Abstract
It has been demonstrated that pregnancy induces the immunomodulation of cytokine responses away from the Th1 paradigm and towards the Th2 paradigm. In this study, we examined the expression of CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2) on decidual CD4+ and CD8+ T cells during the early stages of pregnancy. Examination of the cytokine profile revealed that CRTH2 was expressed on CD4+‐type-2 T helper (Th2-type) and CD8+‐type 2 T cytotoxic (Tc2-type) cells. The percentages of CRTH2+ cells in CD3+/CD4+ T cells and CD3+/CD8+ T cells were significantly higher in the decidua than in the peripheral blood. These results indicate the significance of Th2- and Tc2-type cells of the decidua in the maternal immune system, presumably through their production of cytokines which may contribute to the maintenance of pregnancy.
Keywords: Th2, Tc2, pregnancy, CRTH2, uterus
Introduction
CD4+ effector/memory T cells are classified into two subpopulations: one is the T helper 1 (Th1)-type cells, which are involved in cellular immunity through the production of IL-2, interferon-gamma (IFN-γ) and tumour necrosis factor-beta (TNF-β), the other subpopulation consists of Th2-type cells, which synthesize IL-4, IL-5, IL-6, IL-10 and IL-13, inducing antibody production [1]. Recently, it has been reported that CD8+ cytotoxic T (Tc) cells can be divided into Tc1- and Tc2-type cells, which synthesize IL-2, IFN-γ and TNF-β, and IL-4, IL-5, IL-6, IL-10 and IL-13, respectively [2–5]. Immunopathological conditions, such as HIV infection, autoimmune diseases and allergy, exhibit the polarized Th1 and Th2 responses or Tc1 and Tc2 responses that are believed to be closely implicated in the onset and outcome of these diseases [2–5]. In normal physiological conditions, cytokines produced by Th2-type cells are shown to predominate during pregnancy. Therefore Th1-type immune reactions mediated by cytotoxic T cells which might attack the fetus and trophoblasts are speculated to be suppressed during this period [6], and the data supporting this view have accumulated in experiments with mice [7–11]. However, there are limited data concerning this speculation in humans [12–17]. To study fresh ex vivo cytokine production at a single-cell level, flow cytometry for intracellular cytokines was developed [18]. This method has the capacity to rapidly analyse large numbers of cells, although much skill is required to obtain consistent results.
We have recently cloned the human gene encoding a novel cell surface molecule, CRTH2 (chemoattractant receptor-homologous molecule expressed on T cells), that is predominantly expressed on Th2 cells, basophils and eosinophils in vivo [19,20]. CRTH2 is a G protein-coupled receptor closely related to the members of the N-formyl peptide receptor subfamily. Detection of CRTH2 is easier by flow cytometric analysis than that of intracytoplasmic cytokine molecules.
In the present study we adopted the flow cytometric method to detect the expression of CRTH2 on peripheral blood and decidual CD4+ and CD8+ T cells to monitor Th1 and Th2 balance in pregnancy. The findings demonstrate that CRTH2 is expressed not only on Th2-type cells but also on Tc2-type cells. More importantly, CRTH2-expressing CD4+ and CD8+ T cells significantly increased in the decidua at the site of the maternal–fetal interface. Thus cytokines secreted by decidual Th2- and Tc2-type cells may play critical roles in the maintenance of pregnancy by controlling the immune balance.
Subjects and methods
Subjects
Peripheral blood mononuclear cells (PBMC) were colleted from 25 non-pregnant women (age 29·1 ± 6·9 years (mean ± s.d.)) and 27 induced abortion cases in the first trimester (age 27·0 ± 5·6 years, gestational age at sampling 7·2 ± 1·2 weeks).
Peripheral and decidual mononuclear cell preparations
PBMC were isolated by the standard Ficoll–Hypaque method. Decidual samples obtained from 27 induced abortion cases were separated carefully from villi under a stereomicroscope. The decidual mononuclear cells (leucocytes) were purified by the Ficoll–Hypaque method after homogenization and filtration through a 32-μm nylon mesh, as previously reported [21]. Peripheral blood and decidual mononuclear cells were obtained from the same induced abortion cases. Informed consent was obtained from all subjects.
