Abstract
Chronic active gastritis of the antral mucosa is a characteristic feature of infection with Helicobacter pylori and interactions between bacterial components and inflammatory cells are believed to play an important pathogenic role. Neutrophils stimulated with H. pylori sonicate were demonstrated to release l-selectin (CD62L) expressed on the cellular surface, with a subsequent up-regulation of the β2-integrins CD11b and CD11c, both in a dose- and time-dependent manner, reaching maximum levels after 45–60 min of stimulation. No changes were observed for the CD11a receptor upon stimulation. The activating properties of H. pylori sonicates on neutrophils were heat-labile and susceptible to protease attack, indicating the protein nature of the activating factor. After size fractionation, the major neutrophil-inducing activity was detected in the high molecular weight fraction exhibiting urease activity. Pertussis toxin was unable to inhibit neutrophil activation by the H. pylori protein(s). We conclude that proteins from H. pylori have a potent inflammatory effect on the surface membrane molecules CD62L, CD11b and CD11c essential for transendothelial migration of neutrophils to areas of inflammation. The neutrophil-activating protein(s) act via a pertussis toxin-insensitive mechanism.
Keywords: neutrophils, l-selectin, β2-integrins, Helicobacter pylori
Introduction
A prominent feature of Helicobacter pylori infection of the antral mucosa is a chronic gastritis that in a minority of subjects leads to duodenal ulceration, gastric cancer and MALT-lymphoma. The diversity in clinical outcome, as well as the inflammatory responses leading to ulcer development, may include both bacterial and host factors: (i) H. pylori components cross the epithelial barriere and attract/activate inflammatory cells [1], (ii) H. pylori components act directly on gastric epithelium and induce increased release of cytokines [2], and (iii) the proinflammatory activity of neutrophils induced by H. pylori is well recognized by demonstration of bacterial components exhibiting chemotactic activity [3] and induction of oxidative burst responses [4]. Although both humoral and cellular responses are prominent, the infection persists.
A characteristic feature of intravascular neutrophils, prior to transendothelial migration, is the surface presentation of the lectin CD62L (l-selectin, LECAM-1, Mel-14), which initiates the rolling contact with activated endothelium, mediated through carbohydrate ligands [5]. The rolling is followed by shedding of CD62L upon firm attachment of the neutrophil to the endothelium via β2-integrins, which have been mobilized from intracellular vesicles and have fused with the cellular membrane [6–8]. For neutrophil activation by inflammatory mediators, the up-regulation of CD11b (Mac-1, CR3) and CD11c (p150,95) is closely coordinated. Both are receptors for the complement fragment, iC3b, and bind the same endothelial ligand, intercellular adhesion molecule-1 (ICAM-1; CD54) [9].
In vitro experiments of neutrophils stimulated with H. pylori sonicate have shown up-regulation of the β2-integrin CD11b on the cellular membrane [10,11], whereas CD11c appeared to remain unchanged upon activation [11]. By use of in vivo blocking MoAbs towards neutrophil adherence molecules, a reduced adherence of neutrophils to endothelium was demonstrated for both CD11b and CD11a (LFA-1) with no up-regulation for the latter marker [10]. No changes in the number of CD62L expressed on the cellular membrane could be demonstrated for neutrophils stimulated with H. pylori sonicate [11].
Neutrophil activation by bacterial components often involves trimeric guanine nucleotide-binding proteins (G-proteins) in the cellular membrane, of which the N-formyl-methyl-leucyl-phenylalanine (fMLP) receptor has been most intensely investigated. The toxin from Bordetella pertussis is an enzyme, which binds to a subunit of the G protein and thereby inhibits further intracellular signalling. The mechanism of action of H. pylori components in neutrophil activation is unknown, but an increasing body of evidence points to the protein nature of the neutrophil inducing components of H. pylori [1,11,12]. However, the importance of this finding in the pathogenesis of ulcer development remains to be elucidated.
The aim of the present study was to examine the dynamics of neutrophil proinflammatory markers CD62L and the β2-integrins induced by H. pylori sonicates. The component(s) from the water-soluble extracts of H. pylori mediating neutrophil responses were characterized, including the sensitivity of neutrophil activation to pertussis toxin (PT)-inhibition of membrane G-proteins.
