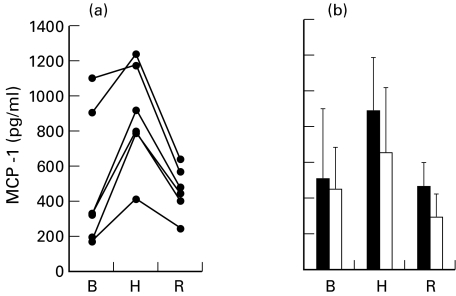
To confirm whether the observed increase in MCP-1 mRNA was followed by an increase in secreted protein, MCP-1 protein was quantified in the supernatants of six fibroblast lines by ELISA. The different cell lines were highly heterogeneous in both the basal and hypoxia-induced levels of MCP-1 (Fig. 3a). In the different lines, the level of secreted protein was increased between 1·4- and 4·2-fold by exposure to hypoxia. The mean level of MCP-1 was significantly increased in hypoxic (P = 0·006) but not in reoxygenated (P = 0·753) fibroblasts compared with normoxic fibroblasts (Fig. 3b). Pretreatment with NAC reduced by 46% (P = 0·005) the mean level of MCP-1 protein in response to hypoxia but did not modify the basal levels (Fig. 3b).
Fig. 3.
MCP-1 protein levels in fibroblast media as analysed by ELISA. Analysis was performed in conditioned media from fibroblasts under basal conditions (B), exposed to hypoxia (H), or reoxygenated 1 h after hypoxia (R). The mean ± s.d. levels of MCP-1 protein secreted by normoxic, hypoxic and reoxygenated cells in six different cells lines are represented (a). The mean values with (□) or without (▪) pretreatment with N-acetyl-L-cysteine (NAC) are represented (b). ELISA determinations were performed in three different samples from each cell line. Mean values were compared by Student's t-test for paired samples.
MCP-1 secreted by fibroblasts is chemotactic for monocytic cells
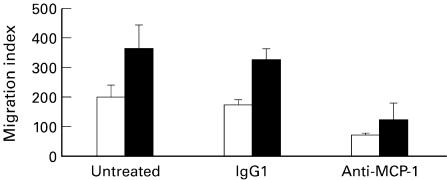
To evaluate the potential biological relevance of MCP-1 secreted by fibroblasts on leucocyte migration, we studied the chemotactic response of monocytic cells to the supernatants of normoxic and hypoxic fibroblasts in a Transwell chemotaxis assay. Fibroblast-conditioned media under both normoxic and hypoxic conditions displayed chemotactic activity for MonoMac1 cells. The mean migration index of MonoMac1 cells to fibroblast-conditioned media was significantly increased in hypoxic compared with normoxic cells (P = 0·015) (Fig. 4). Preincubation of fibroblast-conditioned media with neutralizing anti-human MCP-1 MoAb significantly reduced the migration indices of both normoxic (P = 0·004) and hypoxic cells (P = 0·004). Preincubation with mouse isotype-matched IgG1 did not significantly modify migration indices (Fig. 4).
Fig. 4.
Migration of MonoMac1 monocytic cells in response to fibroblast-conditioned media. Migration index was calculated as the x-fold increase in migration observed over the negative control. Conditioned media were collected from fibroblasts under basal conditions (□) or exposed to hypoxia (▪) (6 h). The mean migration indices from six different fibroblast lines in normoxia and hypoxia are represented. The effect of preincubating media with anti-MCP-1 MoAb or isotype-matched mouse IgG1 is also shown. Error bars indicate the s.d. of the mean from six different fibroblast lines. Mean values were compared by Student's t-test. Statistically significant differences are indicated in Results.
Fibroblast-conditioned media under basal (normoxic) conditions displayed chemotactic activity for MonoMac1 cells. The mean migration index of MonoMac1 cells to fibroblast supernatants was significantly increased in hypoxic compared with normoxic cells (P = 0·015). Preincubation of fibroblast supernatants with neutralizing anti-human MCP-1 MoAb significantly reduced the migration indices of both normoxic (P = 0·004) and hypoxic cells (P = 0·004). Preincubation with mouse isotype-matched IgG1 did not significantly modify migration indices (Fig. 4).
