Abstract
In order to characterize T cell responses in human Helicobacter pylori infection, we have examined proliferative responses and cytokine production by CD4+ and CD8+ T cells isolated from duodenal ulcer patients and asymptomatic H. pylori carriers, after activation with some H. pylori antigens that may be important in disease development. For control purposes, T cells from uninfected volunteers were also examined. The different H. pylori antigens induced only modest proliferative responses in circulating CD4+ and CD8+ T cells from both H. pylori-infected and uninfected individuals. However, circulating T cells from H. pylori-infected subjects produced larger amounts of interferon-gamma (IFN-γ) in response to the Helicobacter antigens than did T cells from uninfected volunteers. Furthermore, CD8+ T cells produced larger amounts of IFN-γ than did CD4+ T cells, on a per cell basis. Most IFN-γ-producing cells from both infected and uninfected volunteers appeared to be naive T cells expressing CD45RA. Increased production of IL-4 and IL-5 was, on the other hand, only seen in a few instances after stimulation of isolated CD4+ and CD8+ T cells. Stimulation of freshly isolated gastric T cells with the different H. pylori antigens did not result in increased proliferation or cytokine production. In conclusion, our results show that several different purified H. pylori antigens induce production of IFN-γ, preferentially by CD8+ cells. Therefore, they suggest that IFN-γ-secreting CD8+ cells contribute significantly to the cytokine response induced by H. pylori infection.
Keywords: Helicobacter pylori, T cells, interferon-gamma, gastric mucosa, human
Introduction
Infection with Helicobacter pylori is strongly associated with the development of active chronic gastritis, duodenal ulcer and primary gastric lymphoma [1,2]. The infection results in a dense infiltration of lymphocytes and granulocytes into the gastric mucosa and development of a macroscopic gastritis in virtually all infected individuals. The evolving inflammation and epithelial destruction might be promoted by a number of antigens produced by H. pylori, such as the vacuolating cytotoxin (VacA), lipopolysaccharide (LPS), and urease [3,4]. In spite of the ongoing gastritis, a large proportion of H. pylori-infected individuals do not experience any symptoms and most of them never develop any ulcers. It has been proposed that the expression of certain genes by the infecting H. pylori strain, especially those located in the so called pathogenicity island [3], may be important in the development of peptic ulcers and other clinical manifestations of the infection. Recent studies have also suggested that host factors may be of fundamental importance in determining the consequences of a Helicobacter infection [5–7].
During the effector phase of specific immune responses, different T helper cell subsets, so called Th1 and Th2 cells defined by characteristic patterns of cytokine production, expand [8]. Th1 cells promote cell-mediated immune responses, mainly through production of interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), whereas the Th2 subset produces IL-4, IL-5, and IL-13 which act on B cells to induce the production of high levels of antibodies and also curtail the action of Th1 cytokines. The T cell cytokines produced are thus likely to be among the host factors that determine the outcome of a H. pylori infection. Earlier studies have suggested that H. pylori-induced inflammation may be a result of polarization towards a Th1-dominated T cell response [9–12]. Whether activated T cells may contribute to protection against symptoms or rather to maintenance of the inflammatory process is, however, not known.
In order to characterize antigen-specific T cell responses in H. pylori-infected humans in greater detail, we examined proliferation and cytokine production from CD4+ and CD8+ circulating T cells after in vitro activation with some of the H. pylori antigens that may play a role in disease development. To evaluate the possible contribution by activated T cells in the development of peptic ulcer disease, the studies were performed with cells from both duodenal ulcer (DU) patients and asymptomatic (AS) H. pylori carriers as well as from healthy, uninfected volunteers. Our studies show a large production of IFN-γ, mainly by CD8+ cells, after stimulation with H. pylori antigens, both in DU patients and AS carriers.
Subjects and methods
Volunteers and collection of specimen
This study was performed with due approval from the Human Research Ethical Committee of the Medical Faculty, Göteborg University, and all volunteers had given informed consent to participate. For studies of T cell proliferation and cytokine secretion, 11 patients suffering from DU (aged 33–76 years; three females), nine AS H. pylori carriers (aged 29–63 years; four females), as well as 11 healthy uninfected volunteers (aged 25–69 years; nine females) were recruited. For studies of CD45 isoforms on activated T cells, four additional AS H. pylori-infected volunteers (aged 32–54 years; two females) and five uninfected volunteers (aged 25–41 years; all females) were recruited.
DU patients were recruited among patients attending the Gastroenterology Unit at Sahlgrenska University Hospital, Göteborg, and the DU diagnosis was confirmed by endoscopy. AS H. pylori carriers were identified by serological screening of healthy blood donors using ELISA [13]. Helicobacter pylori status of DU patients and uninfected volunteers was confirmed using the same ELISA. Neither the AS H. pylori-infected subjects nor the uninfected volunteers had any previous history of gastrointestinal symptoms or illnesses. None of the volunteers had an active ulcer or was on any medication the last 3 weeks before entering the study.
