Abstract
Cachexia is one of the prominent features of advanced tuberculosis (TB) seen in association with increased expression of the monokine TNF-α. Several mycobacterial proteins, including PPD, stimulate TNF-α secretion from monocytes. Host factors that may play a role in cytokine expression from monocytes remain largely unknown. One such factor is the opsonizing antibodies. Monocytes have high-affinity receptors (Fcγ I and Fcγ III) for IgG1 and IgG3 antibodies that mediate antigen uptake. We have reported selective up-regulation of IgG1 (which bind to Fcγ receptors) in advanced TB and have recently shown the ability of PPD-specific IgG1 antibodies to augment TNF-α expression in PPD-stimulated monocytes. These observations have now been extended to other cytokines with semipurified fractions from secreted antigens of Mycobacterium tuberculosis (containing 30 kD and 58 kD) that were devoid of lipids, glycolipids and carbohydrates. In the presence of heat-inactivated TB plasma containing known amounts of antigen-specific IgG1 antibodies, these fractions induced significantly increased TNF-α, IL-6 and IL-10 secretion. Absorption of IgG1 with Protein ‘A’ removed the augmenting activity for TNF-α and IL-6 secretion from the TB plasma samples. In the case of IL-10, removal of IgG1 resulted in increased rather than decreased IL-10 secretion. These results suggest a possible pathogenic role for antibodies in TB by enhancing proinflammatory and blocking down-regulatory cytokines such as IL-10 cytokines during the chronic phase of TB.
Keywords: IgG antibody subclasses, tumour necrosis factor-alpha, IL-6, IL-10, tuberculosis, monocyte
INTRODUCTION
Tuberculosis (TB) is the cause of highest morbidity among tropical diseases over the age of 5 years (source: World Bank, World Development Report, 1993). Although partial protection against disseminated TB is conferred by bacille Calmette–Guérin (BCG) vaccination, the immune determinants that direct the initial establishment of infection and further disease progression are still unclear. As an intracellular pathogen Mycobacterium tuberculosis (Mtb) resides and multiplies within the macrophage (a professional phagocyte) in order to establish disease. Several receptors mediate uptake of Mtb that include, among others, complement receptors [1,2] and mannose receptors [3,4]. Disease progression in TB is associated with fever, cachexia and weight loss. These symptoms are associated with the biological activity of TNF-α[5]. Several mycobacterial components including proteins and glycolipids can directly activate macrophages to release these cytokines [6–10]. This was considered one explanation for the increased expression of TNF-α in advanced TB where there is a high bacterial load. However, host factors such as the opsonizing antibodies that enhance uptake of antigen may also participate in up-regulation of proinflammatory cytokines. High concentrations of opsonic antibodies (IgG1 and IgG3) have been reported in the advanced stages of leprosy and TB [11,12]. Macrophages have high-affinity Fc receptors for IgG1 and IgG3 antibodies [13,14]. Cross-linking of the Fc receptors in the presence of antigen-specific antibodies results in activation of macrophages and subsequent expression of cytokines [15]. Opsonic antibodies have been shown to enhance antigen uptake in leprosy patients [16] and augment PPD-induced TNF-α expression in purified monocytes [17]. This study addresses the role of opsonic antibodies present in patients with TB in the expression of proinflammatory cytokines.
MATERIALS AND METHODS
Antigens
Mtb culture filtrates were prepared as described previously [10]. Briefly Mtb strain H37 Rv was grown in roller bottles in Proskauer Beck medium. After 8–10 weeks of culture, bacilli were removed by sedimentation and filtration. Proteins were precipitated in 60% saturated ammonium sulphate, re-dissolved in H2O and dialysed against ultrapure water. Protein concentration was determined using the Bradford method [18]. Culture filtrate was separated by preparative SDS–PAGE using a PrepCell column (BioRad, Richmond, CA) [19]. Proteins were collected as they eluted from the lower edge of the gel by a pump. Mixing was prevented by a semi-permeable membrane. Approximately 80 fractions were collected. Groups of four adjacent fractions were pooled (a set of 20–24 fractions per run) and then simultaneously concentrated and dialysed using a cylindrical membrane device (Micro-ProDiCon; Spectrum, VWR, South Plainfield, NJ). Removal of lipids and glycolipids was accomplished by fractionation with Triton X-114 that had been preconditioned with endotoxin-free water. Triton X-114 1% was added to the solution to be decontaminated and was vortexed for 1 min. The sample was chilled on ice and vortexed again. It was then warmed to 37°C for 5 min to allow two phases to form, and then centrifuged for 10 s at maximum speed at 37°C. The upper aqueous phase was removed by pipette, taking care not to disturb the lower detergent phase. The above extraction was repeated once. Residual Triton X-114 was removed from the aqueous phase by addition of approx. 0·25 g endotoxin-free BioBeads. BioBeads SM-4 (BioRad) were made endotoxin-free by washing in 2% Triton X-114, methanol, and then endotoxin-free PBS. The beads were mixed with the specimen at 4°C for 1 h and then sedimented. Fractions were subjected to SDS–PAGE, transferred to nitrocellulose and colloidal gold staining was done to determine the approximate molecular size. MoAbs TBC-27 and 8G10 were used to detect alpha antigen and glutamine synthetase by Western blot analysis.
