Abstract
Th2 cells are more abundant than Th1 cells in periodontitis lesions, but the relative importance of the Th1 and Th2 subsets in periodontal disease is not understood. In addition, the role of proinflammatory and anti-inflammatory cytokines in this disease process is unclear. Biopsies were obtained from 10 patients with early onset periodontitis (EOP) and 10 patients with adult periodontitis (AP). From all of the patients in the AP group we were able to obtain and section the gingival tissue to serve as controls. We used polyclonal monospecific antibodies to detect cells expressing IL-2, IL-4, IL-6, IL-10 and IL-15, tumour necrosis factor (TNF-α) and interferon-gamma (IFN-γ) in formalin-fixed, paraffin-embedded sections of granulation tissue from periodontitis lesions. We also employed a series of oligonucleotide probes to detect cells expressing the cytokine transcripts in the same tissue biopsies. Cells that expressed IL-4 or IL-6 were more numerous than cells expressing either IL-2 or IFN-γ. Th2 cells were more numerous in EOP and AP tissues. IL-15 substitutes for IL-2 in a number of biological activities related to the Th1 immune response, and interestingly, in periodontal lesions the IL-15-expressing cells outnumbered IL-2-expressing cells, suggesting that this is the pattern of immune regulation by T cells in the periodontium. The functional balance in the T cell subsets detected by their cytokine profiles underlies the importance of the anti-inflammatory mechanisms taking place in the diseased tissue. The numbers of inflammatory leucocytes that express the anti-inflammatory cytokine IL-10 are much more widely distributed than those that express the proinflammatory cytokines IL-6 and TNF-α. This study suggests that large numbers of infiltrating inflammatory cells as well as accessory cells are involved in the down-regulation of the inflammatory and immune response in periodontitis.
Keywords: periodontitis, leucocyte infiltrate, T cell, B cell, macrophage
INTRODUCTION
Complex inflammatory and immune responses are involved in the progression of periodontal disease [1,2]. B cells and T cells accumulate in large numbers in the periodontal tissues, although until recently we have had little information on cellular synthetic activity and proliferation of these cells. Tissue culture experiments have shown that T cells are involved in the modulation of immunoglobulin synthesis [3–5]. However, the results of these studies do not easily extrapolate to the in vivo situation where complex interactions between a variety of cells of the inflammatory infiltrate occur. When an assessment is made of the role that different cell types play in the sites of inflammation one must be wary of the limitations imposed by merely observing morphology and phenotypic cell surface markers. In the past, immunohistochemical methods have been used for assessments of lymphocyte subsets [6–10], but controversies still exist regarding these reports; particularly, the discrepancies between different workers and interpretation of studies. Gemmell & Seymour [11] have shown an increased proportion of γδ T cells as the infiltrate increases in diseased gingival tissues. Others [12] have shown an increase in T cell numbers in peripheral blood and it has been suggested that homing of these cells to the gingiva may occur during the disease process. Reports from our laboratory [13] have revealed similarities in T cell gene rearrangement profiles between gingival biopsies from the same individual. Therefore, selective localization of different subsets of T cell clones may be occurring that would be consistent with the existence of gingiva-homing memory T cells. However, an alternative possibility exists, that specific T cells proliferate locally within certain tissues (in response to local antigenic stimulation), giving rise to characteristic T cell clones for these regions. Recent reports from our laboratory suggest that this is an unlikely scenario in periodontal tissues due to the absence of proliferation markers noted in the lesional lymphocytes [14,15].
Analysis of the cytokine profiles of the T cell subsets Th1 and Th2 in periodontal tissues have been made using distinctly different strategies. Gemmell & Seymour [10] performed FACs analysis of leucocytes extracted from periodontal tissues, while Yamazaki et al. [7] used an immunochemical method to detect the cells in periodontal lesions, and Manhart et al. [9] utilized a cell blotting technique to trap and identify IL-2- and IL-4-expressing T cells.
While most studies suggest that Th2 cells are more abundant than Th1 cells in periodontitis lesions, they conflict as to the relative importance of the Th1 and Th2 subsets in periodontal disease. A dominant role for Th1 has been suggested by one group, but they failed to detect IL-4-expressing cells [6], while detecting cells typical of Th1 (expressing interferon-gamma (IFN-γ)). Other workers are more convinced of a Th2 role, not only detecting IL-4 expression, but IL-5, IL-6 [7–9] and IL-10 [10]. The paradigms that ‘periodontitis is a B cell lesion’ and the ‘immunoregulatory role of T cells in periodontitis’ have been proposed following such analysis [10,16,17]. Current theories on T cell involvement relate that Th1 cells are tightly localized at sites undergoing an active disease process, whereas the Th2 cells are much more widely distributed throughout the tissue and typify a more quiescent stage of the disease [18]. What remains unclear is the relative importance of proinflammatory and anti-inflammatory mediators in this disease process and the clarification of this was an aim of the current study. To clarify this issue we aimed to use in situ hybridization and immunohistochemistry on early onset periodontitis (EOP) and adult periodontitis (AP) tissues to detect a panel of cell markers and cytokines.
