Abstract
Chronic mucocutaneous candidiasis (CMC) is a rare syndrome characterized by persistent and refractory infections of the skin, nails and mucosal tissues by yeasts of the genus Candida. Defects in the cellular limb of the immune system are well documented in CMC patients, but non-specific immune defects, such as myeloperoxidase deficiency or phagocyte chemotaxis disorders, have also been described. Nonetheless, the underlying defect(s) remains poorly understood, and further studies are required. We studied eight CMC patients without endocrinopathies, who showed (i) low normal proliferative response to phytohaemagglutinin (PHA), (ii) partially defective response to pokeweed mitogen (PWM), and (iii) impaired response to Candida and PPD antigens. Furthermore, peripheral blood mononuclear cells (PBMC) from CMC patients produced lower levels of type-1 cytokines (IL-2 and interferon-gamma) in response to Candida antigens, compared with control individuals. Conversely, we did not observe an enhancement of IL-4 and IL-10 in the patients, suggesting that, even though Th1 cytokines are decreased, the Th2 response is not increased in CMC. Nevertheless, the synthesis of these cytokines was normal when induced by PHA. We also observed an increased antigen-induced apoptosis in lymphocytes from the patients compared with controls, and this applied both to Candida and PPD antigens. Lastly, innate immunity defects were investigated. We observed an impairment of natural killer activity against K-562 target cells in half of the studied patients. These findings corroborate the extensive clinical and laboratory variability of CMC, which requires further studies on a larger number of patients to be better understood.
Keywords: chronic mucocutaneous candidiasis, cellular immunity, cytokines, apoptosis, activation-induced cell death
INTRODUCTION
Chronic mucocutaneous candidiasis (CMC) is a rare and complex disorder, characterized by persistent or recurrent infections of the skin, nails and mucosal tissues by Candida;C. albicans in the majority of the cases [1]. Patients with CMC characteristically do not develop systemic disease and/or septicaemia. The disease is often more severe when presented early in infancy and in patients with disseminated lesions [2–4].
The diversity of clinical features among patients suggests that there are several different disorders still classified under a common clinical denominator. Most reports have shown that the defects are almost exclusively in the cellular branch of the immune system, mainly the specific responses to antigens of Candida species. Some CMC patients present serum factors that inhibit the proliferative responses of peripheral blood mononuclear cells (PBMC) from Candida-sensitized normal subjects [5–9], and a wide spectrum of immune dysfunctions has been observed. The importance of type-1 cytokines in an effective cellular immunity and the requirement of type-2 cytokines in the development of an adequate type-1 response have recently been demonstrated in murine models of candidiasis [10]. Moreover, other recent studies—aimed at clarifying the pathogenic mechanisms of the disease—showed deficient production and secretion of IL-2 by PBMC in response to Candida antigen [11,12].
Therefore, due to the several controversial aspects of CMC, our study proposed to evaluate the proliferative responses and the cytokine synthesis of PBMC after mitogen and antigen stimulation. Furthermore, we also evaluated the lymphocyte apoptosis after non-specific and specific stimulation, and also the natural killer (NK) activity of mononuclear cells
PATIENTS AND METHODS
We studied eight CMC patients (four male and four female) between 3 and 38 years of age, fulfilling clinical criteria for persistent and refractory candidiasis of skin, nails and mucosal tissues. There were six patients with childhood onset, who presented the most severe clinical features, and two female patients with adult onset of the disease. All patients presented oral thrush and nail lesions; five had skin involvement (nos 3, 5, 6, 7 and 8); four oesophageal lesions (nos 1, 2, 3 and 8) and three vulvovaginitis (nos 1, 2 and 3). One patient presented sinusitis by Microsporum canis (no. 8). All patients were submitted to a routine investigation (Table 1). The control group consisted of eight age- and sex-matched healthy individuals. The Ethical Committee of the Hospital das Clínicas da Universidade de São Paulo granted ethical approval, and informed consent of patients or parents was obtained in all cases.
Table 1.