Plasma samples from controls and TB patients
Plasma samples were obtained from pulmonary TB patients (n = 8) with microscopically and culture-proven TB in Karachi, Pakistan. Patients had moderate (PMD) to advanced disease (PAD) that was ranked according to the tissue involvement as described previously [20]. Three healthy PPD skin test-negative donors were included as controls. Venous blood was collected in heparinized syringes. Heparinized blood was separated on a Ficoll layer and the top layer of plasma was carefully removed to avoid mixing with Ficoll. Sterile endotoxin-free conditions were used for handling and separation of blood samples. The plasma samples were distributed in small aliquots and stored at −70°C until further use.
Reagents, MoAbs and conjugates
Escherichia coli endotoxin (lipopolysaccharide (LPS)) and Polymyxin B (PMB) were obtained from Sigma Chemical Co. (St Louis, MO). MoAbs specific for human IgG subclasses were HP 6001 (anti-IgG1) and HP 6047 (anti-IgG3) prepared at the Centers for Disease Control (Atlanta, GA; a gift from the late Dr C. Reimer, Centers for Disease Control). The specificity evaluation and performance characteristics of these antibodies are described in detail elsewhere [21,22]. Goat anti-human IgG (Fc-specific) and goat anti-mouse IgG (H + L chain-specific) conjugated to alkaline phosphatase were purchased from (Jackson ImmunoResearch Labs, Westgrove, PA). Ficoll–Hypaque and Protein A–Sepharose 4B were obtained from Pharmacia (Piscataway, NJ).
Determination of IgG subclass activity in TB plasma
IgG subclass antibodies to the fractions (Fx10 and Fx24) in TB plasma were determined using an ELISA-based assay described in detail previously [13]. Briefly, Immulon 4 plates were coated with 100 μl of antigens at 1 μg/ml in carbonate buffer pH 9·6 for 2 h at 37°C and then overnight at 4°C. PBS containing 5% bovine serum albumin (BSA) was added for 2 h at 37°C to block free sites. Plasma (100 μl) diluted (multiple dilutions) in PBS containing 0·05% Tween 20 and 1·0% BSA was added and incubated for 2 h at 37°C and then overnight at 4°C. MoAbs specific for IgG subclasses were added at saturation concentrations of 1:1000 and further incubated overnight at 4°C. Alkaline phosphatase-labelled goat anti-mouse IgG was then added and incubated for 2 h at 37°C. The plates were finally developed with alkaline phosphatase substrate. The optical density (OD) in the ascending region of the dose–response curve was multiplied by the plasma dilution to give OD units/ml. All sera were run in a single assay to avoid interassay variability.
Absorption of plasma with protein ‘A’ to remove antibody activity
Protein A–Sepharose 4B was reconstituted in PBS and washed several times by repeated suspension and then equilibrated with RPMI to a final concentration of 50% v/v. Aliquots (0·5 ml) of the suspension were prepared and equal volume of plasma was added to the suspension. The tubes were rotated for 30 min at 37°C and the suspension was filtered through a 5·0-ml sterile syringe containing a small layer of sterile glass wool. The clear supernate was collected and distributed in small aliquots and stored at −70°C until further use.
Isolation of peripheral blood mononuclear cells and monocytes
Seven PPD skin test-negative donors (five males and two females) were included in the study. Venous blood was collected in heparinized syringes diluted 1:2 with RPMI 1640 and separated on Ficoll–Hypaque gradient at a centrifugal speed of 1200 rev/min for 30 min at room temperature. Peripheral blood mononuclear cells (PBMC) were isolated from the interface and washed three times in RPMI 1640. Approximately 50 × 106 cells were plated in 100-mm polystyrene tissue culture plates (no. 25020; Corning, Corning, NY) that had been precoated with 1·5 ml of heat-inactivated pooled human serum (PHS) for 30 min at 37°C. After 1 h of incubation at 37°C, plates were washed twice with warm 10% fetal calf serum (FCS) in RPMI 1640. Cold Hanks' balanced salt solution (HBSS; 5 ml) without calcium or magnesium was added and plates were placed in the refrigerator for 10–20 min. Adherent cells were scraped off and suspended in Iscove's modified Dulbecco's medium (IMDM) containing 1% heat-inactivated autologous serum. All serum and plasma samples were heat-inactivated at 56°C for 30 min to minimize any complement-mediated effects in the subsequent monocyte stimulation assay.
Stimulation of monocytes
LPS and culture filtrate fractions (0·1 μg/ml) were used to stimulate purified adherent cells (106 cells/ml) in the presence or absence of PMB (10 μg/ml). For assessing the role of opsonizing antibodies antigens were preincubated with control or TB plasma (1:500) at 37°C for 1 h in 48-well tissue culture plates (Costar, Cambridge MA) in a final volume of 250 μl/well followed by addition of 250 μl of monocytes. Monocytes were incubated at 37°C for 24 h and supernatants were collected and frozen at −70°C in 100-μl aliquots for cytokine assessment.
Assessment of TNF-α, IL-6 and IL-10 secretion
Monoclonal antibody pairs (capture and probe) for TNF-α and IL-10 were purchased from PharMingen (San Diego, CA). For IL-6 assessment antibody pairs were purchased from Endogen (Woburn, MA). Assessment of cytokines in stimulated monocyte supernatants was carried out using the standard methodology recommended by the manufacturer.