Heparinized venous blood (30 ml) was collected from all volunteers. From eleven of them (seven DU patients and four uninfected subjects) 10 antrum and 10 corpus biopsies, each of 2 mm diameter, encompassing the epithelium and lamina propria, were also collected under local anaesthesia using a pair of biopsy forceps.
Purification of H. pylori antigens
Two H. pylori strains, CCUG 17874 and E32, were used for antigen purification [14]. Strains were grown on horse blood agar plates under microaerophilic conditions for 3 days. Membrane proteins (MP) were prepared from strain CCUG 17874 by sonication followed by differential centrifugation as previously described [15]. Urease was isolated from strain E32, followed by purification on a Sepharose CL63 column (Pharmacia, Uppsala, Sweden) and then on a Resource Q FPLC column (Pharmacia) as described [16].
The Helicobacter-specific neutrophil-activating protein (NAP) [17] and a species-specific prominent 26-kD protein, which was recently demonstrated to be an alkyl hydroperoxide reductase homologue, with the gene designation tsaA [18], were recombinantly produced in Escherichia coli; NAP was kindly provided by Dr S. Nyström (AstraZeneca, Umeå, Sweden) and the 26-kD protein was produced by Genome Therapeutic Company (GTC, Cambridge, MA) and kindly provided by AstraZeneca Research Centre in Boston.
The purity of the antigen preparations was demonstrated by SDS–PAGE electrophoresis, followed by coomassie blue staining or transblotting to nitrocellulose membranes and staining with rabbit polyclonal antisera against whole H. pylori bacteria and MoAb specific for the respective proteins [14,19]. All antigen preparations, except MP, were depleted of LPS by incubation with KuttsuClean beads (Maruha Corp., Ibaraki, Japan) for 2 h at room temperature. After this incubation, none of the antigen preparations contained detectable amounts of LPS in the Limulus test, i.e. < 1 ng/ml [20]. When diluted to 5 μg/m, the MP preparation had an LPS activity corresponding to 3 pg/ml of LPS. At this concentration, H. pylori LPS has no detectable effect on cytokine production from human blood mononuclear cells (MNC), but to ensure that the LPS did not interfere with T cell activation, the MP fraction was preincubated with 1 μg/ml of polymyxin B (Sigma, St Louis, MO) at room temperature for 1 h, a procedure that completely inhibits the cytokine production induced by 100 pg/ml of E. coli or H. pylori LPS.
Isolation and fractionation of MNC
Peripheral blood MNC were collected by isopycnic gradient centrifugation on Ficoll–Hypaque (Pharmacia). Gastric lymphocytes were isolated from the gastric biopsies by short-term enzymatic digestion using thermolysine and collagenase as previously described [21].
Peripheral and gastric T cells were isolated from the MNC suspensions by overnight incubation with 2-aminoethylisothiouroniumbromide hydrobromide-treated sheep erythrocytes [22]. CD4+ and CD8+ cells were then further purified from the peripheral blood T cell suspension by incubation together with polystyrene paramagnetic beads (Dynabeads) coated with antibodies to CD4 or CD8, respectively (Dynal AS, Oslo, Norway). The beads were subsequently released from the cells by incubation with a tailor-made F(ab) preparation (Detachabead; Dynal).
The fractionation procedures were evaluated by flow cytometry analyses of the original and resulting cell populations using FITC- or PE-labelled antibodies to CD4, CD8, CD14 and CD19 (Becton Dickinson, San Jose, CA). The resulting CD4+ fractions contained > 97% CD4+ cells and < 2% CD8+ cells, while the CD8 fractions contained > 97% CD8+ cells and < 1% CD4+ cells. The remaining non-T cell fraction obtained after sheep erythrocyte isolation of T cells was used as accessory cells for the T cell stimulations. These cells consisted of approximately one-third B cells (CD19+) and two-thirds monocytes (CD14+).
In vitro stimulation of T cells
For detection of T cell proliferation and cytokine secretion, isolated CD4+ and CD8+ cells were cultured in triplicates at 1·5 × 105 cells/well together with 1·5 × 104 autologous accessory antigen-presenting cells in round-bottomed 96-well plates (Nunc, Roskilde, Denmark) in 200 μl Iscove's medium supplemented with 5% of human AB+ serum, 3 μg/ml l-glutamine, and 100 μg/ml gentamycin. Gastric T cells could not be separated into CD4+ and CD8+ cells due to the limited number of cells isolated from the biopsies, but were cultured at 7·5 × 104 T cells/well together with 7·5 × 103 accessory cells.