MATERIALS AND METHODS
Tissue preparation
The tissues used in this study consisted of 10 granulation tissue biopsies from 10 patients (ages 42–62 years) with AP and 10 biopsies of granulation tissue from 10 patients (ages 24–39 years) with generalized EOP. Diagnostic criteria for EOP were according to Hart et al. [19] and were as follows: patients under the age of 35 years of age, exhibiting attachment loss (AL) of at least 5 mm on at least eight teeth, at least three of which were not first molars or incisors and at least one of which was a first molar. All the patients included in the study had advanced forms of periodontitis, with the exception of one patient with AP who had moderate to severe disease. The pocket depths of the sites biopsied ranged from 6·0 to 9·0 mm in AP patients and 5·8 to 9·6 mm in EOP patients. We also included for comparative purposes one section of gingival tissue from each of the same 10 patients with AP, which was obtained as part of a flap that was raised to obtain the granulation tissue; all the samples were recovered after surgery at periodontitis sites. We were only able to obtain three such flaps from our EOP patients and have not included them in this study. The tissues were immediately fixed in 10% neutral buffered formalin at room temperature. They were embedded in paraffin wax and 5-μ m serial sections were collected (and numbered) on silane-coated glass slides. Adjacent serial sections were used for in situ hybridization (ISH) and immunohistochemistry experiments; a typical set of sections would be analysed as follows: (i) ISH control RNase A-treated section with antisense probe; (ii) ISH sense oligo control; (iii) ISH antisense probe; (iv) IHC specific cytokine antibody; (v) CD4 or CD8 antibody; (vi) CD68 or CD20 antibody; (vii) IHC absorbed anti-cytokine control; (viii) second antibody. Two sections in each sample were used for morphological examination, which was based on haematoxylin and eosin (H–E) staining before ISH and immunohistochemistry.
Tissue handling and fixation prior to sectioning obviously affects the sensitivity of the in situ and immunohistochemistry methods. Rapid fixation of small pieces of tissue seems to be a prerequisite. Prolonged storage in formalin fixative prior to sectioning may be detrimental to the sensitivity of the ISH technique. Furthermore, unmasking of cellular antigens by boiling in EDTA is required for the recognition of the relevant epitopes by the anti-cytokine MoAbs. Polyclonal antibodies also reacted more strongly after this treatment. Care had to be exercised when microwaving sections, because prolonged treatment tended to increase the amount of background staining when the polyclonals were used to detect cytokine-expressing cells without increasing the numbers of positively stained cells in the sections (data not shown).
Oligonucleotide probes
Digoxigenin (DIG)-labelled oligonucleotide (30mer) probe cocktails for IL-2 (BPR 13), IL-4 (BPR 22), IL-6 (BPR 32) and IFN-γ (BPR 216) were purchased from R&D Systems Europe Ltd (Abingdon, UK). For the detection of IL-10 mRNA and IL-15 mRNA we designed and created our own probes. For each probe three strings were chosen of approximately 30–45 nucleotides in length. Both antisense (Probe) and sense strands were synthesized using the cyanoethyl phosphoramidite method on an Applied Biosystems (Foster City, CA, USA) automated DNA synthesizer.
IL-10-probes
(1) 5′ AAGGCTTTGCAACCCAAGTAACCCTTAAATCCT 3′; (2) 5′ GCCTTGCTCTATTTTCACAGGGGAGAAATCGATGACGCGCC 3′; (3) 5′ ATATTGGGATTCTTTCTAAATAGTTCAACGCCGCTCAGTG 3′.
IL-15 probes
(1) 5′ CACTGACAGCCCAAAATGAAGACATGAATGCCAGCCTCAG 3′; (2) 5′ GAGAAAGCAGTTCATTGCAGTAACTTTGCAACTGGG 3′; (3) 5′ TCCTCCAGCTCCTCACATTCCCTTGCAGCCAGATTCTGCTACATCC 3′.