RESULTS
Effect of TB plasma on the release of TNF-α, IL-6 and IL-10 from PPD-stimulated monocytes
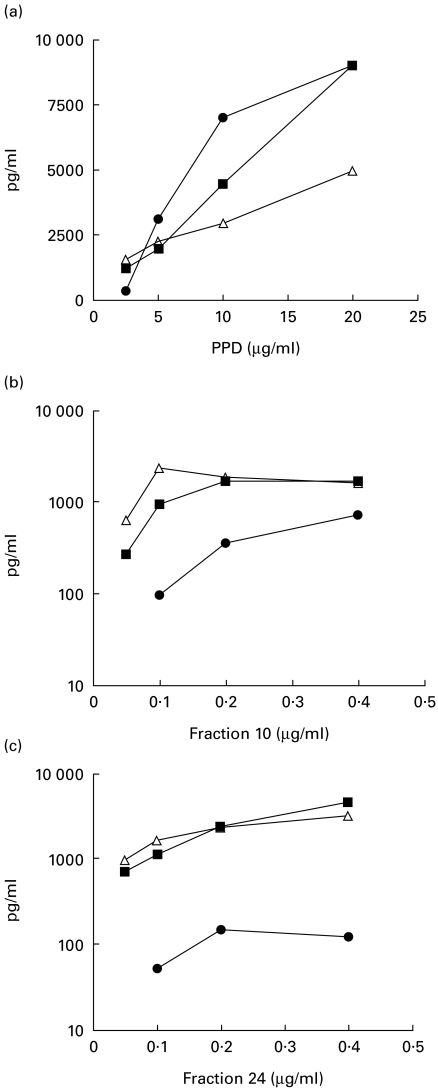
Monocytes stimulated with PPD release TNF-α[10]. We have recently shown that IgG1 anti-PPD antibodies in TB plasma further enhance this secretion [20]. We now extend these studies to analyse the effect of anti-PPD antibodies in TB plasma on PPD-induced expression of IL-6 and IL-10 from purified monocytes. PPD-stimulated monocytes release IL-6 and IL-10 in a concentration-dependent fashion (Fig. 2) similar to that shown with TNF-α. We used a concentration of 10 μg/ml of PPD to analyse further the effect of TB plasma containing PPD-specific antibodies. Table 1 shows the results from six donors. Control plasma had little or no effect on the secretion of TNF-α, IL-6 or IL-10 release from PPD-stimulated monocytes. Thirty-five percent (3/8) TB plasma significantly enhanced IL-6 and 25% (2/8) TB plasma also had a stimulatory effect on IL-10 secretion. As reported previously [20], 50% (4/8) of the TB plasma showed a consistent stimulatory effect on the secretion of TNF-α. PPD is a complex mixture of proteins and glycolipids that may interfere with monocyte activation as well as mask the effects of opsonizing antibodies. We therefore fractionated the secreted antigens in culture medium of Mtb to develop a less heterogeneous antigenic mixture devoid of lipids and glycolipids in order to analyse the role of opsonizing antibodies in monokine release
Fig. 2.
Kinetics of cytokine (TNF-α (•), IL-6 (▪) and IL-10 (Δ)) release from antigen-stimulated monocytes. Purified adherent cells (1 × 106 cells in 500 μl) were stimulated with various concentrations of PPD, Fx10 and Fx24. Cytokines were assessed in the supernatants after 24 h of antigen stimulation using ELISA assays.
Table 1.
Effect of tuberculosis (TB) plasma on PPD-induced monokine secretion
| Stimulant | TNF-α† | (P)* | IL-6† | (P)* | IL-10† | (P)* |
|---|---|---|---|---|---|---|
| Spontaneous | ||||||
| PPD (10 μg/ml) | 46 ± 28 | 6 ± 4 | 1 ± 0 | |||
| + Ctrl plasma | 2274 ± 652 | 1592 ± 590 | 584 ± 371 | |||
| + TB plasma | 2380 ± 768 | 1587 ± 504 | 672 ± 162 | |||
| TB05 | 2118 ± 715 | (0·4) | 1859 ± 722 | (0·11) | 737 ± 103 | (0·46) |
| TB09 | 2148 ± 528 | (0·6) | 1747 ± 629 | (0·17) | 698 ± 75 | (0·46) |
| TB55 | 2337 ± 545 | (0·6) | 1926 ± 667 | (0·046) | 787 ± 124 | (0·46) |
| TB90 | 2354 ± 534 | (0·4) | 2222 ± 1045 | (0·116) | 991 ± 135 | (0·917) |
| TB149 | 5129 ± 1044 | (0·006) | 2828 ± 1602 | (0·028) | 826 ± 193 | (0·173) |
| TB153 | 5086 ± 1410 | (0·007) | 2213 ± 1103 | (0·463) | 672 ± 179 | (0·463) |
| TB157 | 4352 ± 1305 | (0·007) | 2405 ± 1260 | (0·173) | 966 ± 179 | (0·046) |
| TB167 | 4107 ± 949 | (0·037) | 2555 ± 1409 | (0·043) | 1012 ± 118 | (0·028) |
| P versus PPD alone | 0·032 | 0·001 | 0·001 | |||
n = 6. All values are given as pg/ml (mean ± s.e.m.).
Significance was determined by paired t-test in PPD-stimulated monocytes in the presence of control plasma versus TB plasma.