Materials and methods
Monoclonal antibodies and reagents
The MoAbs used for analysis in fluorescence-activated cell sorter were FITC-labelled anti-CD11a and PE-labelled anti-CD11b (Serotec, Oxford, UK), FITC-labelled anti-CD11c and anti-CD62L, and PE-labelled anti-CD14 (Dako, Glostrup, Denmark). The synthetic oligopeptide fMLP, and phorbol myristate acetate (PMA) were from Sigma Chemical Co. (St Louis, MO), while PT (B. pertussis) and Fura-2/AM were obtained from Calbiochem (Bad-Soden, Germany).
Preparation of H. pylori sonicate
Two strains were used: a clinical isolate from a patient with recurrence of an antral ulcer, and the reference strain NCTC 11638. The bacteria were grown under microaerobic conditions on chocolate agar plates (Statens Seruminstitut, Copenhagen, Denmark) for 72 h. The plates were harvested into sterile water, under sterile conditions. The organisms were identified as H. pylori by Gram staining, colony morphology and positive oxidase, catalase and urease reactions.
The bacteria were sonicated on ice using a Labtronic 1510 (B. Braun, Ebersheim, Switzerland) (400 W, three times 45 s, 20 000 Hz). The sonicate was centrifuged at 44 000 g for 1 h at 4°C, and the supernatant was filtered through a 0·22-μm Millipore filter (Sterivex GS, Hedehusene, Denmark). The sonicate was stored in small aliquots at −20°C until use.
Determination of protein concentration
Protein concentration of the H. pylori sonicate was quantified using the BCA Protein Assay reagent kit (Pierce, Rockford, IL). The protocol from the manufacturer was modified for analysis with a Cobas Mira analyser (Roche, Basel, Switzerland) [13].
Fractionation of H. pylori sonicate
The sonicate preparations were fractionated according to size using HiPrep 16/60 Sephacryl S-200 H (Pharmacia Biotech, Uppsala, Sweden). The fraction size was 2 ml and the protein concentration was measured as optical density (OD)280. Each fraction was tested for urease activity by direct reaction with urea (10 g/l), using phenol red as indicator (Statens Seruminstitut). The tests and negative controls were performed overnight to ensure detection of even minor activity.
Fluorescence flow cytometry
Heparinized whole blood from healthy H. pylori-seronegative volunteers was used for the time–response and dose–response investigations. Examination of the protein fractions was performed in duplicate on heparinized whole blood samples and plasma-free cell suspensions, run in parallel. Bacterial sonicate at final concentrations of 1, 10, 100 and 200 μg/ml was incubated with the whole blood or plasma-free cell suspensions for 30 min at 37°C for dose–response study and for examination of the protein fractions. fMLP (10−8 m) and PMA (10 ng/ml) were used as controls. For time–response investigations the clinical strain was used at concentrations of 10 and 100 μg/ml, while NCTC 11638 was tested at a concentration of 100 μg/ml. After stimulation of the cells, fluorescent MoAbs were added and the samples were kept in the dark at 4°C for 30 min. The erythrocytes were lysed (using Becton Dickinson Lysing solution with 2·7% formalin; Becton Dickinson, San Jose, CA), and the leucocytes were washed and fixed. The analysis was performed using a FACScan fluorescence flow cytometer (Becton Dickinson). Mean fluorescence intensity (MFI) was corrected by substracting values of unstimulated control cells. A forward/side light scatter dot plot was used to create a region identifying neutrophils. To exclude monocytes from the region in the analysis, the region was corrected using stimulated and unstimulated samples marked with anti-CD14. The expression of CD11b and CD62L was analysed in histograms derived from this region containing > 95% neutrophils.
Plasma-free cell preparations
Neutrophils from H. pylori-seronegative donors were isolated on Ficoll–Hypaque (1·093 g/cm3) from EDTA–blood in order to exclude the influence of plasma factors in the induction of neutrophil β2-integrins and l-selectin. The cells were washed twice and resuspended in RPMI 1640 at 1 × 107 cells/ml, as assessed by microscopy using Wright's stain. The viability was always > 95% by trypan blue exclusion. The assays were otherwise performed as above.