The Probes (antisense) strands and sense strands were then mixed in equimolar amounts, then labelled with DIG-11-dUTP (Boehringer, Mannheim, Germany) according to the manufacturer's guidelines. Briefly, 1–2 μg probe was 3′-end labelled with a mixture containing 1 μl DIG-11-dUTP, 4 μl of 5× reaction buffer (potassium cacodylate, 500 mm pH 7·2; 10 mm CoCl2; 100 mm DTT) and 2 μl 30 U terminal deoxynucleotidyl transferase (TdT; Gibco BRL, Paisley, UK). The labelling reaction was performed at 37°C for 3–5 h. Labelled probes were then precipitated by the addition of 1/10 volume of 200 mm EDTA, 1/10 volume of 4 m LiCl and three volumes of absolute ethanol. Following centrifugation and one wash in 95% ethanol in 0·1% diethyl pyrocarbonate (Sigma, Poole, UK)-treated double-distilled water, the pellet was dissolved in sterile TE buffer (10 mm Tris–HCl, 10 mm EDTA, pH 7·5) and stored at −20°C. Probe labelling was confirmed by dot blot analysis.
In situ hybridization
All solutions were prepared with 0·1% diethyl pyrocarbonate (Sigma)-treated double-distilled water (DDW) or PBS. Briefly, slides were deparaffinized in xylene, hydrated through descending isopropyl alcohol concentrations, and washed in DDW. The slides were washed in PBS twice for 5 min and then digested with proteinase K (Sigma; 15 μg/ml for the granulation tissue and 60 μg/ml for the pieces of gingiva) in 100 mm Tris–HCl, 50 mm EDTA pH 8·0 for 30 min at 37°C. They were washed twice for 5 min in PBS and, at this stage, pretreatment with RNase A type 1A (Sigma) at 100 μg/ml in 2 × standard saline-citrate (SSC)/10 mm MgCl2 at 37°C for 1 h was performed as a negative control. Post-fixation was performed by incubation in 4% paraformaldehyde at 4°C for 5 min, followed by washing twice for 5 min in PBS. The slides were immersed in prehybridization buffer ( × 2 SSC and 50% formamide) for 2 h at 37°C. Hybridization mixtures for the probes were prepared and hybridization was performed as described in a previous study [20]. As a negative control, sections were incubated with hybridization buffer only or with sense probes. To determine the specificity of probe binding to tissue RNAs, sections were digested with RNase A prior to hybridization. The slides were rinsed in × 4 SSC, sequentially, in × 2 SSC at room temperature twice for 20 min, × 0·1 SSC at 37°C twice for 20 min, then washed in × 2 SSC for 10 min at room temperature.
Immunological detection of hybrids
The immunological detection of the DIG- or fluorescent-labelled oligonucleotide–mRNA complex was performed as described in previous reports [20]. In summary, the slides were placed for 3 h in PBS containing 0·01% Tween 20 (Sigma; PBS–T) and 5% non-fat dry milk (PBS–T–M) including alkaline phosphatase (AP)-conjugated sheep anti-DIG (1:100; Boehringer) or AP-conjugated at room temperature. After thorough rinsing in a PBS–T buffer and 5 min preincubation in an alkaline buffer solution pH 9·0, the AP complex was revealed with a freshly prepared solution of nitroblue tetrazolium, 5-bromo-4-chloro-3-indolylphosphate and levamisole (pH 9·0), overnight. The sections were then washed in double distilled H2O three times before counter-staining with neutral red and mounting in an aqueous mounting medium (DAKO).
Immunohistochemistry
MoAbs and polyclonal antibodies against human IL-2 (MAB 202 and AF-202-NA), IL-4 (MAB 204 and AF-204-NA), IL-6 (MAB 206 and AF-206-NA), IL-10 (MAB 217 and AF-217-NA), and IFN-γ (MAB 285 and AF-285-NA) (R&D Systems) and antibodies to the cluster of differentiation antigens: CD3, CD4, CD8, CD20, and CD68 (Dako, High Wycombe, UK) were obtained from the sources shown. Recombinant IL-2, IL-4 and IL-10 were purchased from R&D Systems and recombinant IL-6 and IFN-γ were gifts from Biogen (Geneva Switzerland). Tissue sections were deparaffinized as described above and immersed in DDW. To inhibit endogenous peroxidase, they were incubated with 3% H2O2 in methanol at 4°C for 15 min. The deparaffinized sections were heated in a conventional microwave oven (650 W) for 3 × 5 min in 0·001 m Di-Sodium EDTA pH 8·0 to unmask the antigen [21]. While undergoing microwave processing, slides were always covered with the solution. The slides were then permitted to cool down to room temperature over a period of 30–60 min, before washing in PBS pH 7·4. They were then blocked and incubated with MoAbs overnight at 4°C, washed in PBS and sequentially incubated with biotin-labelled goat anti-mouse IgG (Vectastain Elite ABC-POD kit; Vector Labs, Burlingame, CA) for 30 min. Polyclonal antibodies were incubated with the sections for 1 h at room temperature. Pre-absorptions of the monospecific polyclonal antibodies were carried out with 50 μg/ml recombinant antigen and 1:50 dilution of antibody for 1 h at room temperature. After washing in PBS the slides were incubated with biotin-labelled rabbit anti-goat IgG (Vectastain Elite ABC-POD kit; Vector Labs) for 30 min. The sections were then treated with DAB substrate kit (Vector Labs) for 2–7 min under microscopic observation and washed in DDW. Finally, the slides were counterstained with haematoxylin and mounted in aqueous mounting medium.