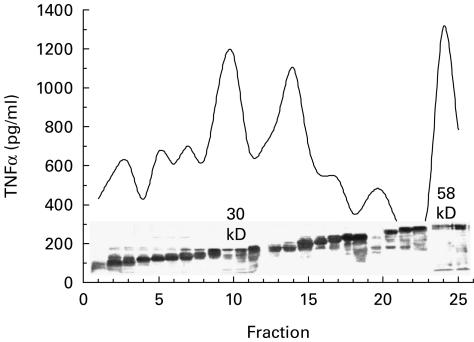
Fractionation of culture filtrate proteins and assessment of TNF-α releasing activity
Figure 1 shows a protein stain of fractions of Mtb culture filtrate. Superimposed is the capacity of each fraction to stimulate production of TNF-α by monocytes. Three fractions (10, 14 and 24) stimulated TNF-α from monocytes of a PPD skin test-negative donor. Fx10 contained alpha antigen (30 kD) and Fx24 glutamine synthetase (58 kD) as detected by MoAbs (TBC-27 and 8G10). These fractions were found to be negative for endotoxin contamination by Limulus lysate (assay sensitivity 0·01–0·04 ng/ml). Fraction 14 contained lipoarabinomannan (LAM) that is known to induce TNF-α via the mannose receptor [2] and was therefore excluded from the study.
Fig. 1.
Fractionation of TNF-α stimulating fractions. Separation of culture filtrate antigens using Prep cell as described. Pooled fractions were run on a 10% acrylamide gel, transferred to nitrocellulose paper and stained with aurodye. Each fraction was also monitored for its capacity to directly stimulate TNF-α in purified adherent cell population. All fractions were tested at a concentration of 0·1 μg/ml.
Dose–response relationship of cytokine release by PPD, Fx10 and Fx24
Figure 2 shows the dose–response relationship of PPD, Fx10 and 24 with three monocyte cytokines: TNF-α, IL-6 and IL-10. As expected, the partially purified Fx10 and 24 were much more potent in stimulating the monocytes' cytokine secretion than PPD. Secretion was optimal at 0·2 μg/ml for both fractions and for all three cytokines. For analysing the effect of opsonizing antibodies a suboptimal concentration (0·1 μg/ml) was selected for the two fractions in the monocyte stimulation assays.
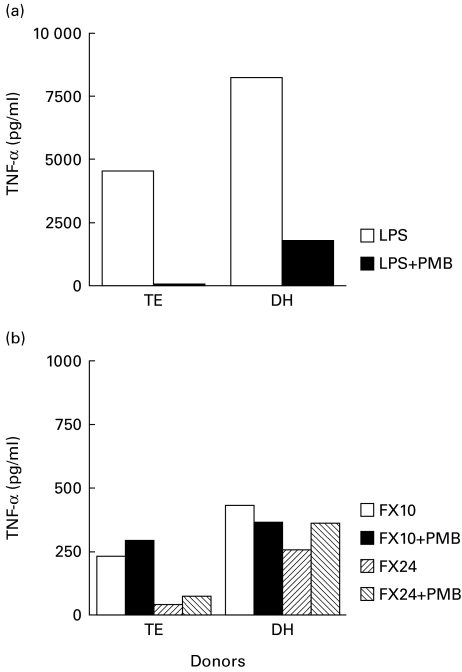
Assessment of LPS contamination in Fx10 and 24
Even extremely low concentrations of LPS contamination in the fractions may contribute to TNF-α secretion. We therefore tested the fractions in the presence of PMB that specifically inhibits LPS-induced TNF-α secretion. Figure 3 shows the results in two PPD skin test-negative donors. As expected, LPS induced high concentrations of TNF-α secretion from purified monocytes. This secretion was inhibited (> 95%) by PMB, indicating that our monocyte system was functioning optimally. Fx10 and 24 at similar concentrations induced much less TNF-α secretion compared with LPS, but this secretion was not inhibited by PMB, further supporting that the fractions were not contaminated by LPS. This assay system was used to test the effect of opsonizing antibodies
Fig. 3.
Functional assessment of adherent cell assay for TNF-α release from two skin test-negative donors (TE and DH). Purified adherent cells (1 × 106 cells in 500 μl) were stimulated with either lipopolysaccharide (LPS; 0·1 μg), Fx10 (0·1 μg) or Fx24 (0·1 μg) in the presence or absence of Polymyxin B (PMB; 10 μg).
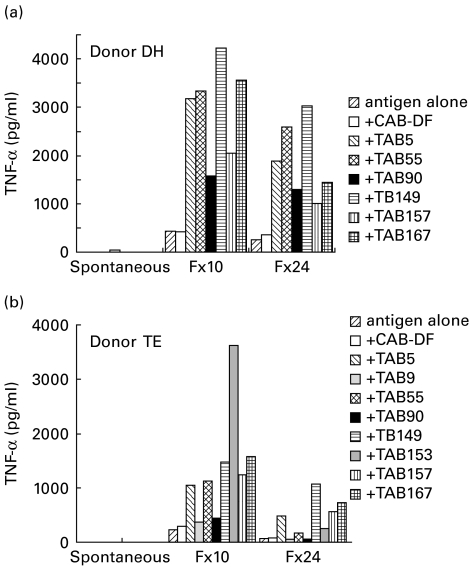
Antibody-induced enhancement of monocyte cytokine secretion in Fx10- and Fx24-stimulated monocytes
TNF-a secretion
Figure 4 shows TNF-α secretion with Fx10- and Fx24-stimulated monocytes in the presence or absence of either control or TB plasma. Spontaneous release by control and TB plasma in the absence of added antigen was <10 pg/ml, ruling out inadvertent LPS contamination of plasma samples. Control plasma showed similar levels of TNF-α secretion to antigen alone in both donors. Several TB plasmas showed significant augmentation of TNF-α secretion.
Fig. 4.
