Abstract
T cell receptors, which recognize antigen peptides on MHC molecules, are essential probes for the analysis of T cell antigen specificity. The identification of paired T cell receptor (TCR) chains, α/β or γ/δ, usually requires the establishment of T cell clones, which is not always available. In this study, we tried, as an alternative method, the paired cloning of TCR α/β genes directly from a single T cell. T cells were sorted as a single cell from which RNA was extracted. Then, TCR α/β CDR3 regions were amplified from the single cell-derived cDNA by reverse transcriptase-polymerase chain reaction to determine their sequences. We successfully identified pairs of TCR α/β genes, and reconstructed the TCR molecule by a bacterial expression system. This strategy makes it possible to obtain recombinant TCR molecules from a single T cell without cellular cloning and promotes the investigation of T cell antigen specificity.
Keywords: single cell PCR, systemic lupus erythematosus, T cell, T cell receptor
INTRODUCTION
Previous studies have reported oligoclonally accumulated T cells in peripheral and/or disease-affected sites of patients suffering from autoimmune diseases [1–3]. The identification of the antigens recognized by these T cells, which has been rarely successful, would be of great help in understanding the pathogenesis of these diseases. An essential probe for the identification T cell antigens is the T cell receptor (TCR), which recognizes antigen peptides together with MHC molecules. Pure TCR clonotypes can be provided by the establishment of T cell clones. However, the establishment of human T cell clones is laborious and difficult in some cases, particularly in cases of T cells with an unknown antigen specificity. Therefore, as an alternative method, we prepared recombinant TCR molecules from a single T cell. Specifically, we amplified TCR α and β genes from single cell-derived cDNAs prepared from peripheral T cells of a patient with systemic lupus erythematosus (SLE). We constructed a single-chain TCR gene using the cloned TCR α and β genes, and expressed it in bacteria. Finally, the product TCR molecule was refolded. This study provides a novel and simple strategy to obtain and reconstruct TCR α and β genes in the correct combination directly from a single T cell of interest without cell cloning. This can be applied to various T cell samples including clinical ones and would be useful in the investigation of antigen specificity.
MATERIALS AND METHODS
Reverse transcription-polymerase chain reaction/single strand conformation polymorphism
Peripheral blood mononuclear cells (PBMC) were obtained from an SLE patient, with informed consent, and from a healthy donor. In each case, a proportion of the cells was subjected to cDNA synthesis and the TCR clonality was analysed by reverse transcription-polymerase chain reaction (RT-PCR) with subsequent separation by single strand conformation polymorphism (SSCP) as described previously [1]. The nucleotide sequences of the primers used for PCR and as a hybridizing probe for the SSCP analysis were as follows: Vα2, 5′-TGGAAGGTTTACAGCACAGC-3′; Va12, AGTGGTCGGTATTCTTGGAAC-3′; Cα, 5′-TGTACCAGCTGAGAGACTCT-3′; Cα probe, 5′-GCAGGGTCAGGGTTCTGGATA-3′ (Fig. 1a).
Fig. 1.
Cloning and expression of the TCR scFv. TCR α genes of Vα12+ T cells and TCR β genes of the two clonally expanded T cells (A22 and P39) were cloned for single strand conformation polymorphism (SSCP), nucleotide sequencing and/or expressing the TCR scFv protein. (a) Location of each primer is indicated by arrows. SS, Signal sequence. (b) The TCR genes of A22 were subcloned to plasmid vectors for expressing the scFv protein. The TCR α and β genes were subcloned to VL and VH sites of 9F12-encoding pCANTAB5E, respectively. The entire gene of TCR α/linker/TCRβ/E-Tag was transferred to EcoRI/BamHI-digested pMAL-c vector. The TCR scFv was expressed as a fusion protein with maltose binding protein (MBP) and E-Tag. After the MBP was cleaved by Factor Xa, the TCR scFv/E-Tag was purified on an affinity column using the anti-E-Tag antibody.