Reagents
The following materials were obtained from Becton Dickinson (San Jose, CA): PE-conjugated MoAbs to CD3, CD56 and IL-4; FITC-conjugated MoAbs to CD4, CD8, CD16, CD20, CD45 and IFN-γ; allophycocyanin (APC)-conjugated MoAb to CD3 and appropriate isotype-matched control immunoglobulin PE-conjugated MoAbs to CD45, IL-5 and IL-13 were purchased from PharMingen (La Jolla, CA). RED670-labelled streptavidin was obtained from Life Technologies (Gaithersburg, MD). MoAb specific for human CRTH2 is described in our reports [19,20].
Flow cytometric analysis
Peripheral and decidual mononuclear cells were suspended in PBS containing 1% fetal calf serum (FCS) and 0·02% sodium azide. After rat serum was added to saturate non-specific binding sites, cells were incubated for 20 min at room temperature with flouorochrome-conjugated MoAbs and biotin-labelled CRTH2/MoAb BM16 (10 μg/ml) [20]. After washing, cells were incubated with RED670-conjugated streptoavidin for an additional 30 min and analysed on a FACSCalibur Cytofluorimeter (Becton Dickinson).
Fractionation of CD4+ or CD8+ cells from PBMC
PBMC were incubated for 20 min with anti-CD4 or CD8 antibody coupled with microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). After washing, CD4+ or CD8+ cells were purified by an MS-positive selection column (Miltenyi).
Staining for intracellular cytokines and surface antigens for flow cytometry
Intracellular cytokines were stained according to the method of Picker et al. [18] with some modifications. Briefly, sorted CD4+ or CD8+ cells were stimulated with phorbol myristate acetate (PMA; 20 ng/ml) and ionomycin (250 ng/ml) in the presence of brefeldin A (10 μg/ml) for 4 h. These mononuclear cells were stained with APC-conjugated CD3 MoAb and biotin-labelled CRTH2/MoAb, washed and then incubated with RED670-conjugated streptavidin for 30 min. Cells were washed and fixed in 4% formaldehyde/PBS at room temperature for 5 min, and then again washed and treated with permealization buffer (Becton Dickinson) at room temperature for 10 min. These fixed and permeabilized mononuclear cells were stained with FITC-labelled anti-IFN-γ MoAb and PE-labelled anti-IL-4, -IL-5 or -IL-13 MoAb. Cells were analysed on a FACSCalibur cytofluorimeter using CellQuest software (Becton Dickinson).
Statistical analysis
The data were analysed by paired t-test and Mann–Whitney U-test. P < 0·05 was considered significant.
Results
CRTH2 expression in fresh peripheral blood and decidual lymphocytes
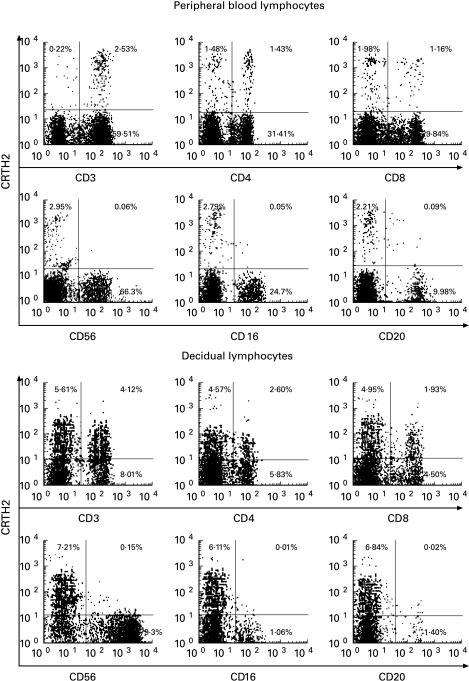
Subsets of lymphocytes in the decidua were different when compared with those in peripheral blood, as shown previously [21,22]. CD3+ T cells markedly decreased and very small numbers of CD16+ natural killer (NK) cells and CD20+ B cells were seen in the decidua, whereas CD16−CD56bright NK cells were dominant (70–80% of total lymphocytes in the decidua during the first trimester of pregnancy). We examined expression levels of CRTH2 in the subsets of decidual lymphocytes with five individual samples.
CRTH2 expression was observed in subsets of CD3+ T cells, CD4+ T cells and CD8+ T cells (Fig. 1), whereas subsets of CD20+ B cells, CD16+ NK cells and CD56bright NK cells did not show significant expression of CRTH2. However, some CD3− cells expressed CRTH2. We already reported that basophils expressed CRTH2 [21,22], so these cells may be basophils. The expression profiles were similar to that of peripheral blood lymphocytes (Fig. 1).
Fig. 1.