Clinical and general laboratory features of the patients
| Patient no., age/sex | First symptoms | Age at diagnosis | PPD DTH | Tp DTH | Ca DTH | SK/SD DTH | IgA | IgE | IgG | IgM | AutoAb | C′3 | C′4 | Leucoc | Eos | Lymph | CD4+ | CD8+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 38/F | 33 years | 36 years | Neg | 10 | 8 | 25 | 185 | 27 | 1200 | 159 | None | 95 | 21 | 5800 | 87 | 1856 | 945 | 393 |
| 2 35/F | 31 years | 33 years | 8 | Neg | Neg | 15 | 257 | 34 | 1635 | 196 | None | 96 | 16 | 8300 | 232 | 2822 | 1343 | 807 |
| 3 25/F | 2 years | 19 years | Neg | Neg | Neg | Neg | 251 | 48 | 2985 | 162 | dsDNA1/1280 | 97 | 19 | 12 900 | 258 | 1574 | 334 | 283 |
| 4 3/F | 18 months | 20 months | NP | NP | NP | NP | 125 | 15 | 1659 | 205 | None | NP | NP | 14 900 | 241 | 4879 | 1678 | 732 |
| 5 6/M | 7 months | 1 year | Neg | Neg | 14 | NP | 289 | 25 | 1870 | 172 | None | NP | NP | NA | NA | 2500 | 975 | 700 |
| 6 38/M | 2 years | 23 years | Neg | 10 | Neg | Neg | 402 | 31 | 1978 | 87 | None | 100 | 40 | 8900 | 9 | 2027 | 446 | 487 |
| 7 7/M | 2 months | 18 months | Neg | Neg | Neg | Neg | 184 | 17 | 1912 | 115 | TPO-92.9 TG-834 | 90 | 30 | 10 900 | 218 | 2986 | 1075 | 478 |
| 8 17/M | 2 years | 15 years | Neg | Neg | Neg | Neg | 400 | 27 | 3048 | 165 | None | NP | NP | 7500 | 0 | 2810 | 1121 | 556 |
NP, Not performed; NA, not available.
Tp, Trichophytin; Ca, candidin; SK/SD, varidase.
Methods
Sample collection
Fasting blood samples were collected in the morning, from clinically stable patients, without associated infections and without systemic anti-mycotic therapy.
Cell separation and cell cultures
PBMC were cultured in RPMI 1640 medium (Sigma, St Louis, MO, USA) with 10% of human AB + serum (Sigma), and stimulated either by Candida metabolic antigen (CMA; Diagnostics Pasteur, Marnes‐la‐coquette, France, code 52952) (5 μg/ml) or Mycobacterium bovis PPD (Staten Serum Institut, Copenhagen, Denmark) (5 μg/ml) for 6 days, or by phytohaemagglutinin (PHA; 2·5 μg/ml; Gibco BRL, Rockville, MD, USA) or pokeweed mitogen (PWM; 5 μg/ml; Sigma) for 3 and 6 days, respectively. Eighteen hours before harvesting, cells were pulsed with 1 μ Ci of 3H-TdR, then harvested and counted in a Beta Plate scintillation counter (Wallac OY, Turku, Finland). The results are presented as stimulation index (SI). Lymphocytes from patients and controls were tested for immunomodulatory effects of patients' sera by comparing lymphocyte responses in normal AB serum and sera from four CMC patients.
Cytokine quantification
Quantification of cytokines in the supernatant was obtained by stimulation of 2 × 106 cells/ml in RPMI/10% AB serum in 24-well plates with PHA 2·5 μg/ml, Candida antigen 5 μg/ml, and the appropriate controls. PBMC from patients and controls were further tested in the presence of patients' serum to evaluate a possible serum-dependent modulatory effect. The supernatant samples were harvested 24 h after PHA stimulation, and 72 h after Candida stimulation, according to preliminary experiments in which harvesting time was determined (data not shown). The supernatants were stored in aliquots at −70°C until quantification of cytokines. Cytokine quantification was performed by a capture ELISA method using kits from Genzyme Diagnostics (Cambridge, MA, USA) (IL-2) and Endogen Inc (Woburn, MA, USA) (IL-4, IL-10 and interferon-gamma (IFN-γ)), following the manufacturer's instructions.
NK activity assay
NK activity was evaluated by a micromethod, as previously described [13], with some modifications. In brief, K-562 target cells were incubated with 100 μ Ci Na[51Cr]O4 (CNEN, São Paulo, SP, Brazil). The effector cells (PBMC) were maintained in RPMI/10% fetal calf serum (FCS) and adjusted to 4 × 106, 2 × 106, 1 × 106, and 5 × 105 cells/ml. PBMC from four patients and four controls were also tested with patients' serum in order to evaluate a possible serum-dependent suppressive effect on the cytotoxic activity. One hundred microlitres of each sample suspension were plated in triplicates in 96-well round-bottomed microplates; 104 previously cromated target cells were added to each well, in order to get 40:1, 20:1, 10:1 and 5:1 effector/target cell ratios, along with the adequate controls. After 4 h, the plates were gently spun and the supernatant collected for radioactivity counting by a gamma counter (Wallac). The lytic activity was calculated and expressed as the percentage of lysed cells for each effector/target cell ratio.