Single-cell PCR
The other portion of the PBMC obtained from the SLE patient was used for single-cell sorting as described in our previous report [4]. Briefly, the PBMC were stained with FITC-conjugated anti-Vα12 antibodies (Anti-Human TCR Vα12 Monoclonal; Endogen, Woburn, MA). The TCR Vα12+ cells were sorted at a ratio of one cell/well using a cell sorter (EPICS Elite; Beckman Coulter, Fullerton, CA), and total RNA was extracted from each of the sorted cells, then converted to cDNA. TCR CDR3 regions were amplified separately by nested PCR as described previously [4]. External primers were identical to those described above. The nucleotide sequence of the internal Vα12 primer was 5′-CTTCACCATCACAGCCTCACA-3′. The nucleotide sequence of the internal Cα primer was identical to that of the Cα probe described above (Fig. 1a). The individual PCR products were subcloned into a plasmid vector (PCR-Script Amp Cloning Kit; Stratagene, La Jolla, CA) for nucleotide sequencing (377 DNA Sequencer; Perkin Elmer/Applied Biosystems, Foster, CA). The CDR3 regions of the TCR β genes were amplified from the identical single cell-derived cDNAs by the semi-nested familial PCR using a common Cβ primer and each of 22 Vβ-specific primers for the first PCR and the same Vβ primers and mixture of internal Cβ l and Cβ2 primers for the second PCR (Fig. 1a). The sequences of the Vβ and Cβ primers had been described previously [5]. The nucleotide sequences of the internal Cβ primers were as follows: Cβ1, 5′-GGGTGGGAACACCTTGTTCAGGT-3′; Cβ2, 5′-GGGTGGGAACACGTTTTTCAGGT-3′. The amplified TCR β genes were subcloned into a plasmid vector for nucleotide sequencing as described above. In addition, TCR β genes were amplified from the healthy donor-derived bulk cDNA, using the Vβ and Cβ primers [5].
Construction of full length genes of TCR α/β chains
To prepare full length cDNAs encoding TCR Vα and Vβ chains, the framework 1–3 of the Vα12 gene was amplified from cDNA derived from a healthy donor's peripheral blood lymphocytes (PBL) by semi-nested PCR, since the Vα12 family is reported to consist of only one gene [6]. The sequence of primers was as follows: 5′ Vα12 external primer, 5′-ATGCTGACTGCCAGCCTGTTGAGGGCAG-3′; 5′ Vα12 internal primer, 5′-CCTCCATCTGTGTTGTATCCAGCATGGCT-3′; 3′ Vα12 primer, 5′-TGTGAGGCTGTGATGGTGAAG-3′. The 3′ Vα12 primer contains a sequence that is complementary to the internal Vα12 primer described above (Fig. 1a). Using this overlapping region, the Vα12 framework gene was connected to the CDR3α gene that had been cloned from a single T cell by PCR. This full-length Vα gene was reamplified with a 5′ primer containing the Sfi I site and a 3′ primer containing the Kpn I site. These primer sequences were as follows: 5′ Sfi I primer, 5′-TTTGGCCCAGCCGGCCCAGAAGGTAACTCAAGCGCAG-3′; 3′ Kpn I primer, 5′-TTTGGTACCGGCACCCTGACCCTTCTGCATATC-3′.
The framework 1–3 of the Vβ2 genes was amplified by the semi-nested PCR from the same single cell-derived cDNA sample that had been used for the CDR3α determination, since Vβ2 consists of multiple subfamilies [6]. The sequences of the primers were as follows: 5′ Vβ2 external primer, 5′-ATGCTGCTGCTTCTGCTGCTTCTGGGGCCA-3′; 5′ Vβ2 internal primer, 5′-GTCGTCTCTCAACATCCGAGCAGGGTT-3′; 3′ Vβ2 primer, 5′-AGGTCAGGCTTGCATGGTTGATGA-3′. The 3′ Vβ2 primer has a sequence complementary to the Vβ2 primer which was used to amplify CDR3β above (Fig. 1a). Similarly, as with the Vα12 gene, the amplified Vβ gene was connected to the CDR3β-encoding gene that had been cloned for sequencing by PCR, using the overlapping region. The full-length Vβ gene was reamplified with a 5′ primer containing the Sa1 I site and a 3′ primer containing the Not I site. The nucleotide sequences of the primers were as follows: 5′ Sal I primer, 5′-TTTGTCGACGTCGTCTCTCAACATCCGAG-3′; 3′ Not 1 primer, 5′-TTTGCGGCCGCGAACACGTTTTTCAGGTCCT-3′.