CRTH2 expression on various populations of peripheral blood and decidual lymphocytes of pregnant subjects. Peripheral blood and decidual mononuclear cells were preincubated with normal rat serum at room temperature for 20 min, then with a combination of biotinylated CRTH2 MoAb with either fluorochrome-conjugated anti-CD4, CD8, CD16, CD20, CD3 or CD56 MoAb. The cells were incubated for 20 min at room temperature, washed, and incubated with RED670-conjugated streptavidin for an additional 30 min. Lymphocytes gated by side and forward light scatters are analysed.
Cytokine production profile in CRTH2+CD4+ and CRTH2+CD8+ T cells
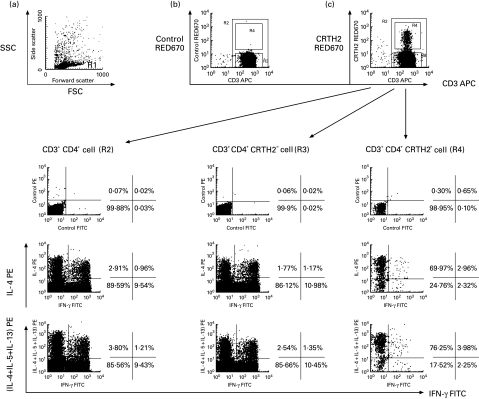
To reveal further subsets of T cells which express CRTH2, CD4+ and CD8+ T cells were characterized by cytokine production profiles. CD4+ T cells and CD8+ T cells were sorted by magnetic separation (purities of CD4+ and CD8+ T cells were 97·8% and 98·1%, respectively) and identified by their characteristic forward and side scatter parameters (Figs 2a and 3a, R1) along with CD3 expression (Figs 2b,c and 3b,c, R2). The cells were then examined for their production of cytokines by staining and flow cytometric analysis. CD4+ T cells were classified into IL-4− IFN-γ+ (Th1)-type cells, IL-4+ IFN-γ+ (Th0) cells and IL-4+ IFN-γ− (Th2)-type cells and others (Fig. 2, lower left). The majority of CD4+CRTH2+ T cells produced IL-4, IL-5 or IL-13, but a few of them were positive for IFN-γ, showing the Th2 phenotype (Fig. 2, lower right). The CD4+CRTH2− T cell population consistently had a distinct cytokine production profile with a slightly higher frequency of Th1-type and markedly lower frequency of Th2-type (Fig. 2, lower middle). These findings demonstrate that CRTH2 is selectively expressed on Th2-type effector cells in vivo.
Fig. 2.
Preferential expression of CRTH2 in Th2 cells in CD4+ peripheral blood lymphocytes. CD4+ cells sorted with magnetic beads were stimulated for 4 h as described in SUBJECTS and METHODS. Cells were then stained with allophycocyanin (APC)-labelled anti-CD3 and biotinylated anti-CRTH MoAb followed by treatment with RED670-conjugated streptavidin. The cells were further fixed, permeabilized and finally stained with FITC-conjugated MoAb to IFN-γ and PE-conjugated MoAb to IL-4, IL-5 and IL-13. The analysis gate was set for lymphocytes by characteristic forward and side scatter parameters ((a) R1). Lymphocyte populations consisting of total CD3+ (R2), CD3+ CRTH2− (R3) and CD3+ CRTH2+ (R4) lymphocytes used as indicated in (c). Control staining of CRTH2 is shown in (b). Isotype-matched fluorochrome-conjugated IgG1 and IgG2a were used as control. The numbers in each quadrant indicate the percentages of respective subpoplations. The expression of intracytokines in CD3+CD4+ T cells (lower left panel), CD3+CD4+CRTH2− T cells (lower middle panel), and CD3+CD4+CRTH2+ T cells (lower right panel) are shown.
Fig. 3.
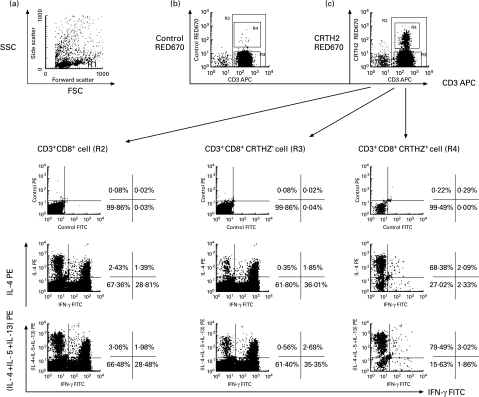
CRTH2 expression in CD8+ subpopulations of peripheral blood lymphocytes. Sorted CD8+ cells were stained and analysed similar to CD4+ cells described in Fig. 2. CD3+ CD8+ T cells (lower left panel), CD3+ CD8+ CRTH2− -T cells (lower middle panel), and CD3+ CD8+ CRTH2+ -T cells (lower right panel) were examined for their production of cytokines.