T cell cultures were initially stimulated with 20, 5, and 1 μg/ml of either MP, urease, 26-kD protein, or NAP; in subsequent experiments 5 μg/ml was used, since 20 and 5 μg/ml yielded similar results. Phytohaemagglutinin (PHA; 10 μg/ml; Murex Diagnostics Ltd, Temple Hill, UK) was used as a positive control, and 5 μg/ml of PPD from Mycobacterium bovis Bacille Calmette–Guèrin (BCG; Statens Seruminstitut, Copenhagen, Denmark) were used to asses antigen-specific T cell activation, since most of the volunteers had been parenterally immunized with the BCG vaccine in childhood.
After culture for 48 h, 100 μl of the culture medium were removed from each well, pooled, and stored at −70°C until further analysed for cytokine content. The collected medium was replaced with fresh medium and the cells cultured for another 3 days. During the last 8–14 h of culture, 1 μCi of 3H-labelled thymidine (Amersham Int. Plc, Aylesbury, UK) was added to each well. The cells were harvested on a glass fibre filter and the amount of incorporated radioactivity was determined in an automated β-scintillation counter (Wallac, Turku, Finland). The stimulation index (SI) was calculated by dividing the ct/min value obtained after stimulation by the ct/min value in corresponding cultures without antigen or PHA.
For detection of CD45 isoforms on activated cells, circulating CD4+ and CD8+ cells were isolated as above, and cultured with the adherent cells from the non-T cell fraction as accessory cells and 5 µg/ml of MP or urease for 20 h. The cells were then cultured for another 6 h with Golgistop (PharMingen, San Diego, CA), and then stained with antibodies to CD45RO, followed by PerCP-conjugated goat antibodies to mouse IgG1, and then FITC-labelled antibodies to CD45RA. After fixation and permeabilization, intracellular IL-4 or IFN-γ was detected with PE-labelled antibodies to the respective cytokine (all antibodies were obtained from Becton Dickinson). To control for unspecific staining by the anti-cytokine antibodies, parallel samples of cells that had been incubated with antibodies to CD45RA and CD45RO were stained with PE-labelled isotype-matched antibodies of irrelevant specificity.
ELISA detection of cytokines
The concentrations of IL-4, IL-5, and IFN-γ were determined in different ELISA tests. Ninety-six-well Maxisorb plates (Nunc) were coated with MoAbs specific for the respective cytokine at 4°C overnight in 0·05 m carbonate buffer pH 9·6. After blocking the plates with 1% bovine serum albumin (BSA) in PBS at room temperature for 1 h, the collected cell culture specimens diluted in PBS–Tween (0·05%) containing 0·1% BSA were incubated in the plates at 4°C overnight. The cytokine concentrations were determined by the stepwise addition of biotinylated antibodies reacting with the respective cytokine, peroxidase-labelled extravidin (Sigma), and o-phenylenediamine substrate (Sigma). The coating and biotin-conjugated antibodies to IL-4 were purchased from PharMingen, the IFN-γ-specific reagents from Chromogenix (Mölndal, Sweden), and the IL-5-specific reagents from ImmunoKontact (Bioggio, Switzerland). Standard curves were constructed using recombinant human cytokines obtained from Genzyme (Cambridge, MA).
Statistical analysis
Wilcoxon signed rank test was used to evaluate differences in cytokine secretion by CD4+ and CD8+ cells, and rank sum test was used for comparisons of H. pylori-infected and uninfected individuals.
Results
Proliferative response by circulating T cells
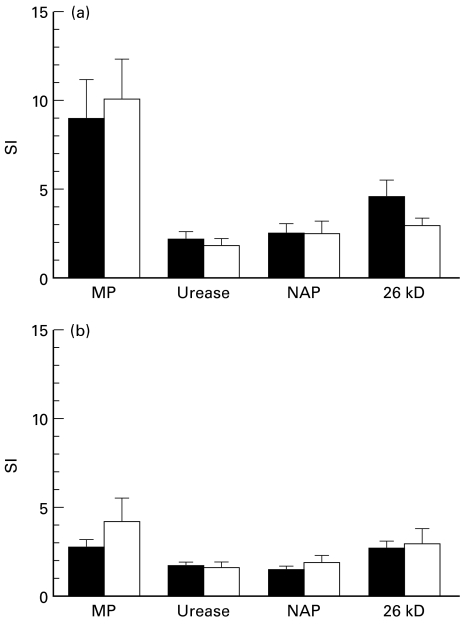
CD4+ and CD8+ T cells isolated from peripheral blood were cultured together with autologous accessory cells and purified H. pylori antigens (MP, urease, NAP, and 26-kD protein), PPD, or PHA and proliferation was assessed 5 days later. When comparing the different H. pylori antigens, the largest proliferation was seen in response to the MP preparation, whereas the responses to urease, NAP and 26-kD protein were all modest (Fig. 1). The CD4+ cells proliferated more strongly to the H. pylori antigens than the corresponding CD8+ cells in all groups of volunteers (Fig. 1). However, the individual variation in the response to each of the antigens was large (e.g. SI ranging from 1 to 35 in the CD4+ T cell response to MP), and there were no differences in the proliferative responses between H. pylori-infected and uninfected individuals, or between AS carriers and DU patients.