Reconstruction of a TCR molecule
In order to express the protein of the TCR scFv, the plasmid vector pCANTAB5SKSN (Expression module/recombinant phage antibody system; Amersham Pharmacia Biotech, Aylesbury, UK), which contains the scFv gene of anti-tetanus toxoid antibody (9F12-2) was used [7]. The gene of the antibody had been inserted as VL-linker-VH. First, the VL-linker-VH gene was digested from the vector using Sfi I and Not I, and was then inserted to pCANTAB5E vector which can express the E-Tag peptide at the C-terminus of the protein of interest (pCANTAB5E/9F12, Fig. 1b). Next the VH gene in pCANTAB5E/9F12 was replaced by the full-length V genes for TCR β genes using Sal I and Not I. Then, the VL gene in pCANTAB5E/9F12 was replaced by the full-length V genes for TCR α genes using Sal I and Not I.
The entire DNA fragment encoding the TCR scFv with the E-Tag (TCR/E: TCR α-linker-TCR β-E) was reamplified by a 5′ primer containing the EcoRI site and a 3′ primer containing the Bcl I site (Fig. 1b). The nucleotide sequences of the primers were as follows: 5′ EcoRI primer, 5′-TTTGAATTCTTGGCCCAGCCGGCCCAGAAGGTA-3′; 3′ Bcl I primer, 5′-TTTTGATCAACGCGGTTCCAGCGGATCCGGATA-3′. The PCR product was digested with EcoRI and Bcl I and subcloned into the EcoRI/Bam HI-digested pMAL plasmid vector (pMAL-c, Profusion Kit; New England Biolabs, Beverly, MA), designated as pMAL/TCR/E. An Escherichia coli strain of DH5a was transformed with pMAL/TCR/E. The TCR scFv, fused with the maltose binding protein (MBP) at its N-terminus and with the E-Tag peptide at its C-terminus, was induced by adding 0·3 mm IPTG to the E. coli. cells (MBP- TCR scFv-E, Fig. 1b). The cells were pelleted and sonicated for extraction of the protein. After the MBP was cleaved by Factor Xa (New England Biolabs), the fusion protein of TCR scFv and E-Tag (TCR scFv/E) was purified by the anti-E-Tag antibody column (RPAS Purification Module; Amersham Pharmacia Biotech).
The protein was refolded principally as described by Tsumoto et al. [8]. First, TCR scFv/E was dialysed against 6 m guanidine–HCI solution containing 100 mm Tris–HCI, 200 mm NaCl, 10 mm 2-mercaptoethanol (2-ME) and 1 mm EDTA. Every 12 h, the dialysing solution was changed in the following order: 2 m guanidine–HCl solution containing 100 mm Tris–HCl, 200 mm NaCl; 1 m guanidine–HCl solution containing 100 mm Tris–HCl, 200 mm NaCl, 375 μm glutathione disulphide and 400 mm l-arginine; and 0 m guanidine–HCl solution containing only 100 mm Tris–HCl and 200 mm NaCl. Finally, the refolded TCR scFv/E, as well as the non-refolded TCR scFv/E, was dialysed against PBS and diluted at 100 μg/ml.
Measurement of surface plasmon resonance
Interaction of the TCR scFv/E with the anti-Vα12 antibody and the anti-Vβ2 antibody (Beckman Coulter) was monitored by the measurement of surface plasmon resonance (SPR670; Nippon Laser & Electronics Lab., Nagoya, Japan). Sensor tips were incubated with 4,4-dithio butyric acid for induction of carboxyls. The carboxyls were then activated with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide for binding of the ligand protein, TCR scFv/E. The refolded and non-refolded TCR scFv were injected on separate channels to be immobilized on the different sites of the same sensor tip. After the unbound activated carboxyls were blocked with 0·5 m glycine/PBS, the anti-Vα12 antibody and the anti-Vβ2 antibody, diluted at 2 μg/ml in PBS, were injected on to the sensor tips prepared as described above.
RESULTS
In this study, our aim was to obtain α and β TCR genes, in the correct combination, from a heterogeneous T cell population, and subsequently reconstruct the TCR genes. Therefore, we applied the single-cell RT-PCR method, as previously reported by us, to peripheral T cells of a patient with SLE [4].