Pertussis toxin treatment
For the measurement of intracellular calcium [Ca2+]i, neutrophils were separated by gradient centrifugation in Ficoll–Hypaque (1·093 g/cm3), washed once and resuspended in saline buffer as described [14]. The cells were adjusted to a final concentration of 1 × 107 cells/ml. PT was added at a final concentration of 1 μg/ml, and the cells were incubated in a waterbath at 37°C. Fura2/AM (2 mg/ml) was added for the last 20 min of incubation. The cells were washed once and resuspended in buffer, and kept at room temperature until use. An untreated cell suspension was handled in parallel.
Chemiluminescence
The measurement of toxic oxygen radicals (TOR) was performed in a 96-well microtitre plate chemiluminescence system with luminol enhancement [15], using a LUMIstar makro luminometer (BMG Lab Technologies, Offenburg, Germany). Each 250-μl well was 1 × 105 neutrophils, 71 μm luminol, H. pylori sonicate (100 μg/ml), fMLP 10−7 m, PMA 10 ng/ml or unstimulated control. The samples were maintained at 37°C. Each analysis was performed in parallel for PT-treated and untreated cells by use of continuous time-resolved chemiluminescence.
Fluorimetry
The fluorescence measurements were performed in a dual detector channel combined steady-state and time-resolved spectrometer (Photon Technologies Int., NJ). The steady state constellation was used for the present experiments. The temperature was maintaned at 37 ± 0·5°C, using a thermostated waterbath. The excitation wavlength was set at 380 nm and the fluorescence emission was measured at 490 nm [14,16].
SDS–PAGE analysis
SDS–Page was performed with 7·5% and 18% Tris-trisine gels using a Mini-PROTEAN II electrophoresis cell (BioRad, Richmond, CA). Each lane consisted of 20 μl of each fraction (approximately 50–100 μg protein in each sample) and unfractioned H. pylori sonicate proteins, plus 40 μl buffered tricene denatured for 5 min at 95°C. The electrophoresis was performed at 130 V, and the gels was fixed in methanol for 30 min. The gels were stained by coomassie blue G-250 (0·025%) solution.
Results
Stimulation of neutrophil adherence molecules
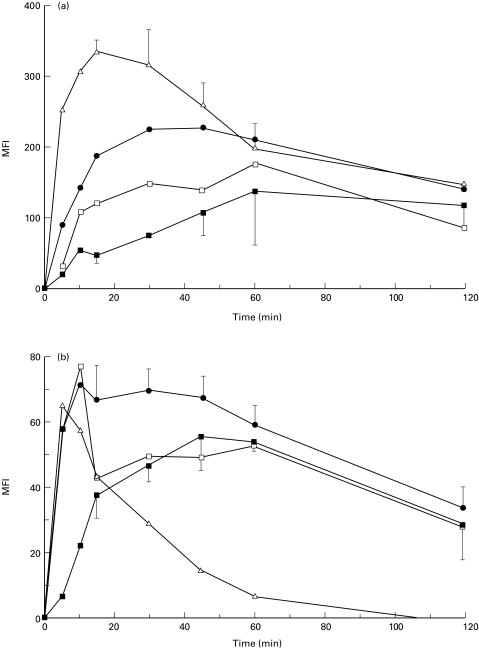
A strong and reproducible up-regulation of adherence molecules on neutrophils was observed with both H. pylori strains. A clear dose-dependent up-regulation of CD11b and CD11c was demonstrated (data not shown), with CD11b displaying the most pronounced change. Kinetic experiments revealed that maximal up-regulation of CD11b was obtained after 60 min (Fig. 1a) and of CD11c after 45–60 min (Fig. 1b). Stimulation with fMLP showed similar kinetics and magnitudes of β2-integrin up-regulation on neutrophils to those obtained with H. pylori sonicate (Fig. 1a,b).
Fig. 1.