Fig. 1.
Proliferative response after stimulation with Helicobacter pylori antigens. Peripheral blood CD4+ (a) and CD8+ T cells (b) from H. pylori-infected volunteers (▪) and healthy uninfected volunteers (□) were cultured with the indicated antigens. Proliferation was assessed by thymidine incorporation after 5 days of culture and expressed as stimulation index (SI), i.e. the proliferation obtained in stimulated cultures divided with the proliferation in unstimulated cultures. Data are pooled from 20 H. pylori-infected subjects (both duodenal ulcer patients and asymptomatic individuals) and from 11 uninfected volunteers, and are expressed as arithmetic means + s.e.m. MP, Membrane protein; NAP, neutrophil-activating protein.
In most volunteers, the response to PPD stimulation was much stronger than that to the H. pylori antigens, and it was also more pronounced in the CD4+ (SI ranging from 2 to 254, mean 45) than in the CD8+ population (SI between 1 and 70, mean 12). The response to PPD did not differ between H. pylori carriers and uninfected individuals (P > 0·05). The PHA-induced proliferation was usually lower in the CD4+ (SI ranging from 8 to 665, mean 179) than in the CD8+ population (SI between 25 and 811, mean 374). In addition, PHA-induced proliferation did not differ between the different study groups (P > 0·05).
Cytokine production by circulating T cells
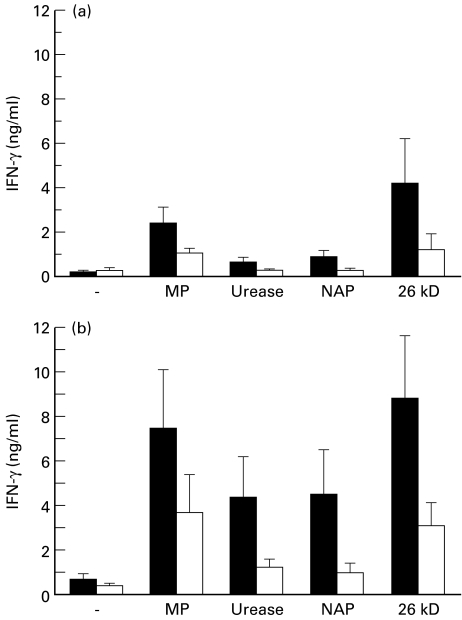
The amounts of secreted IL-4, IL-5, and IFN-γ were determined by ELISA using cell culture supernatants collected 48 h after stimulation. Increased amounts of IFN-γ were detected in T cell cultures from most H. pylori-infected volunteers in response to all the different antigens tested, and both CD4+ and CD8+ T cells responded with IFN-γ production to the H. pylori antigens. Several of the uninfected healthy volunteers also responded to the H. pylori antigens used, although the amounts of IFN-γ secreted were considerably lower in cultures with cells from uninfected individuals, especially when CD8+ cells from infected and uninfected individuals were compared (Fig. 2). Due to the large individual variation, these differences were only significant (P < 0·05) in CD4+ cells stimulated with NAP and in CD8+ cells stimulated with MP. When cultured at the same cell density, CD8+ T cells secreted significantly larger amounts of IFN-γ per cell than CD4+ cells from the respective individual in response to H. pylori antigens (P < 0·01 for all tested antigens). In contrast, the IFN-γ response to both PHA and PPD was higher in the CD4+ than in the CD8+ T cells (P < 0·01 for both).
Fig. 2.
IFN-γ production after stimulation with Helicobacter pylori antigens. Peripheral blood CD4+ (a) and CD8+ T cells (b) from H. pylori-infected volunteers (▪) and healthy uninfected volunteers (□) were cultured with or without the indicated antigens. The IFN-γ concentrations were analysed in culture supernatants collected after 48 h by ELISA. Data are pooled from 20 H. pylori-infected subjects (both duodenal ulcer patients and asymptomatic individuals) and from 11 uninfected volunteers, and are expressed as arithmetic means + s.e.m. MP, Membrane protein; NAP, neutrophil-activating protein.