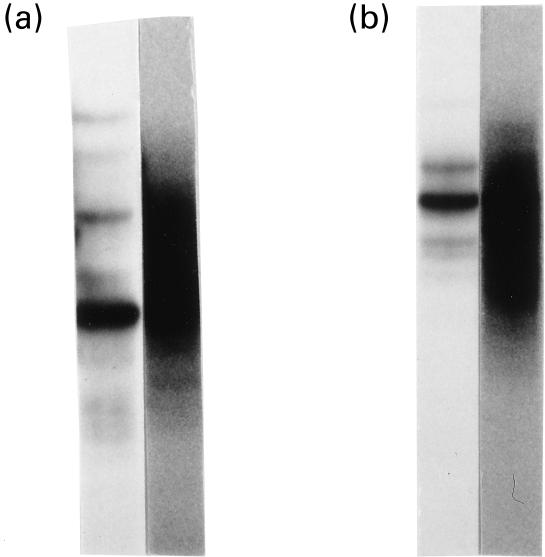
First, we analysed TCR clonotypes of the TCR Vα2 and Vα12 gene families by RT-PCR/SSCP, to which antibodies were available. As shown in Fig. 2, clonally expanded T cells were detected in those TCR Vα families of the PBL derived from the SLE patient. In contrast, the SSCP analysis of PBL from the normal donor showed smear-like broad bands without any distinct bands, which indicate that the Vα2 and Vα12 family of his PBL consisted of extremely heterogeneous T cells without expanding T cell clonotypes.
Fig. 2.
T cell clonality analysis of TCR Vα2 and Vα12 families by reverse transcription-polymerase chain reaction-single strand conformation polymorphism (RT-PCR-SSCP). Bulk cDNA was synthesized from peripheral blood mononuclear cells and TCR α genes carrying Vα2 and Vα12 were amplified by familial PCR. The amplified genes were electrophoresed on a non-denaturing gel to be separated according to their single strand conformations ((a) Vα2; (b) Vα12). The left lanes represent amplified genes from the cDNA of the systemic lupus erythematosus patient and the right lanes are those of a healthy donor.
We sorted one of the two Vα families, the TCR Vα12+ T cells, into a microtitre plate at a ratio of one cell/well. RNA was extracted from each cell separately and then converted to cDNA as described in Materials and Methods. Next, we amplified the TCR α CDR3 genes from the single-cell cDNAs by nested PCR and cloned the products into plasmids separately, so that their sequences could be determined. As shown in Table 1, a group of 10 T cells and a group of three T cells, out of the 18 examined, were found to carry identical TCR α sequences. Their deduced amino acid sequences were YFCA-LSEAT-SGSA (J22) and YFCA-LSEPT-NNAG (J39), designated as A22 and P39, respectively.
Table 1.
Sequences of the TCR α and β genes identified by polymerase chain reaction (PCR) amplification of single cell-derived cDNAs
| α | β | ||||||
|---|---|---|---|---|---|---|---|
| Vα12 | nDn | Jα | Vβ2 | nDn | Jβ | ||
| YFCA | LSEAT | SGSA (J22) | 10/18 | FYIC | RAGQD | SYEQY (J2S7) | A22* |
| YFCA | LSEPT | NNAG (J39) | 3/18 | FYICSA | RAGGT | YNEOF(J2S1) | P39* |
| YFCA | LR | SGSA (J22) | 1/18 | ||||
| YFCA | LKAWAA | SGGG (J45) | 1/18 | ||||
| YFCA | L | RDDK (J30) | 1/18 | ||||
| YFCA | LSDY | NNND (J44) | 1/18 | ||||
| YFCA | LSEALRS | GSEK (J57) | 1/18 | ||||
TCR Vα12+ T cells were sorted at a ratio of one cell/well into a microtitre plate. TCR α genes were amplified from the single cell-derived cDNA in each well by PCR and their CDR3 regions were sequenced. TCR β genes of expanded TCR α clonotypes were analysed similarly.
The expanded T cell clonotypes were named as described.
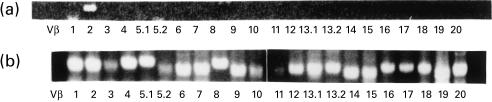
Concentrating on these two expanded clonotypes, we determined the TCR β CDR3 sequences. We amplified TCR β genes from the single-cell cDNAs of the corresponding wells using the 22 TCR Vβ family-specific primers. In both A22 and P39 clonotypes, PCR products of the expected length were detected in only the Vβ2 family. A representative result from the P39 clonotype is shown in Fig. 3a. As a control, TCR β genes were amplified from bulk PBMC-derived cDNA, which contained PCR products corresponding to all of the Vβ families (Fig. 3b). The deduced amino acid sequences of the two expanded clonotypes are as follows: A22, FYIC-RAGOD-SYEQ (J2S7) and P39, YICSA-RAGGT-YNEO (J2S1) (Table 1). Thus, pairs of TCR α/β chains were successfully identified by RT-PCR amplification from single cell-derived cDNAs. In addition, since the amino acid sequences of the TCR nDn regions of A22 and P39 are highly homologous to each other (α-chains, LSEAT and LSEPT; β-chains, RAGQD and RAGGT), they may recognize either highly homologous or identical antigenic peptide–MHC complexes.
