Abstract
Active TB in HIV-1-infected subjects is associated with increased HIV-1-related immunodeficiency and mortality. We assessed plasma viral load in HIV-1-infected patients with pulmonary TB (HIV/TB) and non-TB symptomatic HIV-1-infected patients (HIV). HIV-1 load was higher in HIV/TB compared with HIV at higher CD4 counts (> 500/μl) (P < 0·01), but not at lower CD4 counts (< 500/μl). We also evaluated the status of HIV-1 gene expression in peripheral blood mononuclear cells (PBMC) and serum from HIV/TB and CD4-matched healthy HIV-infected patients (HIV/C) by reverse transcriptase-polymerase chain reaction over a range of CD4 (> 900/μl to <200/μl). HIV-1 RNA in serum and PBMC correlated to one another, and both were markedly higher in HIV/TB compared with HIV/C with higher CD4 counts. Also, during a longitudinal study of anti-tuberculous chemoprophylaxis in HIV-1-infected patients, 10 subjects who developed TB had serologies before, at the time, and after the diagnosis of TB. These HIV/TB patients had an increase in viral load (average 2·5-fold) at the time of diagnosis of TB (P < 0·05). Overall, these data indicate that the transcriptional activity of HIV-1 is enhanced in HIV-1-infected patients with active TB, especially during early HIV-1 disease. As TB often is an early HIV-1 opportunistic infection, it may particularly favour early viral replication and dissemination, and therefore contribute to progression of HIV-1 disease.
Keywords: Mycobacterium tuberculosis, HIV-1, tumour necrosis factor-alpha, transcriptional activation, opportunistic infection
INTRODUCTION
World-wide, TB is the most common co-infection in subjects infected with HIV-1. Over two-thirds of the 15 million cases of dual-HIV/TB infection reported reside in Sub-Saharan Africa [1]. However, as HIV-1 expands in other parts of the world, such as in South-east Asia [2], the interaction between these two pathogens will continue to expand and compound the health issues related to both infections.
In contrast to other HIV-1-associated opportunistic infections (OPI), TB may occur at any level of immunodeficiency [3], and has clearly been shown to be associated with enhanced HIV-1 morbidity and mortality [4]. OPIs in general [5] and TB in particular [6] are associated with enhanced HIV-1 replication. During HIV-1 infection, higher viral load is associated with a more rapid progression of HIV-1 disease [7]. Active [8] but not latent Mycobacterium tuberculosis (MTB) infection [9] is associated with the development of AIDS. However, the true impact of OPIs on HIV-1 disease is still not clear.
Recent studies have elucidated the basis of HIV-1 induction by MTB, and during TB. MTB induces HIV-1 replication in HIV-infected cell lines [10], and in mononuclear cells from HIV-1-infected subjects [11]. Additionally, mononuclear cells from subjects with TB are activated [12], and more susceptible to a productive infection by HIV-1 [13]. Further, the cytokine profile of the tuberculous microenvironment is conducive to HIV-1 replication [14].
To understand the impact of TB on HIV-1 viral load, several different study designs were undertaken. In a cross-sectional case-control study, we found that circulating HIV-1 load was significantly higher in HIV/TB patients compared with CD4-matched symptomatic but non-TB HIV-1-infected subjects, only at higher CD4 levels (> 500/μl). In a longitudinal study of HIV-1-infected patients who developed active TB, a significant increase in viral load at or around the time of diagnosis of TB was documented, which was most notable in cases with a lower initial viral load.
PATIENTS AND METHODS
Study populations
The first study (study 1) was a cross-sectional study of HIV/TB patients undertaken from June to August of 1996. HIV/TB were recruited from the National Tuberculosis Control Program (NTLP) Clinic of Mulago Hospital in Kampala, Uganda. The diagnosis of pulmonary TB was based on chest roentgenography and sputum smear microscopy, and confirmation was by a positive culture of MTB. No subject had received anti-tuberculous treatment or corticosteroid therapy in the preceding 6 months before the study. Patients with extra-pulmonary TB or chronic debilitating diseases, or Karnofsky value <60 were excluded. All patients with active TB were treated with standard short course chemotherapy according to the NTLP protocol.