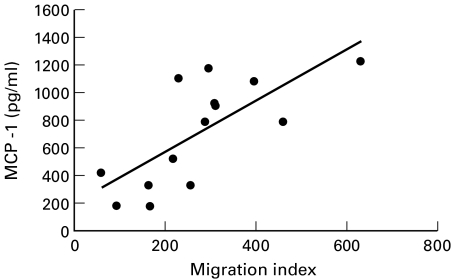
To analyse further the relevance of secreted MCP-1 protein in the migratory responses of monocytic cells we analysed the correlation between migration index and MCP-1 concentration in fibroblast-conditioned media. We found a positive correlation between migration index and MCP-1 concentration (Pearson correlation index: 0·70, P = 0·01) (Fig. 5).
Fig. 5.
Correlation between MCP-1 levels in fibroblast-conditioned media and MonoMac1 migration index. Analysis was performed by Pearson's test in all determinations from six cell lines under both normal and hypoxic conditions. Pearson's correlation index = 0·7, P = 0·01.
Discussion
The potential of resident fibroblasts to initiate or amplify inflammatory responses by releasing multiple proinflammatory factors, including chemokines, has recently received experimental support. Studies of human and animal tissues have demonstrated the presence of chemokines in fibroblasts of a variety of neoplastic, vascular and inflammatory diseases [10,24,25]. Bacterial products such as lipopolysaccharide (LPS) or Borrelia burgdorferi lipoprotein A, inflammatory cytokines such as IL-1 or TNF-α, and growth factors have been demonstrated to induce synthesis of chemokines in cultured fibroblasts [2,3]. One factor common to a large and heterogeneous group of lesions with a vascular and inflammatory component such as wound healing, cardiovascular diseases or connective tissue autoimmune diseases, is the presence of a hypoxic milieu and the generation of ROI. Our data demonstrate that human dermal fibroblasts exposed to hypoxic conditions express a restricted subgroup of chemokines that promote leucocyte migration.
NF-κB is a transcriptional activator for many proinflammatory factor genes, including chemokines [2,12,13]. Redox changes represent a general pathway in the activation of NF-κB by different stimuli [19]. One of the early signalling events induced by hypoxia is an increase in IκBα tyrosine phosphorylation that enhances NF-κB binding to DNA [26]. Our data indicate that, in fibroblasts, activation of NF-κB p65/p50 by redox changes is involved in the induction of chemokine gene expression by hypoxia. We observed the maximal MCP-1 and IL-8 mRNA increase during the hypoxia phase and MCP-1 expression was reduced by pretreatment with the thiol anti-oxidant NAC that antagonizes NF-κB activation by ROI. Since small amounts of ROI may be generated even under a very low oxygen concentration [27,28], they may induce the NF-κB activation and the increase in MCP-1 and IL-8 mRNA transcription observed during hypoxia. However, during reoxygenation, when maximal ROI generation is expected and we observed the strongest NF-κB activation, no further increase in the expression of MCP-1 or IL-8 was detected. A maximal response in the NF-κB-mediated transcription of different proinflammatory genes has also been observed during hypoxia in many other in vitro studies [28–30]. Furthermore, a similar response has been observed in ischaemic myocardium, with maximal MCP-1 mRNA levels achieved during the hypoxia phase and a rapid decline after reperfusion, although the particular myocardial cell type involved was not identified [30]. The reasons why the higher ROI levels and stronger p65/p50 activation observed during reoxygenation do not lead to higher MCP-1 expression levels are not clear from these studies. A possible explanation may relate to the eventual presence of inhibitory factors of NF-κB-induced transcriptional activation. The superinduction of MCP-1 and IL-8 mRNA observed after protein synthesis blockade with CHX is consistent with this hypothesis. Besides the role of NF-κB factors, transcriptional regulation of MCP-1 expression is dependent on multiple transcription factors, including activating protein-1 (AP-1), Sp-1 and a p90 phosphoprotein, and exhibits a cell type-specific pattern [31–33]. Thus, a different balance in the activation of multiple transcription factors could account for the observed differences in chemokine gene transcription during hypoxia and reoxygenation.