Fig. 3.
TCR β gene amplification from single cell-derived cDNA. TCR β genes were amplified from single-cell cDNA by semi-nested familial polymerase chain reaction (PCR), from which expanded TCR α clonotypes had been amplified. (a) The results of Vβ 1–4, 5.·1, 5.2, 6–12, 13.1, 13.2, 14–20 are shown. (b) Bulk peripheral blood mononuclear cell cDNA from a healthy donor was amplified by familial PCR, using the same Vβ and Cβ primers as (a).
Next, we reconstructed the TCR molecule based on the paired TCR genes. To date, TCR molecules have been reconstructed by various methods such as establishment of transfectants and bacterial expression [9–12]. Concentrating on the A22 clonotype, we used a modification of the strategy reported by Hilyard et al. [10] that produced the TCR molecule as a scFv and refolded it. We amplified the framework 1–3 regions of TCR Vα and Vβ genes, from healthy donor's PBL-derived cDNA and from cDNA of the A22 clonotype. The amplified Vα12 framework genes were connected to the A22 CDR3α genes by hybridization at an overlapping region located at the 3′-end of the framework gene and at the 5′-end of the CDR3 gene. Similarly, the entire β variable region gene of A22 was constructed. The entire α and β variable region genes were used for replacement of VH and VL genes in pCANTAB5E/9F12, which produced scFv proteins of anti-tetanus toxoid antibody (9F12) fused with E-tag peptide. Finally, the gene encoding TCR scFv with E-tag was subcloned into pMAL-c, which produced TCR scFv as a fusion protein with MBP and the E-Tag (Fig. 1). After the fusion protein was produced, MBP was cleaved by factor Xa, and the resultant TCR scFv/E protein was purified by the anti-E Tag column and refolded by the gradual decrease of the guanidine–HCl concentration in the dialysing solution and by the addition of glutathione disulphide and l-arginine at the final step.
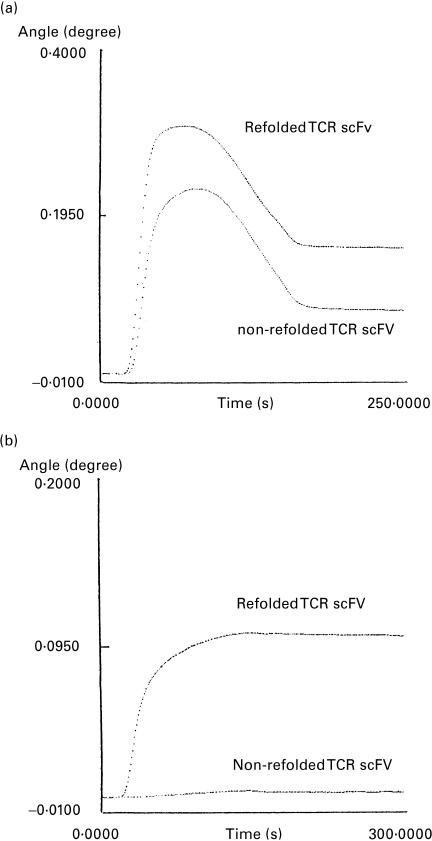
We examined whether the TCR scFv was refolded correctly by measurement of the surface plasmon resonance. The refolded and non-refolded TCR scFv were immobilized on the same sensor tip and their binding to the anti-Vα12 antibody and the anti-Vβ2 antibody was examined. The anti-Vα12 antibody and the anti-Vβ2 antibody can detect the corresponding V family in flow cytometry analysis, indicating that they recognized the epitopes. We applied these antibodies to Western blots and found that they did not bind to the denatured native TCR molecules (results not shown). Thus, these antibodies were considered to recognize conformation-dependent epitopes. As shown in Fig. 4a, the refolded TCR scFv bound to the anti-Vα12 antibody much more strongly than did the non-refolded TCR scFv molecule. Similarly, the refolded TCR scFv showed strong binding to the anti-Vβ2 antibody, whereas the non-refolded TCR scFv failed to bind (Fig. 4b). These results suggest that the TCR scFv proteins were correctly refolded.