Kinetics of up-regulation of CD11b (a), CD11c (b) and shedding of CD62L (c) on neutrophils stimulated with Helicobacter pylori sonicate. The results are expressed as mean fluorescence intensity (MFI) after substracting the values for unstimulated control cells at each time point ± s.d. (a,b) or as a ratio of MFI of stimulated cells/unstimulated cells at each time point (c). The results represents mean of four experiments with neutrophils from H. pylori-seronegative donors. ▪, 28i6 at 100 μg/ml; □, NCTC 11638 at 100 μg/ml; •, fMLP 10−8 mol/l; Δ, PMA 10 ng/ml.
We also observed a dose-dependent shedding of CD62L from neutrophils (Table 1). Kinetic studies showed significant reduction of CD62L after 15 min of incubation and nearly complete shedding after 60 min of incubation (Fig. 1c). CD11a values remained unchanged upon stimulation with either fMLP or H. pylori sonicate (data not shown). Similar experiments were performed with a plasma-free cell suspension, which demonstrated higher values for unstimulated control cells and, consequently, a lower ratio of up-regulation of adherence molecules. Otherwise, an identical pattern was observed (data not shown).
Table 1.
Comparison of potency of different inducers of up-regulation of neutrophil adherence molecules
| Stimulus and concentrations | CD11b MFI | CD11c MFI | CD62L ratio |
|---|---|---|---|
| Reference strain NCTC 11638 | 179 ± 63 | 53 ± 2 | 0·13 |
| 100 μg/ml | (60) | (60) | (60) |
| Clinical strain 28i6 | 125 ± 59 | 43 ± 10 | – |
| 10 μg/ml | (60) | (60) | |
| Clinical strain 28i6 | 139 ± 62 | 55 ± 9 | 0·21 |
| 100μg/ml | (60) | (45) | (60) |
| PMA 10 ng/ml | 316 ± 16 | 43 ± 4 | 0·09 |
| (15) | (15) | (5) | |
| fMLP 10−8m | 228 ± 54 | 30 ± 7 | 0·17 |
| (45) | (30) | (30) |
The results are expressed as the mean fluorescence intensity (MFI) ± s.d. after substracting the values for unstimulated control cells for CD11b and CD11c, or as a ratio of the signals for stimulated and unstimulated control cells for CD62L. The time taken to reach maximum (or minimum) values, in minutes, is given in parentheses. The results are derived as the mean of three experiments with neutrophils from H. pylori-seronegative donors.
Characterization of the neutrophil stimulatory activity of H. pylori sonicate
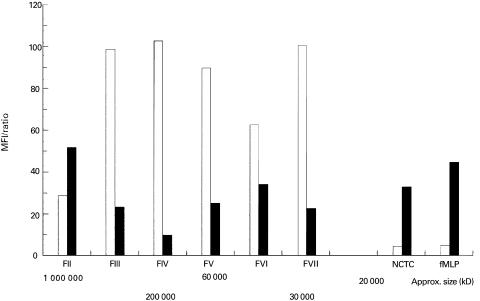
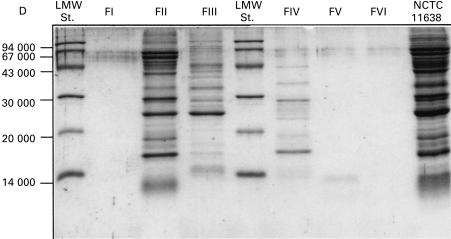
The up-regulation of β2-integrins by H. pylori sonicate was not diniminshed by dialysis, but was impaired by heating the sonicate at 56°C for 30 min (Table 2). Furthermore, the activity was almost completely abolished by heat treatment for 10 min at 100°C and by treatment with pronase E, indicating the protein nature of the activitor. Moreover, markedly reduced activity was observed after treatment of H. pylori sonicate with proteinase K (data not shown). Preliminary size fractionation of sonicate proteins from both H. pylori strains revealed that the majority of the activity resided in the large molecular fractions II + III (molecular size > 200 kD) (Fig. 2). Fraction II had several protein bands on SDS–PAGE dominated by 64, 62, 41, 29, 25 and 16-kD proteins, whereas fraction III had a major band of 25 kD (Fig. 3). The activity in fraction VI and VII was very week, and we were unable to demonstrate the proteins in SDS–PAGE. The fractions containing smaller molecules (< 30 kD) were repeatedly negative for activity, indicating either minimal activity of H. pylori fMLP or negligible concentrations. An important feature of fraction II was a very strong urease activity, whereas fraction III had a weaker urease reaction. All other fractions were urease-negative. It was consistently observed that fractions with activity induced shedding of CD62L and up-regulation of CD11b and CD11c in parallel, suggesting that a common component and mechanism of activation were responsible for changes of all three markers of relevance to neutrophil transendothelial migration.