The control group (HIV) were symptomatic HIV-1-infected patients without a diagnosis of TB who had a Kornofsky score >60. These subjects were recruited from the AIDS support organization (TASO) clinic of Mulago Hospital. HIV were given at least one other non-AIDS diagnosis, most commonly a diagnosis of a local genital infection. Physical examination and a normal chest roentgenogram excluded active pulmonary TB in this group. All subjects in the control group had PPD (Mantoux) skin testing, and only skin test-positive subjects (induration >5 mm) were included in the study. All study subjects signed a consent form approved by the institutional review board of Makerere University, Kampala, Uganda, and received health education about TB and HIV-1 infection.
The second study (study 2) included HIV-1-infected subjects enrolled in a trial of anti-tuberculous preventive chemotherapy between March 1993 and April 1995 [15]. Before enrolment, active TB was ruled out by a physical examination, a chest roentgenogram, and sputum microscopy. Re-examination of enrolled patients was performed at least once every 6 months, or when the subjects developed any symptoms. Of 2635 patients enrolled in this study, 66 developed culture-positive TB. Ten of these latter patients had serial serum samples; 6 months prior to, at or around the time of, and 6 months after the diagnosis of TB. These 10 patients are the subject of the present study. CD4 counts were not available on the majority of these subjects. In addition, during March of 1995, at the time of initial evaluation, five patients who were diagnosed to have TB were excluded from the trial of anti-tuberculous preventive therapy [15]. These incident HIV/TB patients were studied along with a comparable number of CD4-matched, PPD skin test-positive HIV-1-infected subjects separately. Informed consent was obtained from all participants.
HIV-1 load
Circulating HIV-1 RNA in plasma (study 1) and serum (study 2 and incident HIV/TB) was assessed by the Amplicor assay (Roche, Indianapolis, IN), according to the instructions of the manufacturer.
RNA isolation and reverse transcriptase-polymerase chain reaction for HIV-1 and cytokines
In the patients with incident HIV/TB from study 2, heparinized blood was also drawn for HIV-1 RNA analysis in peripheral blood mononuclear cells (PBMC) [11]. PBMC were lysed in Tri-reagent (Gibco BRL, Grand Island, NY) (2 × 106/400 μl) in duplicates. Total RNA was extracted following the instructions of the manufacturer. HIV-1 mRNA expression was assessed by reverse transcriptase-polymerase chain reaction (RT-PCR) of both unspliced (structural, gag/pol), and multiple spliced (regulatory tat/rev) mRNA as before [11].
Tumour necrosis factor-alpha (TNF-α), IL-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF) mRNA expression was also assessed by RT-PCR using oligonucleotide primer pairs (Clonetech, Palo Ato, CA). PCR products were resolved on a 2% agarose gel. A positive control amplified cDNA for each cytokine (Clonetech) was subjected to PCR and resolved along with the samples.
Statistical analysis
Student's t-test was used for comparison of viral loads between groups and in the same individual.
RESULTS
Comparison of HIV-1 viral load in HIV/TB and HIV
In study 1, plasma viral load from HIV/TB (n = 43) was compared with HIV (n = 31). Altogether, the two groups were comparable with respect to gender, age, and average CD4 count (354 + 250 versus 409 + 257). Overall, mean plasma viral load was higher in HIV/TB than in HIV subjects. In both patient groups, when the plasma HIV-1 load and CD4 count for each subject were plotted against one another, a correlation between these parameters was evident (Fig. 1a). This line of correlation was displaced upward in HIV/TB compared with the HIV group, particularly in HIV/TB patients with higher CD4. In fact, when the mean HIV-1 RNA were compared between HIV/TB and HIV patients in three separate CD4 categories (< 200/μl, 200–500/μl, and > 500/μl) (Fig. 1b), the differences in HIV-1 load between HIV/TB and HIV were only significant at CD4 counts >500/μl (P < 0·01, unpaired Student's t-test). These data indicate that the strongest impact of TB on HIV-1 load is at the time when patients are least immunodeficient.