The apoptosis assay
To study the influence of cellular activation on peripheral blood lymphocyte apoptosis, 1 × 106 cells were cultured in RPMI/10% AB + serum in 24-well plates with either 5 μg/ml Candida antigen or 5 μg/ml PPD, or 2·5 μg/ml PHA. Four patient and control samples were also tested with patients' serum in order to evaluate a possible modulatory effect on apoptosis. PBMC from controls and patients were evaluated for spontaneous apoptosis immediately after blood collection and 72 h after antigen or mitogen stimulation. The cells were isolated and 2·5 μg/ml of anti-CD95 antibody (PharMingen, San Diego, CA, USA) were added to 2 × 106 cells/ml. As negative control cells were incubated with culture medium (without anti-CD95) for the same 4 h at room temperature. Cells were then washed twice with cold PBS and resuspended in binding buffer (10 mm HEPES/NaOH, pH 7·4, 140 mm NaCl and 2·5 mm CaCl2) at 106 cells/ml. An aliquot of 100 μl of cell suspension was transferred to a 5-ml cytometer tube, and 5 μl of Annexin V–FITC (PharMingen) and 10 μl of propidium iodide (PI; Sigma) at 50 μg/ml were added to each tube. Cells were then gently vortexed and incubated in the dark for 15 min at room temperature, followed by the addition of 400 μl of binding buffer for analysis in the flow cytometer. The quantification of apoptotic cells was evaluated by Annexin V–FITC binding [14]. The final results were expressed as the percentage of apoptotic cells in the lymphocyte gate (identified by Annexin V–FITC staining and exclusion of PI) per 10 000 counted cells.
Statistical analysis of the data was done by Mann–Whitney test, when the same parameter was compared for patients and controls. We considered significant values ≤0·05.
RESULTS
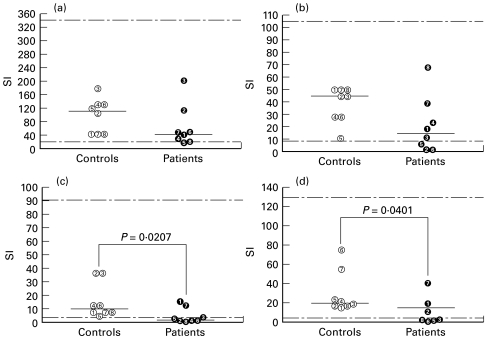
The proliferative lymphocyte responses were analysed after stimulation with PHA, PWM, Candida and PPD antigens (Fig. 1). The majority of the patients (5/8; 62·5%) showed a low proliferative response to Candida antigens (Fig. 1c), but the proliferative response to PPD was also below the lower normal limit (SI < 4·17) in four of seven patients tested (Fig. 1d). These proportions were significantly different in relation to the control group: Candida (P = 0·0207) and PPD (P = 0·0401). The response to PWM was more heterogeneous, as three of them presented low SIs (< 8·42) (Fig. 1b). Nevertheless, all patients presented normal proliferative responses to PHA (> 18·28), despite a trend to lower median SI values (39·78 × 99·94 for patients and controls, respectively) (Fig. 1a).
Fig. 1.
Proliferative response of mononuclear cells from patients and controls under different stimuli. (a) Phytohaemagglutinin (PHA). (b) Pokeweed mitogen (PWM). (c) Candida antigen (CMA). (d) Mycobacterium tuberculosis PPD. Data expressed as levels obtained for each individual and median of stimulation index (SI). The respective normal value limits (percentile 5 and 95) established by the analysis of a normal population studied at LIM/56 are: PHA = 18·28–343·00; PWM = 8·42–107·40; CMA = 3·35–91·40; PPD = 4·17–129·20.