Augmentation of TNF-α secretion in the presence of tuberculosis plasma from purified adherent cells. Purified adherent cells (1 × 106 cells in 500 μl) were stimulated with either Fx10 (0·1 μg) or Fx24 (0·1 μg) in the presence of control plasma or TB plasma or antigen alone. TNF-α was assessed in the supernatants after 24 h of stimulation by ELISA. Spontaneous release of TNF-α by adherent cells was also assessed for plasma samples without any added stimulant (spontaneous), and was <10 pg/ml for all plasma samples.
Comparative release of TNF-α, IL-6 and IL-10 in antigen-stimulated monocytes
Table 2 shows the release of TNF-α, IL-6 and IL-10 to both the mycobacterial fractions (10 and 24) in an individual donor. Antigen stimulation was carried out in the presence of plasma from either healthy control or TB patients. Results are tabulated as percent change in the presence of plasma compared with stimulation with antigen alone (Table 2). The first panel in Table 2 shows the percentage change in TNF-α secretion. TB plasma significantly enhanced TNF-α production in Fx10- and Fx24-stimulated monocytes (P = 0·01). IL-6 production was also significantly enhanced by TB plasma in Fx10-stimulated monocytes (P = 0·004), but only a trend was evident for Fx24-stimulated monocytes (P = 0·06). Significant augmentation of IL-10 responses by TB plasma was observed with Fx24 (P = 0·011) but not with Fx10 (P = 0·092).
Table 2.
Effect of tuberculosis (TB) plasma on antigen-stimulated secretion of TNF-α, IL-6 and IL-10
| TNF-α | IL-6 | IL-10 | ||||
|---|---|---|---|---|---|---|
| Stimulus Antigen alone | Fx10 (30 kD) 233 (pg/ml) % change | Fx24 (58 kD) 42 (pg/ml) % change | Fx10 (30 kD) 926 (pg/ml) % change | Fx24 (58 kD) 268 (pg/ml) % change | Fx10 (30 kD) 106 (pg/ml) % change | Fx24 (58 kD) 49 (pg/ml) % change |
| + Control plasma | 26 | 95 | −42·9 | 9·3 | 33·9 | 0 |
| + TB plasma | ||||||
| TB05 | 356 | 1057 | −4·9 | 3·0 | 12·2 | 10·2 |
| TB09 | 61·7 | 692 | −30 | 21·6 | −5·6 | 132 |
| TB55 | 392 | 317 | 19·2 | 9·3 | 29·2 | 51 |
| TB90 | 88 | 64 | 33 | 90·6 | 107 | 143 |
| TB149 | 545 | 2464 | 69·9 | 23·8 | 105 | 153 |
| TB153 | 1475 | 504 | 235 | 860 | 245 | 16·3 |
| TB157 | 441 | 1247 | 122 | 429 | −10·3 | 316 |
| TB167 | 586 | 1647 | 153 | 154 | 2·8 | 122 |
| TB mean (95% CI) | 493 (125–861) | 999 (343–1655) | 74·7 (0–150) | 198·9 (−54–452) | 60·6 (−12·9–134) | 117·9 (36–200) |
| *P versus control | 0·01 | 0·01 | 0·004 | 0·06 | 0·092 | 0·011 |
Significance determined by one-sample t-test.
The variability in different TB plasma may be related to quantitative as well as qualitative differences in antibodies to each of these fractions. To understand further the role of opsonizing antibodies, concentrations of opsonizing antibodies to each of the fractions were carried out and cytokines were assessed in the presence and absence of IgG1 antibodies to these fractions
Role of opsonic antibodies (IgG1 and IgG3) in cytokine enhancing activity
Affinity depletion of IgG1 antibodies
Figure 5 shows IgG1 and IgG3 antibody concentrations to Fx10 and 24, pre- and post-Protein ‘A’ treatment. Antibody concentrations are expressed as OD units/ml (OD × dilution). Concentrations of IgG1 antibodies were higher to Fx24 than Fx10 (P = 0·02 by paired t-test). Treatment of plasma with Protein ‘A’–Sepharose depleted the levels of IgG1 antibodies to Fx10 and 24 to <5% of pretreatment values (P < 0·003). Much fewer plasma samples were positive for IgG3 antibodies, and as expected Protein ‘A’ treatment resulted in much less removal of IgG3 antibodies from plasma samples (P > 0·5). In two plasma samples (TB153 and TB157) higher concentrations of IgG3 anti-Fx24 antibodies were detected post-treatment, indicating that there may have been competition between different IgG subclasses.
Fig. 5.
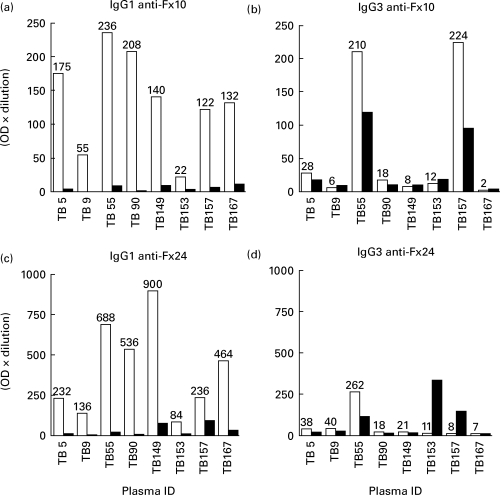
Absorption of IgG1 antibodies from plasma samples obtained from tuberculosis patients. Plasma samples were subjected to protein ‘A’ absorption. IgG1 and IgG3 antibodies were determined in untreated (□) or protein ‘A’-treated plasma (▪) to Fx10 (top panel) and to Fx24 (bottom panel). Antibody activity is expressed as optical density (OD) units/ml (OD × dilution of the plasma).