MCP-1 belongs to the CC chemokine supergene family and it is a specific and potent chemoattractant for monocytes and activated memory T lymphocytes [34,35]. In addition to its chemotactic effects, MCP-1 triggers firm adhesion of monocytes to vascular endothelium [36]. Our data demonstrate that fibroblast-derived MCP-1 is chemotactic for monocytic cells, and that this activity increases after exposure to hypoxia. The chemotactic activity of fibroblasts correlated well with MCP-1 levels and was inhibited by neutralizing anti-MCP-1 MoAb, confirming the role of MCP-1 in this response. A previous study has demonstrated the ability of serum-stimulated fibroblasts to promote transendothelial migration of lymphocytic and monocytic cells which was also inhibited by anti-MCP-1 antibody [37]. MCP-1 is an important factor in the pathogenesis of myocardial ischaemia reperfusion injury, atherosclerosis, and a variety of inflammatory diseases, whose pathogenesis is known to involve infiltration and activation of monocytes and lymphocytes [8–10,30,38,39]. Either blocking NF-κB activation or MCP-1 activity in hypoxic myocardium similarly prevents inflammatory infiltration and tissue injury, which suggests that MCP-1 is a major effector of NF-κB activation in that model [30,40].
Dermal fibroblast activation plays an important role in wound healing and chronic inflammatory diseases characterized by an excessive fibrotic response. Among them, SSc is characterized by early and severe vascular lesions and Raynaud's phenomenon, which lead to repeated cycles of hypoxia/reperfusion of skin and affected organs [20,21]. Although the causes of vascular alterations are not known, ischaemia and ROI generation are thought to play a central pathogenic role. Our data suggest that MCP-1 released by dermal fibroblasts might be a mediator of inflammatory cell infiltration in response to vascular damage.
MCP-1 has also been associated with an excessive fibrotic response in different inflammatory diseases [8–10]. Besides its proinflammatory effects, MCP-1 has been shown to induce transforming growth factor-beta (TGF-β) and procollagen gene expression in fibroblasts by specific stimulation of CCR2 receptor [6]. Interestingly, both TGF-β and procollagen expression have been found to increase in dermal fibroblasts after exposure to hypoxia [41,42], with a temporal sequence compatible with an autocrine role for MCP-1 according to the present findings. Thus, hypoxia-induced MCP-1 gene expression by dermal fibroblasts may be a potential target for the therapy of chronic inflammatory diseases, particularly those in which excessive fibrosis is a deleterious consequence.
Acknowledgments
This work was supported by FIS 98/0356 and 98/0337 (Fondo de Investigaciones Sanitarias), and PM 96/0028 and SAF 96/0186 (Ministerio de Educación y Cultura). J.M.-S. was supported by Instituto de Salud Carlos III. We thank Dr E. C. LeRoy for helpful suggestions and critically reading this manuscript. We also thank Dr A. Comunion for providing skin samples.
REFERENCES
- 1.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–92. [PubMed] [Google Scholar]
- 3.Van Damme J, Proost P, Put W, et al. Induction of monocyte chemotactic proteins MCP-1 and MCP-2 in human fibroblasts and leukocytes by cytokines and cytokine inducers. Chemical synthesis of MCP-2 and development of a specific RIA. J Immunol. 1994;152:5495–502. [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 5.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 6.Keane MP, Arenberg DA, Lynch JP 3rd, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–43. [PubMed] [Google Scholar]
- 7.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–84. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd CM, Minto AW, Dorf ME, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–80. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 1992;89:5371–5. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152:423–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–15. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roebuck KA, Carpenter LR, Lakshminarayanan V, Page SM, Moy JN, Thomas LL. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J Leuk Biol. 1999;65:291–8. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 13.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997;96:2361–7. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 14.Olashaw NE. Inducible activation of RelB in fibroblasts. J Biol Chem. 1996;271:30307–10. [PubMed] [Google Scholar]