Fig. 1.
Viral loads in HIV/TB and HIV. HIV-1 load was assessed in plasma obtained from HIV/TB and symptomatic, non-TB patients (HIV). (a) Viral load is plotted against CD4 counts in HIV/TB (•, n = 43) and HIV (○, n = 31). (b) Mean viral load is compared between HIV/TB (▪) and HIV (□), in three separate CD4 categories: > 500/μl (n = 16 and 7 for HIV/TB and HIV, respectively), 200–500/μl (n = 17 and 12 for HIV/TB and HIV, respectively), and <200/μl (n = 10 and 12 for HIV/TB and HIV, respectively). *P < 0·01 between HIV/TB and HIV.
Correlation of HIV-1 transcriptional activity in PBMC and plasma in HIV/TB patients
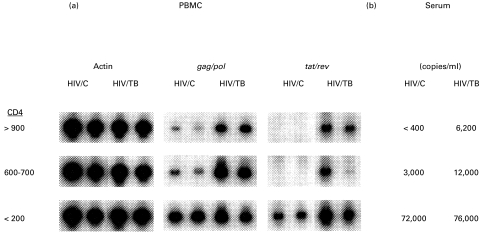
Next, a correlation was sought between HIV-1 RNA in serum and PBMC of patients with incident HIV/TB. HIV-1 transcriptional activity in PBMC and serum of incident HIV/TB (n = 5) were compared with an equal number of CD4-matched, PPD skin test-reactive HIV-1-infected subjects without TB (HIV/C). Duplicate samples of heparinized blood were obtained from each subject and PBMC were prepared. HIV-1 transcriptional activity was assessed by RT-PCR for HIV-1 unspliced (gag/pol) and multiple spliced (tat/rev) messages (Fig. 2a). In addition, circulating HIV-1 load was also measured in serum obtained from these subjects (Fig. 2b). Figure 2 shows data for three pairs of HIV/TB and HIV/C subjects. Both unspliced and multiple spliced HIV-1 mRNA were higher in PBMC from HIV/TB compared with HIV/C, and correlated with serum viral loads. Again, differences between HIV/TB and HIV/C were most notable at highest CD4 counts. Therefore, during active TB, HIV-1 transcriptional activity is increased in both the cellular (PBMC) and acellular (serum) compartments of the blood. We also assessed the expression of cytokines (TNF-α, GM-CSF, and IL-2) mRNA in PBMC from these HIV/TB (n = 5) and HIV/C (n = 4) subjects by RT-PCR. In HIV/TB donors, the expression of TNF-α (Fig. 3) only and not the other cytokines, was up-regulated.
Fig. 2.
HIV-1 RNA in peripheral blood mononuclear cells (PBMC) and serum of HIV/TB and HIV/C. HIV-1 RNA was assessed in three pairs of HIV/TB and HIV/C representing different CD4 categories (shown on left). (a) Duplicate PBMC samples were assessed (by reverse transcriptase-polymerase chain reaction) for HIV-1 unspliced (gag/pol) and multiple spliced (tat/rev) mRNA, and for expression of the housekeeping gene, β-actin. (b) Sera from the same patients were assessed for HIV-1 load.
Fig. 3.
Cytokine gene expression in peripheral blood mononuclear cells (PBMC) of HIV/TB and HIV/C. RNA (1 μg) from PBMC of HIV/TB (n = 5) and HIV/C (n = 4) were assessed for expression of cytokines (tumour necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF0, and IL-2) genes by reverse transcriptase-polymerase chain reaction. An aliquot of RNA was also assessed for expression of the housekeeping gene, GAPDH, to assure equal processing between samples.