Table 2.
Characterization of the activation of EDTA-separated neutrophils stimulated with various physically treated Helicobacter pylori sonicates
| Physical treatment | CD11b MFI | CD62L ratio |
|---|---|---|
| Untreated sonicate | 181 ± 47 | 0·17 ± 0·19 |
| Dialysed sonicate | 165 ± 54 | 0·13 ± 0·17 |
| 56°C for 30 min | 8 ± 34 | 0·99 ± 0·16 |
| 100°C for 10 min | 3 ± 14 | 1·05 ± 0·04 |
| 100°C for 10 min + dialysis | 2 ± 7 | 1·00 ± 0·04 |
| fMLP 10−8m | 283 ± 8 | 0·03 ± 0·08 |
All preparations were employed at a protein concentration of 100 μg/ml. The results for CD11b are expressed as mean fluorescence intensity (MFI) ± s.d., after substracting the values for unstimulated control cells, while the change in CD62L is expressed as a ratio ± s.d. of the signals for stimulated and unstimulated control cells, run in parallel. The results shown are derived from five experiments with donor phagocytes from H. pylori-seronegative volunteers.
Fig. 2.
Up-regulation of adherence molecules on neutrophils stimulated with Helicobacter pylori sonicate fractions at a concentration of 100 μg/ml for 30 min. The results for up-regulation of CD11b are expressed as mean fluorescence intensity (MFI) after substracting the values for unstimulated control cells, whereas results for shedding of CD62L are expressed as a ratio of the MFI of stimulated cells/unstimulated control cells multiplied by 100. The results represent mean of three experiments performed in duplicate with sonicate fractions from two separate column preparations. □, CD62L; ▪, CD11b.
Fig. 3.
SDS–PAGE in an 18% Tris-trisine gel with crude sonicate from Helicobacter pylori NCTC 11638 and with fractions I–VI obtained after separation on a Sephacryl S-200 H column. Molecular weight standards are shown on the left lane.
Effects upon G-proteins
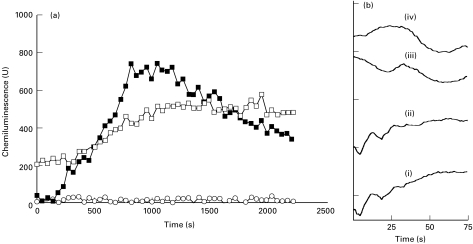
After neutrophil treatment with PT a complete abrogation of the neutrophil chemiluminescence response to fMLP was observed (Fig. 4a). The response to phorbol ester (PMA) was slightly diminished (15–20%) and the peak activities were delayed by 100–150 s (data not shown). Helicobacter pylori sonicate-induced release of TOR was insensitive to PT treatment of the neutrophils (Fig. 4a). The release of [Ca2+]i was measured in parallel. The differences between neutrophils treated with PT and untreated control cells showed increasing numerical values, indicating time-based release of [Ca2+]i in both the PT-treated and untreated cell populations (data not shown). The role of G-protein-dependent signalling measured by calcium release is assessed from the initial 60–90 s of the reaction. Using fMLP activation of the EDTA-separated neutrophils, the results confirmed a highly reduced release of [Ca2+]i in the PT-treated cells (Fig. 4biii) compared with the PT-untreated cells. Neutrophils activated by H. pylori sonicate proteins showed an almost unaltered initial release of [Ca2+]i PT-treated neutrophils (iv) compared with the activation of the PT-untreated cells (ii) (Fig. 4B).