Fig. 4.
Reactivity of the reconstructed TCR molecules to the antibodies against their variable region. The refolded and non-refolded TCR scFv was immobilized on the same sensor tip of SPR670. (a) Anti-TCR Vα12 antibody and (b) anti-TCR Vβ2 antibody were injected at the starting point (0 s). Representative results are shown.
DISCUSSION
In this study, we have described the paired cloning of TCR Va and Vβ chains of a single T cell isolated from a clinical specimen of PBL collected from a patient with SLE, and the subsequent reconstruction of the TCR. Since the establishment of T cell clones is unnecessary, our cloning strategy can be applied to various T cell samples easily and widely, and provides a new tool to investigate T cell antigen specificity.
The first step to identify the TCR α/β gene pair by this method is single-cell sorting of T cells. A cell sorter is convenient for this aim. However, if a cell sorter is not available, cell sorting by magnetic beads such as MACS (Magnetic Cell Sorting, Miltenyi Biotec, Bergisch Gladbach, Germany) and following manual limiting dilution can be used as an alternative.
Recently, single-cell PCR methods using genomic DNA have been reported [13–15]. However, genome amplification from a single cell should start, at most, from only two copies of template genes. Our strategy uses cDNA derived from a single cell that contains multiple copies of mRNA. Thus, the single cell-derived cDNA could be directly used as templates for multifamilial PCR. In fact, the cDNA from a single cell was sufficient to examine more than 20 Vβ families (Fig. 3b).
To determine the combination of Vα and Vβ gene segments, it would be convenient to sort T cells by a particular Vα-specific antibody, then amplify the Vβ gene segment, since the TCR gene system has only 32 Vβ gene families but has a greater number of Vα families [6]. However, at present only a limited number of Vα family-specific antibodies are commercially available. An increased number of Vα family-specific antibodies would increase the potential application of this method.
The primary aim of the current study was to identify the pair of TCR α and β genes of a particular T cell without cellular cloning. We amplified multiple genes (TCR α and β) from a single cell, and therefore were able to identify the TCR α/β combination of the clonally expanding T cell whose antigen is not known. Compared with animal and/or model experiments using hybridomas and cell lines, methods used to analyse particular T cell clonotypes in human clinical specimens have not been established. The results of this study present a new strategy to examine antigens of expanding T cell clonotypes in human clinical samples. Currently, we are attempting to screen antigens of clonally expanding T cells from either rheumatoid arthritis or SLE. Our results suggest that it is not difficult to increase the number of TCR for reconstruction.
It is important to determine whether our reconstructed TCR molecule recognizes its specific ligand, the MHC–peptide complex. Unfortunately, we were not able to examine its antigen-specific response in the current study, because we were cloning TCR α/β gene pairs with unknown antigens. Alternatively, we demonstrate that the reconstructed TCR molecule was recognized by conformation-dependent anti-Vα12 and anti-Vβ2 antibodies. It is noteworthy that several groups have already succeeded in reconstructing TCR molecules of T cell clones or hybridomas in vitro using methods similar to those described here. In those reports, the reconstructed TCR molecules are capable of both recognizing their specific MHC/peptide ligands and blocking the response of the original T cells to the ligands [10–12]. The TCR of the clonally expanded T cells have been selected by their affinity to their ligands in vivo. Thus, the reconstructed TCR molecule in this study is probably capable of recognizing its specific MHC/peptide.
Recently, the interaction between TCR and the MHC–peptide complex has been investigated not only at the cellular response level, but also at the molecular level. For example, the interaction of TCR and the MHC–peptide complex was examined by surface plasmon resonance [10,12]. Further, a tetramer MHC–peptide complex was found to react to the TCR molecules quite well [16,17]. This strategy would confirm the antigen recognition of reconstructed TCR after detecting their MHC/peptide ligands. In this way, TCR molecules prepared by our method may promote the characterization of T cells with an unknown antigen specificity by their use as probes to search for T cell antigens through molecular interactions.