Increased HIV-1 load at the time of diagnosis of TB
In study 2, 10 patients had serum evaluations for HIV-1 load 6 months before, at or around the time, and 6 months after diagnosis of TB. Initial HIV-1 loads in these subjects varied over a wide range (2000–650 000 copies/ml). However, there was on average a 2·7-fold (range 1–7·4-fold) increase in HIV-1 load (P < 0·05, paired t-test), at or around the time of diagnosis of TB compared with initial viral levels (data not shown). Five subjects with initial viral loads <50 000 copies/ml had on average a 3·3-fold increase in viral load (Fig. 4), and three out of these five patients died, 2–6 months after the completion of chemotherapy, presumably from HIV-1-related causes. By questioning the families of patients, it was determined that deaths were not due to recurrence of TB or trauma. HIV-1 load increased in these three subjects subsequent to initiation of chemotherapy (two-, two-, and >10-fold higher at follow up compared with the time of diagnosis of TB). By contrast, the group of five patients who had initial HIV-1 loads that were >50 000 had on average a 2·5-fold increase in viral load (data not shown), and four of five were alive at the end of the study. The limitation of the numbers in each category do not allow a firm conclusion to be made on these data, but it appears that a higher fold increase in viral load, rather than the absolute level of viral load prior to or at the time of diagnosis of TB, was associated with a worse prognosis.
Fig. 4.
Serial HIV-1 RNA in the serum of HIV-1-infected patients with baseline viral loads <50 000 who developed TB. On the abscissa the time points when blood was drawn from the patient and the date when the patient was diagnosed with TB (TB) are identified. TB* are those subjects who subsequently died.
DISCUSSION
These data further establish the impact of TB in HIV-1 disease. Under stringent conditions, i.e. when HIV-1-infected patients with active pulmonary TB were compared with symptomatic HIV-1-infected subjects without TB, viral load was significantly higher in the TB patients only at ‘normal’ levels of CD4 (> 500/μl). This finding was corroborated in the cases with incident HIV/TB; the differences in viral load in both PBMC and serum between HIV/TB and healthy HIV-infected subjects were most notable at higher CD4 counts. At all levels of CD4, ΤΝF-α mRΝΑ in PBMC was constitutively expressed in HIV/TB subjects only. Also, whereas all HIV-infected patients who developed TB had higher viral loads at the time of diagnosis of TB, those with low initial HIV-1 levels (< 50 000) had a more dramatic increase in their viral loads and died from presumable HIV-1-related causes more frequently.
Other HIV-1-related OPIs have also been shown to be associated with increased HIV-1 replication both at the site of opportunistic infections [5], and in the circulation [16]. However, in contrast to other OPIs, TB occurs at all CD4 levels during HIV-1 infection [3]. In fact other studies have shown that the viral load threshold necessary for development of TB has been estimated to be over a log lower than that of other OPIs [16]. In this study 25% of HIV/TB had CD4 counts >500/μl, indicating that a significant number of HIV-1-infected subjects develop TB when the immune system is ‘intact’. Whereas this phenomenon may predominantly reflect the virulence of MTB, in part it also signifies that factors other than or in addition to the number of CD4 cells may be required for continued immunosurveillance against MTB infection. Alternatively, during HIV-1 infection CD4 cells may have functional defects that are conducive to development of TB. In the subgroup of HIV/TB patients with CD4 counts >500/μl, TB was associated with an approximately 20-fold higher viral load compared with HIV. By contrast, viral load was comparable in HIV/TB and non-TB symptomatic HIV whose CD4 was < 500/μl. However, the convergence of viral loads in HIV/TB and HIV with CD4 < 500/μl may also relate to the fact that HIV/TB patients with extrapulmonary and disseminated TB, who are likely to have lower CD4 counts, were excluded from this study.