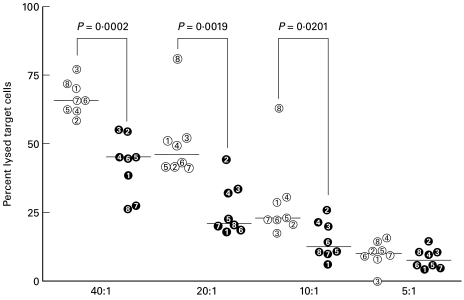
The patients' PBMC showed lower NK activity than those of the controls (Fig. 2). Since NK activity was low, as were T cell responses, it was decided to investigate whether cytokines, known to activate both subsets, were involved in these immunological disturbances.
Fig. 2.
Natural killer activity of peripheral blood mononuclear cells from chronic mucocutaneous candidiasis (CMC) patients (filled symbols) and healthy controls (open symbols). Results presented as the percentage of lysed target cells at different effector:target (E:T) cell ratios for individual patients and median group values.
The analysis of IL-2 production by PBMC of the patients (Fig. 3a) revealed significantly lower levels than those found for the control group; i.e. 4/8 patients and only 1/8 control secreted low levels of IL-2, when stimulated by Candida antigen (P = 0·0281). There was no statistical difference upon PHA stimulus. IFN-γ levels (Fig. 3b) also showed a highly significant difference among patients and controls when stimulated by Candida antigen; i.e. 5/8 patients and none of the controls produced low levels of IFN-γ (P = 0·0047). Conversely, data from IL-4 and IL-10 failed to show any statistical difference among the groups, under any conditions (Fig. 3c,d).
Fig. 3.
Production of IL-2 and IFN-γ in response to stimulation with phytohaemagglutinin (PHA) and Candida antigen (CMA). Peripheral blood mononuclear cells from patients with chronic mucocutaneous candidiasis (P) and controls (C) were stimulated in culture and supernatants assessed for IL-2 (a), IFN-γ (b), IL-4 (c), and IL-10 (d). Results are presented as levels obtained for each individual and median group values. Bg, Background (unstimulated cytokine production).
The analysis of patient and control lymphocyte apoptosis (Fig. 4) under different stimulation conditions showed that the behaviour of peripheral blood lymphocytes was similar in both situations: in basal conditions, stimulated by suboptimal doses of anti-CD95 antibody (Fig. 4a) and when stimulated by PHA, enhanced or not by anti-CD95 antibody (Fig. 4b). However, when cells were stimulated by Candida antigen, there was a trend to higher levels of apoptosis in the patient group (5·75 versus 9·10), that achieved a statistically significant difference when the stimulation was enhanced by anti-CD95 antibody (8·85 versus 19·4) (P = 0·007) (Fig. 4c). The same trend of activation-induced cell death was observed upon PPD stimulation (3·10 versus 7·60), which was also significant when enhanced by anti-CD95 (5·0 versus 16·0) (P = 0·0205) (Fig. 4d).
Fig. 4.
Quantification of lymphocyte apoptosis from patients and controls in response to different stimulation conditions: (a) basal; (b) phytohaemagglutinin (PHA); (c) Candida antigen (CMA); (d) PPD. Results are presented as apoptosis levels obtained for each individual and group median of percent values.
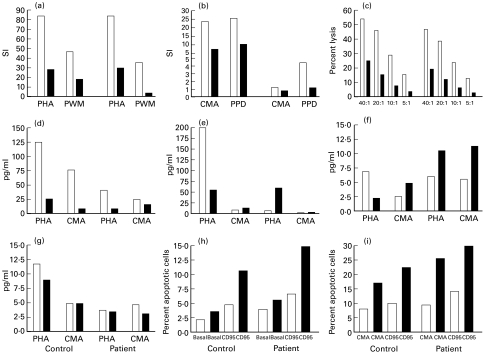
We performed another series of experiments comparing serum from four patients with AB serum; only patient no. 6 serum modulated several functions of the immune response, such as the proliferation of patient and control PBMC under all stimuli (Fig. 5a,b). Patient no. 6 serum suppressed both his NK activity and the control subject's (Fig. 5c). The analysis of cytokine synthesis modulation showed (i) a suppression of IL-2 secretion under PHA and Candida stimuli for control and patient (Fig. 5d), (ii) an enhancement of IL-4 (Fig. 5f) under Candida stimuli for control and patient, and (iii) a suppression of IFN-γ (Fig. 5e) under PHA for control of patient no. 6. There was no influence of patients' sera over IL-10 secretion (Fig. 5g). Modulation of apoptosis occurred only for patient no. 6 under CD95 (Fig. 5h), CMA and CMA-CD95 stimuli (Fig. 5i), in which the patient's serum enhanced the activation-induced cell death.