Similarly, total CD8+ T cells were classified into Tc1-, Tc0-and Tc2-type cells according to expression profiles of IL-4 and IFN-γ (Fig. 3, lower left). Most CRTH2+ cells with CD8+ maker were classified as cells with typical Tc2-type phenotypes because they generated IL-4, IL-5 and IL-13 but not IFN-γ (Fig. 3, lower right). In contrast, the CD8+CRTH2− T cell population consisted of Tc1- and Tc0-type cells, and little Tc2-type cells (Fig. 3, lower middle), demonstrating Tc2-type cells predominantly produce CRTH2 in the CD8+ T cell population in vivo.
Expression of CRTH2 on peripheral blood and decidual CD4+ and CD8+ T cells
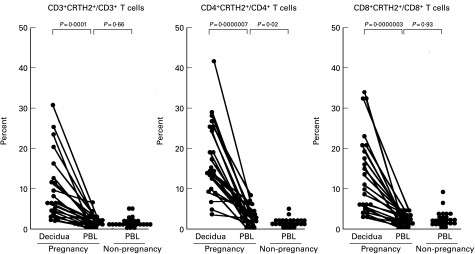
Frequencies of CRTH2-expressing cells in CD3+, CD4+ and CD8+ cell populations were compared between lymphocytes from the decidua and peripheral blood. Decidual and peripheral blood lymphocytes from 27 women at the early stages of pregnancy and peripheral blood lymphocytes from 25 non-pregnant women were analysed in expression of CD3, CD4, CD8 and CRTH2 by flow cytometry.
The percentages of CD3+, CD4+ and CD8+ cells among peripheral blood lymphocytes did not differ significantly between pregnant and non-pregnant women. However, the percentages of CRTH2+ cells in peripheral blood CD4+ cells of pregnant women were significantly higher than those of non-pregnant women (3·75 ± 2·75% versus 2·08 ± 1·06%, P = 0·006), while CRTH2+ cells in peripheral blood CD8+ T cells of pregnant subjects were the same as non-pregnant women (Table 1 and Fig. 4).
Table 1.
Percentage of CRTH2+ T cells in peripheral blood and decidual T cells during early pregnancy
| Early pregnancy (n = 23) | Non-pregnancy (n = 25) | ||
|---|---|---|---|
| Decidua (%) | PBL (%) | PBL (%) | |
| CD3+/lymphocytes | 11·0 ± 8·37 | 73·7 ± 10·6 | 76·7 ± 10·2 |
| CD3+CRTH2+/CD3+ | 10·5 ± 8·54 | 2·08 ± 1·54 | 1·91 ± 1·12 |
| P = 0.0001 P = 0.66 | |||
| CD4+/lymphocytes | 5·0 ± 3·45 | 39·8 ± 9·13 | 45·1 ± 8·91 |
| CD4+CRTH2+/CD4+ | 18·0 ± 9·30 | 3·43 ± 2·44 | 2·08 ± 1·06 |
| P = 0.0000007 P = 0.02 | |||
| CD8+/lymphocytes | 6·70 ± 5·43 | 25·0 ± 6·81 | 24·5 ± 9·51 |
| CD8+CRTH2+/CD8+ | 14·6 ± 9·91 | 2·38 ± 1·62 | 2·43 ± 1·96 |
| P = 0.0000003 P = 0.93 | |||
Data summarized from the results shown in Fig. 4. Probabilities calculation by paired t test and Mann‐Whitney U test are shown.
Fig. 4.
Comparison of CRTH2+ cells of CD3+ T cells, CD4+ T cells, and CD8+ T cells, peripheral blood cells and decidual cells. Lymphocytes from peripheral blood and decidua were stained with a combination of biotinylated anti-CRTH2 MoAb with either FITC-labelled anti-CD3, CD4 or CD8 MoAb, and analysed with a flow cytometer. Dots linked with a line represent samples from the same individual.
In decidual lymphocytes, the percentages of CD3+, CD4+ and CD8+ cells were approximately 1/6, 1/9 and 1/4 of those of the peripheral blood, respectively (Table 1), as expected from bias of lymphocyte contents in the decidua. The percentages of CRTH2-expressing CD3+, CD4+ and CD8+ T cells were seen at a significantly higher frequency in the decidua than in the peripheral blood (Table 1 and Fig. 4).