In contrast to IFN-γ, IL-4 and IL-5 were rarely detected after H. pylori antigen stimulation. Thus, CD4+ cells from only one of the H. pylori-infected volunteers responded with IL-4 production to MP, urease, and the 26-kD protein, and CD4+ cells from three infected volunteers responded to the NAP stimulation (data not shown). IL-5 production could not be detected from cells from any of the volunteers after stimulation with the different H. pylori antigens. CD4+ cells from most volunteers did, however, produce moderate levels of IL-4 (mean 51 ng/ml, range 7–200 ng/ml in H. pylori-infected and 71 ng/ml, range 3–219 ng/ml in H. pylori-negative individuals) and IL-5 (mean 39 ng/ml, range 11–162 ng/ml in H. pylori-infected and 27 ng/ml, range 6–42 ng/ml in H. pylori-negative individuals) after stimulation with PHA, but there was no significant difference between H. pylori-infected and uninfected individuals.
CD45RA and CD45RO expression on cytokine-producing cells
The relatively high frequency of H. pylori-negative subjects responding to the different H. pylori antigens tested made us examine the composition of responding T cells with regard to memory and naive subsets. We used three-colour flow cytometry to determine the expression of CD45RA and CD45RO on IFN-γ- and IL-4-containing cells after activation with MP or urease. In keeping with our previous results, IL-4-containing cells were rarely detected after these stimulations, whereas IFN-γ-containing cells were found in T cells isolated from several volunteers (Table 1). In agreement with the levels of secreted IFN-γ, the frequencies of IFN-γ+ cells were higher among CD8+ cells than CD4+ cells (Table 1). Furthermore, the frequencies of IFN-γ+ cells were somewhat higher in T cells isolated from infected than from uninfected volunteers, both in the CD4+ and CD8+ populations.
Table 1.
Frequencies of IFN-γ-containing cells after stimulation of circulating CD4+ and CD8+ T cells, isolated from Helicobacter pylori-infected or uninfected volunteers, with H. pylori membrane proteins (MP) or urease
| H. pylori-infected | Uninfected | |||
|---|---|---|---|---|
| CD4+ | CD8+ | CD4+ | CD8+ | |
| MP | 0·5 (0·2–0·9)* | 1·2 (0·2–2·7) | 0·2 (0·1–0·4) | 0·6 (0·3–1·1) |
| 3/4† | 3/4 | 3/5 | 3/5 | |
| Urease | 0·7 (0·4–1·0) | 1·3 | 0·1 | 0·3 |
| 2/4 | 1/3 | 1/5 | 1/5 | |
Percentage of IFN-γ-containing cells determined by flow cytometry, mean (range) of responding individuals.
Frequency of responders.
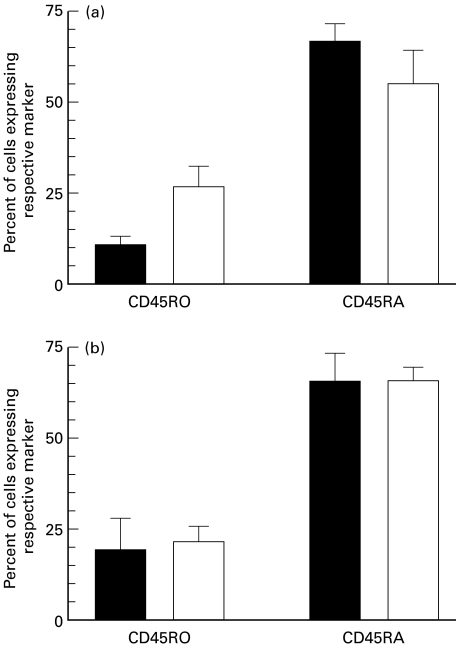
When the expression of CD45RO and CD45RA by IFN-γ+ cells activated by MP or urease was analysed, the proportion of cells positive for CD45RA was always higher than that of CD45RO+ cells. This was true for both CD4+ and CD8+ cells, and for both H. pylori-infected and uninfected individuals. However, it was only among CD8+ cells stimulated with MP that frequencies of IFN-γ+ were high enough to compare adequately H. pylori-infected and uninfected volunteers (Fig. 3). The distribution of CD45RO and CD45RA was very similar on IFN-γ-containing cells from the responding individuals in both groups of volunteers (three in each group). Furthermore, the culture conditions employed did not alter the overall distribution of CD45RO and CD45RA on CD8+ cells. Double-positive CD45RA+ RO+ cells also made up about 10% of the IFN-γ+ cells in both groups. Taken together, this suggests that the responding cells were mainly naive T cells in both H. pylori-infected and uninfected subjects. However, the distribution of CD45RA and CD45RO on the IFN-γ-containing cell population was similar to that expressed by all T cells in the respective populations, indicating that there was no specific subpopulation that was preferentially activated by the H. pylori antigens.
Fig. 3.
CD45RO and CD45RA expression on CD8+ T cells after stimulation with Helicobacter pylori membrane protein (MP). Circulating CD8+ cells isolated from H. pylori-infected volunteers (▪) and healthy uninfected volunteers (□) were stimulated in vitro with H. pylori MP for 20 h, and the expression of CD45RO and -RA as well as intracellular IFN-γ was determined using flow cytometry. Cell surface expression of CD45RO and -RA was determined on IFN-γ+ cells (a) and on all CD8+ T cells in the same cultures (b). Data are expressed as arithmetic means + s.d. of three individuals in each group.