Fig. 5.
Modulation of control and patient immune functions by chronic mucocutaneous candidiasis (CMC) patients’ sera. (a) Phytohaemagglutinin (PHA) and pokeweed mitogen (PWM)-induced lymphoproliferation. (b) Candida antigen (CMA) and PPD-induced proliferation. (c) Natural killer (NK) cytotoxic activity at different effector:target ratios. (d) IL-2 secretion induced by PHA and CMA. (e) IFN-γ secretion. (f) IL-4 secretion. (g) IL-10 secretion. (h) Basal and CD95-enhanced lymphocyte apoptosis. (i) CMA and CMA-CD95-induced lymphocyte apoptosis. SI, Stimulation index. □, AB serum (fetal calf serum for NK activity assay); ▪, serum from patient no. 6.
DISCUSSION
The inconclusive reports of cellular immune response of CMC patients reflect the clinical and immunological variability of these patients. Some reports describe the heterogeneity of the immunological disturbances, suggesting the existence of several variants (or several defects) giving rise to the various clinical manifestations [1].
Due to the dominant role of the cellular compartment of the immune system in the defence against fungi, several parameters of the immune response of patients with CMC were evaluated. These were mainly the T cell response to Candida antigens, as well as to mitogens, and the NK activity that showed variable disturbances. Furthermore, cytokine secretion and apoptotic activation-induced cell death were also investigated.
Despite the depressed proliferative response from PBMC cultured in medium with AB serum to metabolic products of the yeast present in skin and mucous membranes, some patients presented a wider disturbance of the specific immune response, failing to recognize PPD antigen and even PWM, as observed previously [1,7,15]. However, it is important to stress that the serum of one out of four patients clearly modulated immune reactivity, suppressing the proliferation of his own PBMC and of the control subject.
The NK activity against some fungal infections is important [16–20] and therefore it was evaluated. These reports demonstrated that direct activity, as well as NK-derived IFN-γ, are important in the natural immunity against fungi. The indirect effect—modulated by cytokines—is mainly secondary to nitric oxide (NO) production by macrophages. Indeed, half of the patients showed depressed NK activity, suggesting that some of them could present multiple immunological defects. Moreover, serum from patient no. 6 suppressed the immune activity of NK cells. However, none of the patients has so far presented clinical manifestations that are frequently associated with decreased NK activity, such as neoplasia, herpetic or Cryptococcal infections.
In addition, disturbances in the amplification and effector mechanisms of the immune response, or an inadequate Th2 responsiveness pattern could lead to suppression of cell-mediated processes, maintaining predominantly humoral responses. Therefore, the absence of an efficient cellular immune response in patients with persistent infectious processes by yeasts of the genus Candida might be due to defects in the generation or maintenance of cellular memory response, and this defect could be linked to disturbances in the production of cytokines or antigen-induced apoptotic mechanisms. Cytokine production induced by non-specific stimuli, such as PHA, was preserved as previously reported [15,21].
Conversely, we observed a significant decrease in the production of Th1 cytokines, without concomitant Th2 enhancement, especially after stimulation with C. albicans antigen. Contrary to other reports [11,12], none of our patients presented a typical Th2 pattern with high levels of IgE and eosinophilia. It should be noted that serum from patient no. 6 suppressed both mitogen- and antigen-induced lymphocyte proliferation and the production of Th1-type but not Th2-type cytokines. This serum also enhanced stimulation-induced apoptosis and decreased NK function. It is important to stress that the cytokine disturbance did not relate to the proliferative response, nor to the clinical features of the patients. The fact that some patients secreted adequate concentrations of the Th1 cytokines can be a consequence of the heterogeneity of the disease or, on the other hand, of the clinical status at the time of evaluation. Considering that these defects are not consistently found in CMC patients, they are unlikely to be the only or the main underlying cause of susceptibility to persistent Candida infections.