Discussion
In addition to their characteristics of cytokine production, Th1- and Th2-type cells differentially express a number of molecules such as cytokine receptors, chemokine receptors and adhesion molecules which are considered to play important roles in the development, site-specific recruitment and effector functions of each subset [21–28]. Chemokines play key roles in the leucocyte recruitment processes. Several chemokine receptors have been reported to be selectively expressed on either Th1- or Th2-type cells [29–37]. For example, chemokine receptors CXCR3 and CCR5 were found to be mainly expressed in Th1-type cells, whereas Th2-type cells have been shown to express selectively CCR3, CCR4 and CCR8. However, subset specificity of these molecules in vivo has not been fully established [34–38].
In the present study, in addition to the confirmation of our previous report that CRTH2-expressing CD4+ T cells showed pure Th2-type phenotypes in vivo [19], we interestingly found that CRTH2-expressing CD8+ T cells showed pure Tc2-type phenotypes in vivo. This is the first demonstration of CRTH2 expression in Tc2-type cells. Our results suggest that CRTH2 serves as a surface marker for circulating Th2- and Tc2-type effector populations, because it is expressed in not all but substantial populations of these subsets. CRTH2 may be clinically useful in monitoring the fluctuation of Th2- and Tc2-type cells in circumstances such as atopic dermatitis, HIV infection, cancer and pregnancy.
Since the fetus is semiallogeneic to the mother, considerable modulation of the maternal immune responses is thought to occur in order for pregnancy to be successfully carried to term. Wegman et al. hypothesized that in pregnant women, cytokines produced by Th2-type cells predominate over those produced by Th1-type cells, which contribute to the maintenance of pregnancy [6]. Recently, we reported that IL-4-producing CD3+ T cells increased in early pregnancy decidua, and that the ratio of IFN-γ-producing CD3+ T cells to IL-4-producing CD3+ T cells was lower in the decidua than in the peripheral blood [16]. A decrease in production of leukaemia inhibitory factor (LIF), IL-4 and IL-10 by decidual T cells has been reported in women with unexplained recurrent abortions in comparison with that in women with normal gestation [15]. These observations imply that Th2-type cytokines in the uterus are necessary for maintenance of normal pregnancy during the first trimester. It remains to be clarified, however, which subset, Th2- or Tc2-type cells, increases in the pregnant uterus. We showed here that both CRTH2-expressing CD4+ and CD8+ T cells appeared more frequently in early human pregnancy decidua than in peripheral blood, presumably suggesting that both Th2- and Tc2-type cells increase in early human pregnancy decidua. We already reported that the majority of CRTH2-expressing CD4+ and CD8+ T cells in fresh peripheral blood have phenotypes of CD45RA− and CD45RO+, indicating that CRTH2 is mainly expressed in an activated state of effector/memory T cells [19]. In the decidua, most of the T cells are CD45RA−CD45RO+ effector/memory T cells [39] and express activation marker, CD69 and HLA-DR [21]. These data demonstrate that the T cells in the decidua are sufficiently differentiated to secrete Th2-type cytokines. A confirmation of this idea requires further studies to examine directly the frequencies of decidual Th2- and Tc2-type cells in normal pregnancy and unexplained recurrent abortion cases.
We have also reported that circulating Th1-type cells significantly increased in preeclamptic patients [17]. Monitoring of CRTH2 on circulating CD4+ and CD8+ T cells may be useful in the prediction of preeclampsia, because the serum IL-2 concentration in subjects between 11 and 13 weeks pregnancy who developed preeclampsia after 28 weeks of pregnancy was shown to be significantly higher than those in normal pregnancy subjects [40].
In conclusion, results indicate that CRTH2 may be useful as a marker of Th2- and Tc2-type effector cells in peripheral blood. More importantly, frequencies of CRTH2-expressing CD4 and CD8 T cells significantly increase during early pregnancy decidua at a greater rate than in peripheral blood, suggesting that both Th2- and Tc2-type cells play important roles in the maintenance of pregnancy. Identification of Th2- and Tc2-type cells in local tissues such as inflamed sites, embryo implantation sites, and cancer-invading sites provides critical information on the clinically relevant immune conditions.