Gastric T cell responses
In order to evaluate to what extent the responses among circulating T cells reflect local gastric T cell activity, T cells were isolated from gastric mucosal biopsies from some of the volunteers, and directly cultured together with peripheral blood accessory cells and H. pylori antigens. In contrast to peripheral blood T cells, no increase in proliferation or cytokine production could be detected after stimulation of the gastric T cells with the different H. pylori antigens or PPD, regardless of the H. pylori status of the volunteers (Table 2). The functional capacity of these cells was, however, demonstrated by activation with PHA, which induced a strong proliferation of gastric T cells and production of IFN-γ in all volunteers (Table 2). However, the gastric T cells did not produce detectable amounts of IL-4 or IL-5, even after polyclonal stimulation.
Table 2.
Proliferation and IFN-γ production after stimulation of gastric T cells from Helicobacter pylori-infected and uninfected individuals with H. pylori membrane proteins (MP), urease or phytohaemagglutinin (PHA)
| Unstimulated | H. pylori MP | Urease | PHA | |||||
|---|---|---|---|---|---|---|---|---|
| H. pylori+ | H. pylori− | H. pylori+ | H. pylori− | H. pylori+ | H. pylori− | H. pylori+ | H. pylori− | |
| Stimulatory index* | 1 | 1 | 1·3 ± 0·9 | 1·0 ± 0·8 | 0·8 ± 0·4 | 1·3 ± 1·2 | 210 ± 247 | 25 ± 22 |
| IFN-γ (ng/ml) | 0·60 ± 1·2† | 0·15 ± 0·11 | 0·11 ± 0·06 | 0·15 ± 0·10 | 0·10 ± 0·06 | 0·15 ± 0·11 | 3·6 ± 4·8 | 7·0 ± 10·2 |
| n | 7 | 4 | 7 | 4 | 5 | 4 | 7 | 4 |
Calculated by dividing the proliferation after stimulation by the proliferation in corresponding unstimulated cultures, mean ± s.d.
Given as mean ± s.d.
Discussion
In this study, we show that stimulation with different purified H. pylori antigens results in the production of Th1-type cytokines from both CD4+ and CD8+ T cells isolated from the circulation of H. pylori-infected individuals. In contrast to the prominent production of IFN-γ after stimulation with H. pylori antigens, very little IL-4 and IL-5 could be detected. Earlier studies of the cytokine regulation of immune responses in Helicobacter infection have focused on the balance between Th1 and Th2 cells [3,23]. Our study confirms the previous notion that H. pylori gastritis is associated with a Th1-type response, but more importantly, it suggests a significant contribution of CD8+ cells in the production of IFN-γ in H. pylori infection. Even though IFN-γ was produced by both CD4+ and CD8+ T cells, CD8+ cells usually produced larger amounts of IFN-γ than CD4+ cells on a per cell basis. In agreement with this notion, CD8+ cells constitute a substantial part of the lymphocytes infiltrating the H. pylori-infected gastric mucosa [24,25], and after polyclonal stimulation with PHA, a large proportion of gastric CD8+ cells produce IFN-γ in vitro [26]. The existence of functional subpopulations analogous to the Th1 and Th2 types of CD4+ cells among CD8+ T cells has been documented during recent years [27,28], and might contribute to the polarization of immune responses. The large production of IFN-γ by CD8+ T cells after H. pylori stimulation suggests an important role for these cells in the H. pylori-infected gastric mucosa. Whether IFN-γ-secreting CD8+ T cells mainly promote inflammatory reactions or if they have protective functions still remains to be elucidated.
CD8+ cells recognize peptides presented by MHC class I molecules, which have been considered to present mainly endogenously synthesized peptides. More recent studies, however, demonstrate the possibility that exogenous antigens may escape from phagosomes into the cytosol and be loaded onto MHC class I [29], and that orally delivered soluble proteins can induce MHC class I-restricted CD8+ T cell responses [30]. Furthermore, binding of peptide antigens to heat shock proteins (hsp) has been demonstrated to channel them to the MHC class I antigen presentation pathway [31,32]. The hsp produced by H. pylori have been shown to be expressed on the bacterial surface [33], and may help directing the immune response to other H. pylori antigens towards MHC class I presentation.