- 15.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–87. [PMC free article] [PubMed] [Google Scholar]
- 16.Blake DR, Winyard PG, Marok R. The contribution of hypoxia-reperfusion injury to inflammatory synovitis: the influence of reactive oxygen intermediates on the transcriptional control of inflammation. Ann NY Acad Sci. 1994;723:308–17. [PubMed] [Google Scholar]
- 17.Remick DG, Villarete L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J Leuk Biol. 1996;59:471–5. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GR, Volpe CM, Russo CA, Stoler DL, Miloro SM. The anoxic fibroblast response is an early-stage wound healing program. J Surg Res. 1995;59:666–74. doi: 10.1006/jsre.1995.1221. [DOI] [PubMed] [Google Scholar]
- 19.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wigley FM, Flavahan NA. Raynaud's phenomenon. Rheum Dis Clin North Am. 1996;22:765–81. doi: 10.1016/s0889-857x(05)70300-8. [DOI] [PubMed] [Google Scholar]
- 21.Peng SL, Fatenejad S, Craft J. Scleroderma: a disease related to damaged proteins? Nature Med. 1997;3:276–8. doi: 10.1038/nm0397-276. [DOI] [PubMed] [Google Scholar]
- 22.Frade JMR, Llorente M, Mellado M, et al. The amino-terminal domain of the CCR2 chemokine receptor acts as coreceptor for HIV-1 infection. J Clin Invest. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lain de Lera T, Folgueira L, Martin AG, et al. Expression of IkappaBalpha in the nucleus of human peripheral blood T lymphocytes. Oncogene. 1999;18:1581–8. doi: 10.1038/sj.onc.1202455. [DOI] [PubMed] [Google Scholar]
- 24.Konishi T, Okabe H, Katoh H, Fujiyama Y, Mori A. Macrophage inflammatory protein-1 alpha expression in non-neoplastic and neoplastic lung tissue. Virchows Arch. 1996;428:107–11. doi: 10.1007/BF00193938. [DOI] [PubMed] [Google Scholar]
- 25.Tomita H, Egashira K, Kubo-Inoue M, et al. Inhibition of NO synthesis induces inflammatory changes and monocyte chemoattractant protein-1 expression in rat hearts and vessels. Arterioscler Thromb Vasc Biol. 1998;18:1456–64. doi: 10.1161/01.atv.18.9.1456. [DOI] [PubMed] [Google Scholar]
- 26.Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–30. [PubMed] [Google Scholar]
- 27.Khan S, O'brien PJ. Modulating hypoxia-induced hepatocyte injury by affecting intracellular redox state. Biochim Biophys Acta. 1995;1269:153–61. doi: 10.1016/0167-4889(95)00112-6. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka K, Shimizu K, Sun X, et al. Hypoxia, but not reoxygenation, induces interleukin 6 gene expression through NF-kappa B activation. Transplantation. 1997;63:466–70. doi: 10.1097/00007890-199702150-00023. [DOI] [PubMed] [Google Scholar]
- 29.Ji YS, Xu Q, Schmedtje Jf., Jr Hypoxia induces high-mobility-group protein I (Y) and transcription of the cyclooxygenase-2 gene in human vascular endothelium. Circ Res. 1998;83:295–304. doi: 10.1161/01.res.83.3.295. [DOI] [PubMed] [Google Scholar]
- 30.Ono K, Matsumori A, Furukawa Y, et al. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79:195–203. [PubMed] [Google Scholar]
- 31.Hanazawa S, Takeshita A, Amano S, et al. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:9526–32. [PubMed] [Google Scholar]
- 32.Ueda A, Okuda K, Ohno S, et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–63. [PubMed] [Google Scholar]
- 33.Freter RR, Alberta JA, Hwang GY, Wrentmore AL, Stiles CD. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-kappaB and a 90-kDa phosphoprotein coactivator. J Biol Chem. 1996;271:17417–24. doi: 10.1074/jbc.271.29.17417. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–59. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 37.Denton CP, Shi-Wen X, Sutton A, Abraham DJ, Black CM, Pearson JD. Scleroderma fibroblasts promote migration of mononuclear leucocytes across endothelial cell monolayers. Clin Exp Immunol. 1998;114:293–300. doi: 10.1046/j.1365-2249.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–7. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 40.Morishita R, Sugimoto T, Aoki M, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-kappaB binding site prevents myocardial infarction. Nature Med. 1997;3:894–9. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 41.Falanga V, Qian SW, Danielpour D, Katz MH, Roberts AB, Sporn MB. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991;97:634–7. doi: 10.1111/1523-1747.ep12483126. [DOI] [PubMed] [Google Scholar]
- 42.Falanga V, Martin TA, Takagi H, et al. Low oxygen tension increases mRNA levels of alpha 1 (I) procollagen in human dermal fibroblasts. J Cell Physiol. 1993;157:408–12. doi: 10.1002/jcp.1041570225. [DOI] [PubMed] [Google Scholar]