REFERENCES
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1994;7:145–7. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Sad S. The expanding universe of T cell subsets—Th1, Th2 and more. Immunol Today. 1996;17:138–4. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–5. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 4.Adkis M, Simon HU, Weigl L, Kreyden O, Blaser K, Adkis CA. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–7. [PubMed] [Google Scholar]
- 5.Clerici M, Shearer GM. A Th1/ Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–1. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 6.Wegmann TG, Lin H, Guilbert L, Mosmann TL. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–7. [PubMed] [Google Scholar]
- 8.Delassus S, Coutinho GC, Saucier C, Darche S, Kourilsky P. Deferential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994;152:2411–2. [PubMed] [Google Scholar]
- 9.Chaouat G, Meliani AA, Martal J, Raghupathy R, Elliot J, Mosmann T, Wegmann T. IL-10 prevents naturally occurring fetal loss in the CBAxDBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is occurred by in vivo injection of IFN-t. J Immunol. 1995;154:4261–8. [PubMed] [Google Scholar]
- 10.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TG, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFNγ responses and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–5. [PubMed] [Google Scholar]
- 11.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. J Immunol. 1996;156:653–6. [PubMed] [Google Scholar]
- 12.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–3. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthiesen L, Ekerfelt C, Berg G, Ernerudh J. Increased numbers of circulating interferon-gamma and interleukin-4-secreting cells during normal pregnancy. Am J Reprod Immunol. 1998;39:362–7. doi: 10.1111/j.1600-0897.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 14.Russell AS, Johnston C, Crew C, Maksymowych WP. Evidence for reduced Th1 function in normal pregnancy: a hypothesis for the remission of rheumatoid arthritis. J Rheumatol. 1997;24:1045–5. [PubMed] [Google Scholar]
- 15.Piccinni M-P, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nature Med. 1998;4:1020–4. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2 and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol. 1999;42:240–5. doi: 10.1111/j.1600-0897.1999.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 17.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picker LJ, Singh MK, Zdraveski S, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1. [PubMed] [Google Scholar]
- 19.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–8. [PubMed] [Google Scholar]
- 20.Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, Nakamura M, Takano S. CRTH2 an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Letters. 1999;459:195–9. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 21.Saito S, Nishikawa K, Mori T, Narita N, Enomoto M, Ichijo M. Expression of activation CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2Rα) and IL-2Rβ on T cells of human decidua at an early stage of pregnancy. Immunology. 1992;75:710–2. [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa K, Saito S, Morii T, et al. Accumulation of CD16−CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol. 1991;3:743–5. doi: 10.1093/intimm/3.8.743. [DOI] [PubMed] [Google Scholar]
- 23.Annunziato F, Manetti R, Tomasevic I, et al. Expression and release of LAG-3-encoded protein by human CD4+ T cells are associated with IFN-gamma production. FASEB J. 1996;10:769–7. doi: 10.1096/fasebj.10.7.8635694. [DOI] [PubMed] [Google Scholar]
- 24.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–8. [PubMed] [Google Scholar]
- 25.Pernis A, Gupta S, Gollob KJ, Garfein E, Coffman RL, Schindler C, Rothman P. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–7. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 26.Austrup F, Vestweber D, Borges E, et al. P- and E-selection mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–8. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 27.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–2. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lnzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–7. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim CH, Broxmeyer HE. Chemokines. Signal lamps for trafficking of T and B cells for development and effector function. J Leuk Biol. 1999;65:6–1. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer J-M. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–8. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 34.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1 s) and Th2s. J Exp Med. 1998;187:129–3. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–8. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 36.Zingoni A, Soto H, Hedrick JA, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–5. [PubMed] [Google Scholar]
- 37.Bernardini G, Hedrick J, Sozzani S, et al. Identification of the CC chemokines TARC and macrophage inflammatory protein-1 beta as novel functional ligands for the CCR8 receptor. Eur J Immunol. 1998;28:582–8. doi: 10.1002/(SICI)1521-4141(199802)28:02<582::AID-IMMU582>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Annunziato F, Cosmi L, Galli G, Beltrame C, Romagnani P, Manetti R, Romagnani S, Maggi E. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leuk Biol. 1999;65:691–9. doi: 10.1002/jlb.65.5.691. [DOI] [PubMed] [Google Scholar]
- 39.Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ito A, Ichijo M. A study of CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol Letters. 1994;40:193–7. doi: 10.1016/0165-2478(93)00019-a. [DOI] [PubMed] [Google Scholar]
- 40.Hamai Y, Fuii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, Taketani Y. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-α levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38:89–9. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]