Acknowledgments
We are indebted to Dr Hiroshi Furukawa (University of Tokyo), Mr Yasuhiko Nagasaka (Beckman Coulter) for their advice in using the cell sorter. We thank Ms Hiroko Sasakawa, Ms Yuka Onoki, Ms Sayaka Yukii, Ms Kayoko Osumi, Ms Yumiko Ajiri (St. Marianna University) and Ms Sumiyo Kawamura (Japanese Red Cross Central Blood Centre) for their technical assistance. This work was supported in part by grants from the Ministry of Health and Welfare and the Ministry of Education of Japan.
REFERENCES
- 1.Yamamoto K, Sakoda H, Nakajima T, et al. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol. 1992;4:1219–23. doi: 10.1093/intimm/4.11.1219. [DOI] [PubMed] [Google Scholar]
- 2.Mato T, Masuko K, Misaki Y, et al. Correlation of clonal T cell expansion with disease activity in systemic lupus erythematosus. Int Immunol. 1997;9:547–54. doi: 10.1093/intimm/9.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Iwabuchi S, Takatori M, et al. Clonotype analysis of T cells in patients with autoimmune and viral hepatitis. Hepatology. 1997;25:1070–6. doi: 10.1002/hep.510250504. [DOI] [PubMed] [Google Scholar]
- 4.Kurokawa M, Furukawa H, Yabe T, et al. Frequency of clonally expanding T cells evaluated by PCR from a single cell. J Immunol Methods. 1999;224:203–8. doi: 10.1016/s0022-1759(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin ‘superantigens’ with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–5. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Sato K, Suzuki S, Sasakawa H, Kurokawa M, Nishioka K, Yamamoto K. Mammalian expression of single chain variable region fragments dimerized by Fc regions. Mol Biol Rep. 1995;21:141–6. doi: 10.1007/BF00997236. [DOI] [PubMed] [Google Scholar]
- 8.Tsumoto K, Shinoki K, Kondo H, Uchikawa M, Juji T, Kumagai I. Highly efficient recovery of functional single-chain Fv fragments from inclusion bodies overexpressed in Escherichia coli by controlled introduction of oxidizing reagent—application to a human single-chain Fv fragment. J Immunol Methods. 1998;219:119–29. doi: 10.1016/s0022-1759(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire C, Malissen B, Mazza G. Characterization of T cell receptor single-chain Fv fragments secreted by myeloma cells. Eur J Immunol. 1996;26:2410–6. doi: 10.1002/eji.1830261022. [DOI] [PubMed] [Google Scholar]
- 10.Hilyard KL, Reyburn H, Chung S, Bell JI, Strominger JL. Binding of soluble natural ligands to a soluble human T-cell receptor fragment produced in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9057–61. doi: 10.1073/pnas.91.19.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter Y, Kurucz I, Brinkmann U, Jung SH, Lee B, Segal DM, Pastan I. Construction of a functional disulfide-stabilized TCR Fv indicates that antibody and TCR Fv frameworks are very similar in structure. Immunity. 1995;2:281–7. doi: 10.1016/1074-7613(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 12.Plaksin D, Polakova K, McPhie P, Margulies DH. A three-domain T cell receptor is biologically active and specifically stains cell surface MHC/peptide complexes. J Immunol. 1997;158:2218–27. [PubMed] [Google Scholar]
- 13.Zhang L, Cui X, Schmitt K, Hubert R, Navidi W, Arnheim N. Whole genome amplification from a single cell: implications for genetic analysis. Proc Natl Acad Sci USA. 1992;89:5847–51. doi: 10.1073/pnas.89.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snabes MC, Chong SS, Subramanian SB, Kristjansson K, DiSepio D, Hughes MR. Preimplantation single-cell analysis of multiple genetic loci by whole-genome amplification. Proc Natl Acad Sci USA. 1994;91:6181–5. doi: 10.1073/pnas.91.13.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells D, Sherlock JK, Handyside AH, Delhanty JD. Detailed chromosomal and molecular genetic analysis of single cells by whole genome amplification and comparative genomic hybridisation. Nucl Acids Res. 1999;27:1214–8. doi: 10.1093/nar/27.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bieganowska K, Hollsberg P, Buckle GJ, et al. Direct analysis of viral-specific CD8+ T cells with soluble HLA-AS/Tax11-19 tetramer complexes in patients with human T cell lymphotrophic virus-associated myelopathy. J Immunol. 1999;162:1765–71. [PubMed] [Google Scholar]