Effect of IgG1 antibody depletion on cytokine-enhancing activity of TB plasma
The effect of removal of IgG1 antibodies from TB plasma on cytokine secretion in antigen-stimulated monocytes is shown in Table 3a (Fx10) and Table 3b (Fx24). Removal of IgG1 resulted in highly significant decreases in antigen-induced TNF-α and IL-6 release to Fx10 as well as Fx24 when compared with pretreatment TB plasma. TB plasma on the other hand showed an opposite effect in antigen-stimulated monocytes on IL-10 release. In the case of Fx10 there was significant enhancement of IL-10 release in IgG1-absorbed TB plasma (P < 0·006). With Fx24, although the mean increase was >50%, overall significance was not achieved (P = 0·17). Interestingly, three TB plasma (TB55, TB153 and TB157) that showed the most enhanced IL-10 release in the absence of IgG1 contained high concentrations of IgG3 antibodies to the two fractions. It is therefore tempting to suggest that IgG3 antibodies may up-regulate IL-10 secretion in the absence IgG1 antibodies. However, results with both fractions for IL-10 are consistent in that they support that IgG1 plays little or no role in enhancing IL-10 secretion, while the most significant impact of IgG1 is on TNF-α secretion. These studies highlight the role that opsonic antibodies may play in cytokine balance during mycobacterial infections.
Table 3a.
a. Effect of protein ‘A’ absorbtion of tuberculosis (TB) plasma on antigen-stimulated (Fx10) monocyte section of cytokines
| TNF-α | IL-6 | IL-10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma ID | Pre | Post | % change | Pre | Post | % change | Pre | Post | % change |
| TB05 | 738 | 94 | −87·2 | 881 | 536 | −39·1 | 148 | 242 | +63·5 |
| TB09 | 364 | 66 | −81·8 | 674 | 820 | +26·7 | 122 | 146 | +19·6 |
| TB55 | 1026 | 290 | −71·7 | 1104 | 785 | −28·8 | 122 | 234 | +91·8 |
| TB90 | 656 | 180 | −72·5 | 1232 | 776 | −37 | 194 | 215 | +10·8 |
| TB149 | 1291 | 464 | −64 | 1539 | 1032 | −32·9 | 191 | 220 | +15·1 |
| TB153 | 1156 | 701 | −39·3 | 3107 | 1885 | −64·8 | 417 | 440 | +5·5 |
| TB157 | 1057 | 453 | − 7·1 | 2036 | 1225 | −39·8 | 98 | 172 | +75·5 |
| TB167 | 1362 | 556 | −59·1 | 2336 | 1127 | −51·7 | 116 | 140 | +20·6 |
| Mean (95% CI) | 605 (450–761) | 587 (190–983) | −50 (−81·276–18·974) | ||||||
| *P versus prior to protein A absorption | P < 0·0001 | P < 0·009 | P < 0·006 | ||||||
Expression of all TNF-α and IL-6 was significantly decreased following absorption of plasma using protein A. A trend towards increased expression of IL-10 is evident.
Significance determined by paired t-test using Fisher's two-tailed analysis.
Table 3b.
b. Effect of protein ‘A’ absorbtion of TB plasma on antigen-stimulated (Fx24) monocyte section of cytokines
| TNF-α | IL-6 | IL-10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma ID | Pre | Post | % change | Pre | Post | % change | Pre | Post | % change |
| TB05 | 88 | 0 | −100 | 276 | 9·8 | −96·4 | 58 | 132 | +127 |
| TB09 | 350 | 0 | −100 | 326 | 143 | −56 | 140 | 175 | +25 |
| TB55 | 211 | 9·1 | −95·6 | 276 | 84·5 | −69 | 87 | 148 | +70 |
| TB90 | 83 | 0 | −100 | 511 | 184 | −63·9 | 148 | 135 | −8·7 |
| TB149 | 742 | 16·1 | −17·8 | 873 | 359 | −58·8 | 154 | 194 | +25·9 |
| TB153 | 287 | 305 | 6·27 | 2573 | 193 | −92·4 | 63 | 550 | +773 |
| TB157 | 585 | 4·5 | −99·2 | 1394 | 359 | −74·2 | 180 | 159 | −11·6 |
| TB167 | 632 | 32·5 | −94·8 | 671 | 316 | −52·9 | 25 | 57·5 | +130 |
| Mean (95% CI) | 326 (92·1–560) | 656 (30·3–1282) | − 60·7 (− 224·8–50·94) | ||||||
| *P versus control | P = 0·013 | P = 0·04 | P = 0·17 | ||||||
Expression of all TNF-α and IL-6 was significantly decreased following absorption of plasma using protein A. A trend towards increased expression of IL-10 is evident.
Significance determined by paired t-test using Fisher's two-tailed analysis.