In a wider context, production of cytokines in CMC may be compared with the fungal disease paracoccidioidomycosis. Our own data on this mycotic disease suggest several points of convergence. In the chronic form of the latter, there are typical mucosal lesions, mainly in the upper respiratory tract. Therefore, the fungal antigens chronically stimulate these patients; yet a decrease in the proliferative response to some Paracoccidioides brasiliensis products—but not to mitogens or to other non-related antigens, e.g. C. albicans antigens—was observed [22,23]. A decreased production of Th1 cytokines (IL-2 and IFN-γ) and preservation of IL-10 synthesis was also observed (G. Benard, personal communication). On the other hand, patients presenting recurrent vulvovaginal candidiasis also showed a decrease in the proliferative response and in IFN-γ synthesis when stimulated by the yeast, and this was more pronounced in the follicular phase [24]. We can conclude that in these chronic mycoses the predominance of a non-protective response might occur, with high titres of antibodies.
It is also possible that apoptosis could favour the selective depletion of the T lymphocytes responsible for the immunological memory to Candida sp., explaining the defective immune response among CMC patients. Our findings support this possibility, as patients' antigen-specific stimulated lymphocytes expressed activation-induced cell death at significantly higher levels than the controls, when enhanced by anti-CD95 MoAb in suboptimal doses. It is important to stress that CD95 expression is higher in memory cells (CD45RO+) [25], influencing memory cell depletion [26]. Our observation of higher levels of apoptotic cell death among patients suggests that some disturbance of cell signalling could be leading to a dissociation of the pathways for the expression of CD95 and its ligand [27,28] and cytokine synthesis [29,30], favouring apoptosis instead of proliferation. In contrast, it is also possible that activation-induced cell death could be due to repeated stimulation by widely disseminated antigens, presented by antigen-presenting cells [31]. A horizontal analysis of the data showed that antigen-specific activation-induced cell death inversely correlated with antigen-specific proliferation and IFN-γ secretion. This finding corroborates the idea that apoptosis is an important phenomenon in the pathogenesis of CMC, at least in our patients. Moreover, serum from patient no. 6 enhanced apoptosis by his own and control lymphocytes. The enhanced antigen-induced apoptosis described here opens a new approach to the understanding of more specific immunodeficiency diseases and chronic relapsing infections.
Acknowledgments
We are grateful for the invaluable statistical assistance of Isac de Castro. We are also grateful to Carolina de Almeida Zomignan for the suppression assays and Drs Gabriela Ribeiro dos Santos and Gil Benard for the critical reading of the paper.
REFERENCES
- 1.Hong R, Clement LT, Gatti RA, Kirkpatrick CH. Disorders of the T-cell system. In: Stiehm ER, editor. Immunologic disorders in infants and children. Philadelphia: Saunders; 1996. pp. 339–408. [Google Scholar]
- 2.Kauffman CA, Shea MJ, Frame PT. Invasive fungal infections in patients with chronic mucocutaneous candidiasis. Arch Int Med. 1981;141:1076–9. [PubMed] [Google Scholar]
- 3.Bodey GP. Candidiasis: pathogenesis, diagnosis and treatment. New York: Raven Press; 1993. [Google Scholar]
- 4.Germain M, Gourdeau M, Hébert J. Case report: familial chronic mucocutaneous candidiasis complicated by deep Candida infection. Am J Med Sci. 1994;307:282–3. doi: 10.1097/00000441-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Paterson P, Semo R, Blumenschein G, Swelstad J. Mucocutaneous candidiasis, anergy and a plasma inhibitor of cellular immunity: reversal with amphotericin B. Clin Exp Immunol. 1971;9:595–602. [PMC free article] [PubMed] [Google Scholar]
- 6.Lehner T, Wilton JMA, Ivanyi L. Immunodeficiencies in chronic mucocutaneous candidiasis. Immunology. 1972;22:775–87. [PMC free article] [PubMed] [Google Scholar]
- 7.Valdimarsson H, Higgs JM, Wells RS, Yamamura M, Hobbs JR, Holt PJL. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973;6:348–61. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- 8.Fischer A, Ballet JJ, Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharide antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978;62:1005–13. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podzorski RP, Gray GR, Nelson RD. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990;144:707–16. [PubMed] [Google Scholar]