Fig. 4.
(a) Time-resolved chemiluminescence of neutrophils induced by sonicate proteins from Helicobacter pylori strain NCTC 11638 at 100 μg/ml. ▪, Control cells; □, pertussis toxin (PT)-treated neutrophils; ○, fMLP 10−8 mol/l PT-treated neutrophils. Representative of four experiments. (b) Time-resolved fluorimetry emission measured at 490 nm. The curves represent changes for stimulated neutrophils after substracting values of unstimulated control cells, measured in arbitrary units. (i) fMLP 10−8 mol/l PT-untreated cells; (ii) H. pylori strain NCTC 11638 at 100 μg/ml PT-untreated cells; (iii) fMLP 10−8 mol/l PT-treated cells; (iv) H. pylori strain NCTC 11638 at 100 μg/ml PT-treated cells. Representative of four experiments.
Discussion
Clinical and experimental observations support the view that H. pylori attracts inflammatory cells to the site of infection with subsequent tissue damage, and that eradication treatment, which eliminates H. pylori from the stomach, resolves gastritis and cures duodenal ulcer lesions [17,18]. An understanding of the exact mechanism(s) by which H. pylori induces a chronic inflammatory response, and its resultant gastric mucosal injury, should lead to improved approaches to preventing and curing H. pylori gastritis.
Although rarely showing signs of mucosal invasiveness [19], H. pylori infection of the antral mucosa is followed by a strong humoral and cellular immune response in the host. It is believed that soluble factors released from the bacteria enter the lamina propria [1], and inflammatory responses ensue. A prerequisite for transendothelial migration of circulating resting neutrophils into the site of inflammation is the transformation into activated adhesive neutrophils, and relevant changes of the major β2-integrin CD11b have been reported after stimulation with H. pylori components [10,11,20]. The strong CD11b up-regulation by bacterial components underlines a crucial function of CD11b in bacterial infections. As a receptor for iC3b, it is involved in complement-dependent phagocytosis and lysis of bacteria, as major mechanisms for eradicating bacteria. CD11c seems to have similar functions. Thus, it is not surprising that we found CD11c on neutrophils induced by H. pylori sonicate as well. In an assay closely related to ours, Enders et al. [11] were not able to induce CD11c up-regulation by H. pylori extracts, but minor differences in concentrations, strains or experimental conditions could explain the lack of agreement. The change in CD11b expression was higher than that of CD11c, which may reflect the relative importance of the two membrane structures to the migration of the neutrophils across the endothelium [10].
The interaction of CD11b on neutrophils with its counter-receptor CD54 on the endothelial cell is responsible for the firm sticking of neutrophils to the endothelium [21]. CD54 is constitutively expressed on blood vessel endothelium in stomach biopsy samples, and in H. pylori-associated gastritis CD54 expression is significantly correlating with the increase in intensity of mucosal inflammation [22]. Transendothelial migration is a highly regulated process, and the separate events are often induced by the same active components. Consequently, CD11b up-regulation without CD62L shedding by bacterial products has not been reported, except by Enders et al. [11] with H. pylori extracts. We found a nearly complete shedding of CD62L from neutrophils comparable to the activity of other well-described factors (fMLP, PMA). Moreover, we observed that shedding of CD62L and up-regulation of CD11b and CD11c were obtained in the same fractions of bacterial sonicate, which is more in agreement with the general role of bacterial components in neutrophil adherence. Although the kinetics of CD62L reduction was not identical for our clinical strain and the reference strain, NCTC 11638, both preparations induced maximal and comparable responses after 60 min.