DISCUSSION
Antibodies conventionally are considered to play little or no role in defence against mycobacteria and the role of antibodies in pathogenesis is yet to be determined. We have recently reported that IgG1 anti-PPD antibodies augmented TNF-α secretion in PPD-stimulated monocytes, suggesting a role for antibodies in disease symptomatology associated with TNF-α in advanced TB [17]. This study extends these observations to another proinflammatory cytokine IL-6 and macrophage down-regulatory cytokine IL-10. Both these cytokines are considered to play an important role in B cell differentiation and class switching [23]. The effects of eight TB plasma on IL-6 and IL-10 secretion were not as consistent or dramatic in PPD-stimulated monocytes as those observed with the partially purified Fx10 and Fx24. There was considerable variability within donors and an even greater variability was introduced by the complexity of PPD, which is a complex mixture of proteins and glycolipids prepared from the spent culture medium of Mtb. To have a more defined system we fractionated antigens from culture filtrate containing the known 30-kD fibronectin binding alpha antigen [24] and 58-kD glutamine synthetase [25]. Both these proteins have previously been demonstrated to stimulate directly monocyte production of TNF-α[19,26]. Care was taken to exclude glycolipids and endotoxin from these preparations that may influence cytokine expression. Furthermore, to observe the effect of antibodies the antigenic fractions were used at suboptimal concentrations. The extent of TNF-α augmentation in the presence of antigen-specific antibodies with both fractions was significantly greater than that observed previously with PPD. In the case of Mtb 30-kD alpha antigen, binding to plasma fibronectin has previously been shown to enhance its capacity to stimulate release of TNF-α[26]. We did not observe such enhancement in the presence of at least three control plasmas (data not shown). The contribution of other receptors (mannose receptor or complement receptors) should also be minimal, if any, as LAM-containing fractions were excluded and heat inactivation of plasma samples was carried out to deactivate complement components. Thus the main contribution in antigen uptake should be mediated by the Fc receptors in this system. Antigen-induced expression of TNF-α, IL-6 and IL-10 was significantly enhanced only in the presence of TB plasma.
That IgG1 antibodies played a significant role in the enhancement of proinflammatory cytokines was further confirmed by absorption of plasma samples with Protein ‘A’, which selectively binds to the Fc portion of human IgG1, IgG2 and IgG4 but not to IgG3 antibodies [27,28]. Depletion of IgG1 either had no effect on the enhancing activity of IL-10 secretion or in some cases showed a significant increase in IL-10 secretion. Interestingly, three plasmas that resulted in a significant increase of IL-10 also had the highest concentrations of IgG3 antibodies. This effect of IgG3-containing plasma was restricted to IL-10 only, suggesting a role for IgG3 in the up-regulation of IL-10. Although IgG2 and IgG4 antibodies are also removed by Protein ‘A’ treatment, these subclasses do not bind to Fc receptors on monocytes. IgG2 antibodies are prominently directed to carbohydrate antigens and IgG4 is present in minute amounts, making it unlikely for either one to compete for antigen in immune complex-mediated uptake.
Similar effects of IgG1 antibodies on TNF-α and IL-6 are not surprising, since co-ordinate expression of these two cytokines has been observed in several systems [23]. This may be due to utilization of the same signalling pathway by TNF-α and IL-6 [29,30]. The signalling pathway for IL-10 is known to be distinct from the proinflammatory cytokines [31]. It is tempting to speculate that IgG3 may be activating via that pathway. However, further studies with purified IgG subtypes and blocking of Fcγ receptors are warranted to draw a firm conclusion. These experiments are now in progress.
The main proinflammatory cytokines in TB are TNF-α (produced by activated macrophages and T cells) and interferon-gamma (IFN-γ) by CD4, CD8, γδ, and natural killer cells [32,33]. Both of these cytokines participate in granuloma formation [34,35]. TNF-α also synergizes with IFN-γ in its tuberculostatic activity [36]. However, steady TNF-α production during chronic TB is not without cost to the host, resulting in cachexia and weight loss in advanced disease. These effects are counteracted by inhibitory cytokines such as IL-10 and transforming growth factor-beta, which down-regulate macrophage activity [37,38]. The balance between these cytokines may thus determine the pathogenic/protective axis. Our findings therefore have several important clinical implications, particularly for advanced TB where opsonizing antibodies are raised and may be aggravating the disease pathology by, first, amplifying the proinflammatory cytokine circuit and second, by interfering with the secretion of down-regulatory cytokines such as IL-10.
Acknowledgments
The authors wish to thank Dr Ghaffar Dawood for providing patient material, Mrs Maqboola Dojki and Miss Shabnum Karim for excellent technical help. Secretarial help by Miss Regina Paul is gratefully acknowledged. We would also like to acknowledge Mr Iqbal Azam (Community Health Sciences Department, Aga Khan University) for help with statistical analysis.