The pathogenesis of TB during HIV-1 infection includes both reactivation of prior MTB infection, and progressive primary MTB infection [17]. TB in early HIV-1 disease resembles reactivation disease, and thus is most amenable to preventive therapy. Considering the effect of TB on viral load, it can therefore be argued that efforts directed at TB chemoprophylaxis should be particularly targeted to HIV-1-infected patients who have higher CD4 counts. Whereas such subjects remain at risk of re-infection with MTB and development of primary TB throughout the course of their HIV disease, they will benefit most from chemoprevention of TB, as this aborts TB resulting from reactivation of endogenous MTB infection. This issue is of obvious practical importance in countries where both MTB and HIV-1 infection are endemic and where chemoprophylaxis for all HIV-infected patients is not feasible.
Development of TB during HIV-1 infection is associated with intense immune activation [12], and an increase in HIV-1 enhancing proinflammatory cytokines, such as TNF-α. MTB and its components induce proinflammatory cytokines in mononuclear cells [14,18]. Increased spontaneous release of the proinflammatory cytokines IL-1β, IL-6 and ΤNF-α in bronchoalveolar cells from HIV-infected and uninfected patients with active TB has been reported [19]. In patients with HIV/TB high circulating TNF-α levels correlated with a poor response to anti-tuberculous therapy [20], and sustained plasma TNF-α activity, but not other parameters of activation, was associated with persistent viraemia [21]. MTB and PPD induce the transcriptional activity of HIV-1 that is in part abrogated by neutralization of ΤΝF-α[18]. In the present study, we found a constitutive expression of TNF-α mRNA, but not IL-2 or GM-CSF mRNA, in PBMC of HIV/TB patients compared with control subjects. Therefore it appears that regardless of the level of immunodeficiency, active TB is conducive to excess expression of TNF-α. As immunotherapeutic agents directed at inhibition of TNF-α are assessed, a possible role for inhibition of HIV-1 expression during active TB may become apparent. In preliminary data, we have observed that both antibody to ΤΝF-α and Pentoxiphylin, which inhibits the transcriptional activation of TNF-α, inhibit PPD-induced HIV-1 p24 release from PBMC of patients with active TB in vitro (Toossi, unpublished). Recently, Wallis et al. conducted a controlled trial of Pentoxifylline in HIV-infected patients with TB [22]. Whereas trends towards reduced in vitro induced TNF-α production and improved performance scores were noted, no effect on body mass, CD4 count, or survival was observed. However, the efficacy of any immunotherapy directed at TNF-α should be considered with regard to the CD4 level of HIV/TB patients. Thus, anti-TNF adjunctive therapy in HIV/TB may be most beneficial in patients with higher CD4 counts. Studies of more potent TNF-α inhibitors in HIV-infected patients with TB need to be performed.
Our findings corroborate the findings of Goletti et al. [6] that in HIV-infected subjects, development of active TB is associated with increased viral load. However, in our study the increase in viral load was not reversible subsequent to treatment of TB in all cases. In fact, lower initial viral load was associated with higher induction of HIV-1, and more deaths. Deaths were attributed to progressive HIV-1 disease as they were not due to trauma or recurrence of TB. However, due to the limitation in the number of patients who developed TB, a firm association between the pre-TB level and subsequent induction of HIV-1 during TB, and HIV-1-related mortality can not be ascertained in this study. In a recent epidemiologic study of the impact of TB on survival of HIV/TB patients, we have observed that compared with CD4-matched HIV-infected subjects, mortality in HIV/TB patients with CD4 counts >200/ml are most significantly affected (Whalen, unpublished).
In conclusion, active TB is associated with increased viral load in HIV-1-infected patients, which is of particular significance to HIV-1-infected patients with an intact immune system. The virologic and immunologic consequences of the expansion of HIV-1 activity during TB need to be further explored.
Acknowledgments
This study was supported by grants from the National Institute of Health (AI 45244 and HL 51636). H.M.-K. was the recepient of a Fogarty grant from NIH 1996–98.