The nature of inflammatory activity induced by H. pylori has been suggested to be heterogeneous, including both the direct actions of bacterial components and indirect stimulation by inducing the release of epithelial or macrophage-derived inflammatory mediators (e.g. tumour necrosis factor-alpha (TNF-α), IL-6, IL-8 and others) [23,24]. The main effects measured in our assays were the inducing activities of H. pylori sonicate protein(s), as no major differences were observed between whole blood and separated cell preparations. Proinflammatory effects of certain extracts of H. pylori can be partially attributed to H. pylori-derived urease, as observed by direct immunohistochemistry [1], recombinant protein [25] or urease-deficient mutants [26]. In contrast, Yoshida et al. [10] observed very weak activity of urease in a model of neutrophil adherence to human umbilical vein endothelial cells. In our system the strongest activities were obtained in the two fractions with urease positivity, which makes H. pylori urease one of the possible components responsible for neutrophil proinflammatory activation.
Lipopolysaccharides have been suggested to stimulate neutrophils [27], but generally H. pylori lipopolysaccharides display weak activation of human neutrophils [28]. In the present study, the data obtained were not consistent with lipopolysaccharides having any role in neutrophil adherence activation. Evans et al. [20] described a neutrophil-activating factor of 150 kD composed of identical subunits, which was obtained, upon column fractionation, as aggregates in high molecular weight fractions as well as in low molecular weight fractions, as single subunits of 15 kD. The nature of neutrophil stimulation by this neutrophil-activating protein has not been elucidated. Our fraction II with multiple bands on SDS–PAGE also had a strong protein band at approx. 16 kD, whereas fraction III had only one major band of approx. 25 kD, which is not different from our previous report of a chemotactic protein from H. pylori [3].
The molecular mechanism of action for neutrophil activation by H. pylori has not previously been described. Trimeric guanine nucleotide-binding proteins (G-proteins) relay signals from several membrane receptors, the fMLP receptor being most intensely investigated [29]. Engaging the receptor leads to activation of different intracellular effectors. The PT from B. pertussis is an enzyme, which binds to a subunit of the G-protein and thereby inhibits further intracellular signalling [29,30]. As expected, we observed a complete inhibition by PT of fMLP-induced neutrophil activation in both systems examined, whereas no inhibition of H. pylori sonicate activation could be observed. This is consistent with a membrane receptor mechanism unrelated to G-proteins and different from that of the fMLP receptor. The proadhesive property of H. pylori extract in another experimental model was not due to fMLP, as assessed by lack of inhibition by receptor agonist [10], and based on cross-incubation techniques of neutrophils we previously concluded that fMLP-like components were not part of the activity in H. pylori sonicate [31]. Our novel information with the use of PT supports the idea that the active protein(s) from H. pylori is not using a classical G-protein-mediated cellular activation.
In conclusion, our results indicate strong neutrophil activation by H. pylori components inducing β2-integrin up-regulation and release of CD62L, partly supporting and partly contrasting with previous reports. The major component(s) responsible is of protein nature, and the size was estimated to be > 200 000 kD by gel filtration, although, upon electrophoretic analysis under reducing conditions, it was < 65 kD. The active fractions were positive for H. pylori urease, which is known to have antigenic properties, but these fractions contain several proteins, which makes other proteins possible candidates too. A novel finding was the lack of inhibitory action of PT on neutrophil activation by bacterial sonicate, suggesting a mechanism of action unrelated to the classical fMLP type through membrane G-proteins. More studies are necessary to characterize the neutrophil membrane receptor for the soluble protein from H. pylori.
Acknowledgments
The expert technical assistance of Anne Elbaek and Barbara Hansen is highly appreciated. The study received grant support from King Chr. X Foundation and County of Northern Jutland.