REFERENCES
- 1.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1999;144:2771–80. [PubMed] [Google Scholar]
- 2.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 3.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomann from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–8. [PubMed] [Google Scholar]
- 4.Schlesinger LS, Kaufman TM, Lyer S, Hull SR, Marchiando LK. Difference of mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–75. [PubMed] [Google Scholar]
- 5.Dinarello CA, Cannon JG, Wolff SM, et al. Tumor necrosis factor (Cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–50. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valone SE, Rich EA, Wallis RS, Ellner JJ. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and Mycobacterial antigens. Infect Immun. 1988;58:3313–5. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedland JS, Hartley JC, Hartley CGC, Shattock RJ, Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–8. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall BG, Chambers MA, Wangoo A, Shaw RJ, Young DB. Production of tumor necrosis factor and nitric oxide by macrophages infected with live and dead mycobacteria and their suppression by an interleukin-10-secreting recombinant. Infect Immun. 1997;65:1931–5. doi: 10.1128/iai.65.5.1931-1935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toossi Z, Young T-G, Averill LE, Hamilton BD, Shiratsuchi H, Ellner JJ. Induction of transforming growth factor beta 1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–8. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallis RS, Amir-Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: The monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–52. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain R, Kifayet A, Chiang TJ. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–5. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain R, Dawood G, Abrar N, et al. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Lab Immunol. 1995;2:726–32. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber HS, Douglas D, Nusbacher J, Kochwa S, Rosenfield RE. IgG subclass specificity of human monocyte receptor sites. Nature. 1971;229:419. doi: 10.1038/229419a0. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CL, Abraham GN. Characterization of the Fc receptors for IgG on a human macrophage cell line, U937. J Immunol. 1980;125:2735–41. [PubMed] [Google Scholar]
- 15.Heller T, Gessner JE, Schmidth RE, Klos A, Bautsch W, Kohl J. Fc receptor type I for IgG on macrophages and complement mediate the inflammatory response in immune complex peritonitis. J Immunol. 1999;162:5657–61. [PubMed] [Google Scholar]
- 16.Hasan R, Dockrell HM, Jamil S, Chiang TJ, Hussain R. Antigen-coated latex particles as a model system for probing monocyte responses in leprosy. Infect Immun. 1993;61:3724–9. doi: 10.1128/iai.61.9.3724-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain R, Shiratsuchi H, Ellner JJ, Wallis RS. PPD-specific IgG1 antibody subclass upregulate TNF α expression in PPD stimulated monocytes. Possible link with disease pathogenesis in tuberculosis. Clin Exp Immunol. 2000;119:449–55. doi: 10.1046/j.1365-2249.2000.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Wallis RS, Paranjape R, Philip M. Identification by two dimensional gel electrophoresis of a 58-kilodalton tumor necrosis factor-inducing protein of Mycobacterium tuberculosis. Infect Immun. 1993;61:627–32. doi: 10.1128/iai.61.2.627-632.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain R, Hasan R, Khurshid M, Sturm AW, Ellner JJ, Dawood G. Pulmonary tuberculosis in a BCG vaccinated area: relationship of disease severity with immunological and hematological parameters and drug resistance patterns. Southeast Asian J Trop Med Public Health. 1996;27:257–62. [PubMed] [Google Scholar]
- 21.Hussain R, Poindexter RW, Wistar R, Reimer CB. Use of monoclonal antibodies to quantify subclass of human IgG. I. Development of two-site immunoenzymometric assays for total IgG subclass determinations. J Immunol Methods. 1986;93:89–96. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- 22.Hussain R, Poindexter RW, Ottesen EA, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define antigen specific (anti-filarial) IgG subclass antibodies. J Immunol Methods. 1996;94:73–80. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- 23.Bendtzen K. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Letters. 1988;19:183–92. doi: 10.1016/0165-2478(88)90141-1. [DOI] [PubMed] [Google Scholar]
- 24.Harth G, Lee B-Y, Clemens DL, Hortwitz MA. Novel insights into genetics, biochemistry, and immunochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–47. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harth G, Clemens DL, Horwitz MA. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci USA. 1994;91:9342–6. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aung H, Toossi Z, Wisnieski JJ, et al. Induction of monocyte expression of TNF α by the 30 kD alpha antigen of Mycobacterium tuberculosis, and synergism with fibronectin. J Clin Invest. 1996;98:1261. doi: 10.1172/JCI118910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hjelm H, Hjelm K, Sjoquist J. Protein ‘A’ from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Letters. 1972;28:73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- 28.Recht B, Frangione B, Franklin E, van Loghem E. Structural studies of a human gamma3 myeloma protein (GOE) that binds Staph protein A. J Immunol. 1981;171:917–23. [PubMed] [Google Scholar]
- 29.Pan XQ, Darby C, Indik ZK, Schreiber AD. Activation of three classes of non receptor tyrosine kinases following Fc gamma receptor cross linking in human monocytes. Clin Immunol. 1999;90:55–64. doi: 10.1006/clim.1998.4644. [DOI] [PubMed] [Google Scholar]
- 30.Bermudez LE, Young KS. Tumor necrosis factor alpha stimulates mycobactericidal/mycobacteriostatic activity in human macrophages by a protein kinase C-dependent pathway. Cell Immunol. 1992;144:258–68. doi: 10.1016/0008-8749(92)90243-i. [DOI] [PubMed] [Google Scholar]
- 31.Oswald IP, Wynn TA, Sher A, James SL. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as costimulatory factor for interferon gamma induced activation. Proc Natl Acad Sci USA. 1992;89:8676–80. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerksiek KM, Pamer EG. T cell responses to bacterial infections. Curr Opin Immunol. 1999;11:400–5. doi: 10.1016/S0952-7915(99)80067-3. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa T, Uchida H, Kusumoto Y, Mori Y, Yamamura Y, Hamada S. Increase in tumor necrosis factor alpha and interleukin-6 secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991;59:3021. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bermudez LE, Kaplan G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 1995;3:22–27. doi: 10.1016/s0966-842x(00)88864-2. [DOI] [PubMed] [Google Scholar]
- 35.Kindler V, Sappino AP, Grau GE, Piquet PF, Vassali P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 36.Flesch IEA, Kaufmann SHE. Activation of tuberculostatic macrophage functions by interferon gamma, interleukin 4 and tumor necrosis factor. Infect Immun. 1990;58:269. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in Mycobacterial infection. J Immunol. 1997;158:315–21. [PubMed] [Google Scholar]
- 38.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]