In the present study, exclusion of accessory cells from the T cell cultures resulted in the loss of T cell responses, demonstrating that the responses recorded were not a result of unspecific polyclonal activation of the T cells. Nevertheless, many of the uninfected individuals did respond to H. pylori stimulation, although with lower levels of IFN-γ production than the infected subjects. This made us examine the expression of CD45RA and CD45RO in the responding cell populations, to get an estimate of the proportions of naive and memory cells. Most IFN-γ-producing cells activated by H. pylori antigens expressed CD45RA, a marker of naive T cells, in both infected and uninfected individuals. The distribution of CD45RA and CD45RO on IFN-γ-producing cells was, however, similar to that of all T cells in the respective populations, regardless of IFN-γ production. Therefore, it seems as if no selected T cell population was preferentially activated by the H. pylori antigens. The reason for T cell activation in uninfected subjects may be earlier antigen exposure by low levels of H. pylori, due to the generally high percentage of infected individuals in the population. Alternatively, the H. pylori antigen preparations might activate accessory cells to produce substances which in turn preferentially stimulate CD8+ cells to secrete IFN-γ. Another possibility is that the MP preparation contains a component with superantigen properties. This is suggested by the fact that there was a high proportion of naive T cells among the stimulated cells and a requirement for the presence of accessory cells in order to induce T cell activation. This possibility will be addressed in forthcoming experiments.
At variance with our findings, previous studies have suggested that H. pylori-infected individuals have down-regulated H. pylori-specific T cell responses in the circulation compared with healthy individuals [24,34,35]. However, the antigens used in those studies were inactivated whole bacteria or whole cell lysates, of which at least the latter have been shown to contain immunomodulating substances [36]. The different antigen preparations used in previous and in our study may explain the differences between the results obtained, since we used purified or recombinant antigens in addition to the MP preparation and since all our antigen preparations were devoid of LPS activity.
The mechanisms by which the H. pylori-induced antral inflammation might have ulcerogenic effects are still unclear. We therefore analysed whether there were any differences in the T cell response between DU patients and AS H. pylori carriers. In this relatively small study there were no qualitative or quantitative differences in the T cell responses in the circulation to any of the studied antigens between DU patients and AS carriers. The ulcerogenic capacity of different H. pylori strains has been correlated to their capacity to produce the vacuolating cytotoxin and their expression of the genes in the pathogenicity island [3]. In addition, CagA, one of the proteins encoded by the pathogenicity island, seems to be one of the most immunogenic H. pylori antigens [10,37]. Unfortunately, we did not have access to CagA or VacA proteins and thus could not evaluate their potential to induce T cell activation in our system.
In contrast to the circulating T cells, freshly isolated gastric T cells were not activated when stimulated with H. pylori antigens in vitro. This could be due to specific requirements of antigen-presenting cells by gastric T cells, which are not met by the peripheral blood accessory cells used in this study. Alternatively, a local down-regulatory mechanism, analogous to what has already been described for intestinal T cells [38], might be operating also among gastric T cells. To our knowledge, antigen-specific activation of gastric T cells directly after isolation from the mucosa has not been accomplished by any research group. However, the presence of H. pylori-specific T cells in the gastric mucosa has been demonstrated by Délios et al. [10], who showed that T cell clones reacting with H. pylori proteins could be established from human gastric biopsies. Furthermore, studies by our group and others have demonstrated increased levels of IFN-γ mRNA as well as increased frequencies of IFN-γ-containing cells in H. pylori-infected human stomach mucosa [9,39,40].
In conclusion, our results show that several different purified H. pylori antigens induce production of IFN-γ, preferentially by CD8+ cells. Therefore, they suggest that IFN-γ-secreting CD8+ cells contribute significantly to the cytokine response induced by H. pylori infection.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grant 16X-013055) and by AstraZeneca Research Center, Boston, MA. We thank all volunteers who participated in this study. We are grateful to Dr Annika Hamlet for valuable help with recruitment of volunteers. The skilful technical assistance of Ingela Ahlstedt, Mikael Innocenti and Kristina Retteli, as well as the help from the staff at the Gastroenterology Unit at Sahlgrenska University Hospital, is gratefully acknowledged.