REFERENCES
- 1.Mai UEH, Perez-Perez GI, Allen JB, et al. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leucocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–25. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieder G, Hatz RA, Moran AP, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–30. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen H, Andersen LP. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992;33:738–42. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen H, Andersen LP. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992;103:1747–53. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence MB, Springer TA. Leucocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 6.Borregaard N, Kjeldsen L, Sengeloev H, Diamond MS, Springer TA, Anderson HC, Kishimoto TK, Bainton DF. Changes in subcellular location and surface expression of l-selectin, alkaline phosphatase and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leuc Biol. 1994;56:80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Sengeloev H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N. Subcellular location and dynamics of Mac-1 in human neutrophils. J Clin Invest. 1993;92:1467–76. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieu P, Arnanout MA. Structural basis and regulation of β2-integrin interactions. In: Ward P, Fantone JC, editors. Lung biology in health and disease. Vol. 89. New York: M. Dekker; 1996. pp. 1–42. [Google Scholar]
- 9.Kishimoto TK, Anderson DC. The role of integrins in inflammation. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York: Raven Press; 1992. pp. 353–406. [Google Scholar]
- 10.Yoshida N, Granger DN, Evans DJ, Jr, Evans DG, Graham DY, Anderson DC, Wolf RE, Kvietys PR. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–40. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 11.Enders G, Brooks W, von Jan N, Bayerdörffer E, Hatz R. Expression of adhesion molecules on human granulocytes after stimulation with Helicobacter pylori membrane proteins: comparison with membrane proteins from other bacteria. Infect Immun. 1995;63:2473–7. doi: 10.1128/iai.63.7.2473-2477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaser MJ. Hypothesis on the pathogenenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–7. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 13.Hill HD, Straka JG. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988;170:291–4. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- 14.Richter J, Andersson R, Edvinsson L, Gullberg U. Calcitonin gene related peptide (CGRP) activates human neutrophils—inhibition by chemotactic peptide antagonist BOC-MLP. Immunology. 1992;77:416–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa H, Suzuki K, Nakaji S, Sugawara K. Analysis and assessment of the capacity of neutrophils to produce reactive oxygen species in a 96-well microplate format using lucigenin- and luminol-dependent chemiluminescence. J Immunol Methods. 1997;210:1–10. doi: 10.1016/s0022-1759(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 16.Abe F, Mitsui M, Karaki H, Endoh M. Calcium compartments in vascular smooth muscle cells as detected by aequorin signal. Br J Pharmacol. 1995;116:3000–4. doi: 10.1111/j.1476-5381.1995.tb15955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss SF, Legons S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–70. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705–8. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 19.Andersen LP, Holck S. Possible evidence of invasiveness of Helicobacter (Campylobacter) pylori. Eur J Clin Microbiol Infect Dis. 1990;9:135–8. doi: 10.1007/BF01963640. [DOI] [PubMed] [Google Scholar]
- 20.Evans DJ, Evans DG, Takemura T, et al. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–20. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann GA, Prescott SM, McIntyre TM. Endothelial cell interaction with granulocytes: tethering and signaling molecules. Immunol Today. 1992;13:93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 22.Hatz RA, Rieder G, Stolte M, Bayerdörffer E, Meimarakis G, Schildberg FW, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908–19. doi: 10.1053/gast.1997.v112.pm9178683. [DOI] [PubMed] [Google Scholar]
- 23.Crowe SE, Alvarez L, Dytoc M, et al. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree JE. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:46–55. [PubMed] [Google Scholar]
- 25.Harris PR, Mobley HLT, Perez-Perez GI, Blaser MJ, Smith PD. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–25. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 26.Makristathis A, Rokita E, Labigne A, Willinger B, Rotter ML, Hirschl AM. Highly significant role of Helicobacter pylori urease in phagocytosis and production of oxygen metabolites by human granulocytes. J Infect Dis. 1998;177:803–6. doi: 10.1086/517814. [DOI] [PubMed] [Google Scholar]
- 27.Craig PM, Territo MC, Karnes WE, Walsh JH. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992;33:1020–3. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen H, Birkholz S, Andersen LP, Moran AP. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J Infect Dis. 1994;170:135–9. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- 29.Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–72. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 30.Farfel Z, Bourne HR, Liri T. The expanding spectrum of G protein diseases. N Eng J Med. 1999;340:1012–20. doi: 10.1056/NEJM199904013401306. [DOI] [PubMed] [Google Scholar]
- 31.Nørgaard A, Nielsen H, Andersen LP. Activation of human phagocytes by Helicobacter pylori. A novel interaction with neutrophils and monocytes distinct from that of N-formylated oligopeptides. Zbl Bakt. 1993;280:86–92. doi: 10.1016/s0934-8840(11)80943-6. [DOI] [PubMed] [Google Scholar]