- 10.Mencacci A, Del Sero G, Cenci E, Fè d'Ostiani C, Bacci A, Montagnoli C, Kopf M, Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J Exp Med. 1998;187:307–16. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilic D, Cant AJ, Abinun M, Calvert JE, Spickett GP. Chronic mucocutaneous candidiasis. I. Altered antigen-stimulated IL-2, IL-4, IL-6 and interferon-gamma (IFN-γ) production. Clin Exp Immunol. 1996;105:205–12. doi: 10.1046/j.1365-2249.1996.d01-764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobrynski LJ, Tanimune L, Kilpatrick L, Campbell DE, Douglas SD. Production of T-helper cell subsets and cytokines by lymphocytes from patients with chronic mucocutaneous candidiasis. Clin Diagn Lab Immunol. 1996;3:740–5. doi: 10.1128/cdli.3.6.740-745.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillow PJ, Hegarty J, Ramsden C, Davison AM, Will EJ, Giles GR. Changes in human natural killer activity early and late after renal transplantation using conventional immunosuppression. Transplantation. 1982;33:414–20. doi: 10.1097/00007890-198204000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MHJ. Annexin V for flow cytometry detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 15.Kirkpatrick CH. Chronic mucocutaneous candidiasis. J Am Acad Dermatol. 1994;31(3 part 2):S14–S17. doi: 10.1016/s0190-9622(08)81260-1. [DOI] [PubMed] [Google Scholar]
- 16.Levitz SM, Dupont MP, Smail EH. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun. 1994;62:194–202. doi: 10.1128/iai.62.1.194-202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn CA, Washburn RG. Anticryptococcal activity of NK cell-enriched peripheral blood lymphocytes from human immunodeficiency virus-infected subjects: responses to IL-2, IFN-γ and IL-12. J Infect Dis. 1995;172:1023–7. doi: 10.1093/infdis/172.4.1023. [DOI] [PubMed] [Google Scholar]
- 18.Peraçoli MT, Fortes MR, da Silva MF, Montenegro MR. Natural killer cell activity in experimental paracoccidioidomycosis of the Syrian hamster. Rev Inst Med Trop São Paulo. 1995;37:129–36. doi: 10.1590/s0036-46651995000200007. [DOI] [PubMed] [Google Scholar]
- 19.Gulay Z, Imir T. Anti-candidial activity of natural killer (NK) and lymphokine activated killer (LAK) lymphocytes in vitro. Immunobiology. 1996;195:220–30. doi: 10.1016/S0171-2985(96)80041-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. IL-12 and IL-18 synergistically induce the fungicidal activity of murine exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–9. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanarek DAM. São Paulo (SP), University of de São Paulo; 1997. Contribuição para o estudo da Candidíase Mucocutânea Crônica: Aspectos clínicos e avaliação da função imune mediada por linfócitos T e macrófagos. [Dissertation] [Google Scholar]
- 22.Benard G, Hong MA, Del Negro GMB, Batista L, Shikanai-Yasuda MA, Duarte AJS. Antigen-specific immunosuppression in paracoccidioidomycosis. Am J Trop Med Hyg. 1996;54:7–12. doi: 10.4269/ajtmh.1996.54.7. [DOI] [PubMed] [Google Scholar]
- 23.Benard G, Mendes-Giannini MJS, Juvenale M, Miranda ET, Duarte AJS. Immunosuppression in Paracoccidioidomycosis: T cell hyporesponsiveness to two Paracoccidioides brasiliensis glycoproteins that elicit strong humoral immune response. J Infect Dis. 1997;175:1263–7. doi: 10.1086/593694. [DOI] [PubMed] [Google Scholar]
- 24.Corrigan EM, Clancy RL, Dunkey ML, Eyers FM, Beagley KW. Cellular immunity in recurrent vulvovaginal candidiasis. Clin Exp Immunol. 1998;111:574–8. doi: 10.1046/j.1365-2249.1998.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–8. [PubMed] [Google Scholar]
- 26.Böhler T, Nedel S, Debatin KM. CD95-induced apoptosis contributes to loss of primed/memory but not resting/naive T cells in children infected with human immunodeficiency virus type 1. Pediatr Res. 1997;41:878–85. doi: 10.1203/00006450-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA. Fas ligand mediates activation-induced cell death in human lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Kharib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:441–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 29.Musci MA, Latinis KM, Koretzky GA. ‘Signaling events in T lymphocytes leading to cellular activation or programmed cell death. Clin Immunol Immunopathol. 1997;83:205–22. doi: 10.1006/clin.1996.4315. [DOI] [PubMed] [Google Scholar]
- 30.Latinis KM, Carr LL, Peterson EJ, Norian LA, Eliason SL, Kocetzky GA. Regulation of CD95 (Fas) ligand expression by TcR-mediated signaling events. J Immunol. 1997;158:4602–11. [PubMed] [Google Scholar]
- 31.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–8. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]