REFERENCES
- 1.Dye C, Scheele S, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S. WHO gives southeast Asia a health warning. Lancet. 1999;354:1010–1. doi: 10.1016/S0140-6736(05)76623-X. [DOI] [PubMed] [Google Scholar]
- 3.Lucas S, Nelson AM. Tuberculosis: pathogenesis, protection, and control. Washington, DC: ASM Press; 1994. Pathogenesis of tuberculosis in human immunodeficiency virus-infected people; pp. 503–13. [Google Scholar]
- 4.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 5.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–60. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 6.Goletti D, Weissman D, Jickson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 7.Fahey JL, Taylor JM, Manna B, Aziz N, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1591–600. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Chaisson RE, Gallant JE, Keruly JC, Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. AIDS. 1988;12:29–33. doi: 10.1097/00002030-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Manoff SB, Farzadegan H, Munoz A, Astemborski JA, Vlahov D, Rizzo RT, Soomon L, Graham NM. The effect of latent Mycobacterium tuberculosis infection on human immunodeficiency virus (HIV) disease progression and HIV RNA load among injecting drug users. J Infect Dis. 1996;174:299–308. doi: 10.1093/infdis/174.2.299. [DOI] [PubMed] [Google Scholar]
- 10.Lederman MM, Georges DL, Kusner DJ, Mudido P, Giam CZ, Toossi Z. Mycobacterium tuberculosis and its purified protein derivative activate expression of the human immunodeficiency. J Acquir Immun Defic Syndr Hum Retrovir. 1994;7:727–33. [PubMed] [Google Scholar]
- 11.Toossi Z, Nicolacakis K, Xai L, Ferrari N, Rich E. Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-infected individuals in vitro. J Acquir Immun Defic Syndr Hum Retrovir. 1997;15:325–31. doi: 10.1097/00042560-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Vanham G, Edmonds KE, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toossi Z, Sierra Madero JG, Blinkhorn RA, et al. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177:1511–6. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrait V, Cadranel J, Esvant H, Herry I, Morinet P, Mayaud C, Israel-Biet D. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. J Immunol. 1997;159:2824–30. [PubMed] [Google Scholar]
- 15.Whalen CC, Johnson JJ, Okwera A, Hom DL, Huebner R, Mugyenyi P, Mugerwa RD, Ellner JJN. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case western Reserve University Reserch Collaboration. Engl J Med. 1997;337:801–8. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 16.Romeu J, Balague M, Marfil S, Gatell JM, Puig T, Arno A, Sierra G, Colet B. Short term risk for AIDS indicator diseases predicted by plasma HIV-1 RNA and CD4+ lymphocytes. Scand J Infect Dis. 1999;31:37–42. doi: 10.1080/00365549950161862. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PF, Bloch AB, Davidson PT, Snider PE., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–50. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 18.Toossi Z, Xia L, Salvekar A. Transcriptional activities of Human Immunodeficiency Virus (HIV) by Mycobacterium tuberculosis in human monocytes. Clin Exp Immunol. 1999;117:324–30. doi: 10.1046/j.1365-2249.1999.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakata K, Rom WN, Honda Y, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh SM, Hung CC, Chen MY, Sheng WH, Chang SC. Dynamics of plasma cytokine levels in patients with advanced HIV infection and active tuberculosis: implications for early recognition of patients with poor response to anti-tuberculosis treatment. AIDS. 1999;13:935–41. doi: 10.1097/00002030-199905280-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Shattock RJ, Acheampong JW, Lal RB, Folks TM, Griffin GE, Butera ST. Sustained plasma TNF-alpha and HIV-1 load despite resolution of other parameters of immune activation during treatment of tuberculosis in Africans. AIDS. 1999;13:2231–7. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wallis RS, Nsubuga P, Whalen CC, et al. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J Infect Dis. 1996;174:727–33. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]