REFERENCES
- 1.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–71. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Blaser MJ. Pathophysiology of Helicobacter pylori induced gastritis and peptic ulcer disease. Am J Med. 1997;102:200–7. doi: 10.1016/s0002-9343(96)00273-2. [DOI] [PubMed] [Google Scholar]
- 3.Telford JL, Covacci A, Rappuoli R, et al. Immunobiology of Helicobacter pylori infection. Curr Opin Immunol. 1997;9:498–503. doi: 10.1016/s0952-7915(97)80101-x. [DOI] [PubMed] [Google Scholar]
- 4.Harris PR, Mobley HLT, Perez-Perez GI, et al. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–25. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi M, Czinn S, Redline R, et al. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–38. [PubMed] [Google Scholar]
- 6.Sakagami T, Dixon M, O'rourke J, et al. Atrophic gastric changes in both H. felis and H. pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–48. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton KA, Ringler SR, Danon SJ. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonisation in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 9.D'elios MM, Manghetti M, De Carli M, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 10.D'elios MM, Manghetti M, Almerigogna F, et al. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–5. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 11.Abbiati C, Meucci G, Vecchi M, et al. Gastric mucosal levels of cytokines according to Helicobacter pylori infection and presence of duodenal ulcer. Gut. 1997;41(Suppl. 1):A27. [Google Scholar]
- 12.Mohammadi M, Nedrud J, Redline R, et al. Murine CD4 T-cell response to Helicobacter infection: Th1 cells enhance gastritis and Th2 cells reduce bacterial load. Gastroenterol. 1997;134:1848–57. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 13.Hamlet AK, Erlandsson KIM, Olbe L, et al. A simple, rapid and highly reliable capsule-based 14C urea breath test for diagnosis of H. pylori infection. Scand J Gastroenterol. 1995;30:1058–63. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 14.Lindholm C, Osek J, Svennerholm A-M. Quantification of conserved antigens in Helicobacter pylori during different culture conditions. Infect Immun. 1997;65:5376–80. doi: 10.1128/iai.65.12.5376-5380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bölin I, Lönroth H, Svennerholm A-M. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface exposed protein. J Clin Microbiol. 1995;33:381–4. doi: 10.1128/jcm.33.2.381-384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson A, Quiding-Järbrink M, Lönroth H, et al. Antibody-secreting cells in the stomach of symptomatic and asymptomatic Helicobacter pylori infected subjects. Infect Immun. 1998;66:2705–12. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans Dj, Jr, Evans DG, Takemura H, et al. Characterisation of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–20. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomb JF, White O, Kerlavage AR, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–47. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 19.Thoresson A-C, Hamlet A, Çelic J, et al. Differences in surface‐exposed antigen expression between Helicobacter pylori strains isolated from duodenal ulcer patients and from asymptomatic subjects. J Clin Microbiol. 2000;38:3436–41. doi: 10.1128/jcm.38.9.3436-3441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto T, Ede'n S, Nordenstam G, et al. Endotoxin levels in sera of elderly individuals. Clin Diagn Lab Immunol. 1994;1:684–8. doi: 10.1128/cdli.1.6.684-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson A, Lönroth H, Quiding-Järbrink M, et al. Induction of B cell responses in the stomach of Helicobacter pylori infected subjects after oral cholera vaccination. J Clin Invest. 1998;102:51–56. doi: 10.1172/JCI22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxon A, Feldhaus J, Robins RA. Single step separation of human B and T cells using AET treated SRBC rosettes. J Immunol Methods. 1976;12:285–8. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- 23.Nedrud JG, Czinn SJ. Helicobacter pylori. Curr Opin Gastroenterol. 1997;13:71–78. [Google Scholar]
- 24.Fan XJ, Chua A, Shahi CN, et al. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–84. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatz RA, Meimarakis G, Bayerdörffer E, et al. Characterisation of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1996;31:222–8. doi: 10.3109/00365529609004870. [DOI] [PubMed] [Google Scholar]
- 26.Bamford KB, Fan XJ, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori infection have a T helper cell 1 phenotype. Gastroenterol. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 27.Croft M, Carter L, Swain SL, et al. Generation of polarised antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 29.Kovacsovic-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 30.Lefrancois L, Altman JD, Williams K, et al. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–32. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 31.Ciupitu A-MT, Petersson M, O'donnel CL, et al. Immunisation with a lymphocyte choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685–91. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 33.Eschweiler B, Bohrmann B, Gerstenecker B, et al. In situ localisation of the 60 k protein of Helicobacter pylori, which belongs to the family of heat-shock proteins, by immuno-electron microscopy. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;280:73–85. doi: 10.1016/s0934-8840(11)80942-4. [DOI] [PubMed] [Google Scholar]
- 34.Karttunen R. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin Exp Immunol. 1991;83:396–400. doi: 10.1111/j.1365-2249.1991.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma SA, Miller GG, Perez-Perez GI, et al. Humoral and cellular immune recognition of Helicobacter pylori proteins are not concordant. Clin Exp Immunol. 1994;97:126–32. doi: 10.1111/j.1365-2249.1994.tb06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knipp U, Birkholz S, Kaup W, et al. Partial characterisation of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect Immun. 1996;64:3491–6. doi: 10.1128/iai.64.9.3491-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crabtree JE, Taylor JD, Wyatt JI, et al. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–5. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 38.Khoo UY, Proctor IE, Macpherson AJS. CD4+ T cell down-regulation in human intestinal mucosa. Evidence for intestinal tolerance to luminal bacterial antigens. J Immunol. 1997;158:3626–34. [PubMed] [Google Scholar]
- 39.Karttunen R, Karttunen T, Ekre H-PT, et al. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindholm C, Quiding-Järbrink M, Lönroth H, et al. Local cytokine response in Helicobacter pylori infected subjects. Infect Immun. 1988;66:5964–71. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]