Cell counts
We used an eyepiece grid to select the fields prior to counting. In order to check that we could locate the same area accurately we first counted the cells within a field, and then moved the slide and repositioned it to locate the same field and counted the cells again. A second individual also counted the same fields, and the results gave an agreement >95%. To make this procedure less laborious images were captured with a digital camera (Olympus DP 10 mounted on an Olympus microscope) and stored on a computer for future reference and cell counting. This made identification of the same field in different tissue sections much easier since a captured image and a new microscope field could be viewed simultaneously. In calculating the ratios only the total number of cells in 12 fields for each tissue section were compared; these were chosen because of the presence of a marked inflammatory infiltrate in the original H–E tissue reference section. The cell counts were also averaged and expressed as cells per field.
RESULTS
In the 30 biopsy specimens used in the study we were able to detect the presence of the mRNA encoding each of the cytokines, or the proteins themselves, i.e. IFN-γ, IL-4, IL-6, and IL-10, in replicate tissue sections. Also in the adjacent sections it was possible to detect IL-15 mRNA and tumour necrosis factor-alpha (TNF-α) mRNA. In a subset of six tissue sections from this group (three EOP, two AP and one AP gingiva) we located a few cells that expressed IL-2 mRNA, where the protein was also detected in the adjacent serial tissue section, in what we considered to be the same cells. While every tissue biopsy contained some IL-2-expressing cells, many of the fields were devoid of IL-2-expressing cells.
Close inspection of the adjacent serial tissue sections enabled an approximate discrimination of the cell types stained by the cytokine-specific antibodies or mRNA probes. The mRNA and protein indicative of IL-2, IL-15 and IL-4 expression were observed in T cells. IL-6, IFN-γ and TNF-α expression was observed in T cells and in fibroblast-like cells, which may have included some tissue macrophages, and IL-10 expression was observed in many B cells, T cells, fibroblasts and macrophages (Figs 1–3).
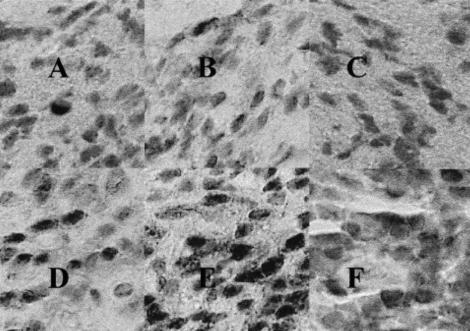
Fig. 1.
Serial sections of granulation tissue from a patient with early onset periodontitis (EOP) showing: immunohistochemical staining with (A) goat monospecific antibodies to IL-2 antigen (anti IL-2); (B) goat monospecific anti-IFN-γ; (C) monospecific goat anti-IL-6, which had been preabsorbed with human recombinant IL-6; (D) monospecific goat anti-IL-4; (E) monospecific goat anti-IL-6; (F) and monospecific goat anti-IL-10; mag. ×400. Similar results were seen in the tissues of all the patients in the study.
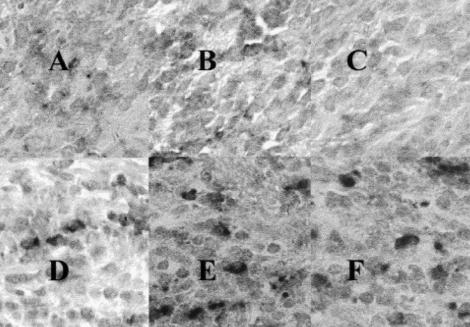
Fig. 3.
Serial sections from the same granulation tissue from the patient with early onset periodontitis (EOP), as for Figs 1 and 2, showing: immunohistochemistry and in situ hybridization with specific digoxigenin-labelled oligonucleotide probes. The panels represent: (A) a section stained with anti-CD3 (pan-T cell marker); (B) the detection of tumour necrosis factor-alpha (TNF-α) mRNA-expressing cells; (C) the detection of IL-15 mRNA-expressing cells; (D) a immunohistochemistry, second antibody (negative) control; (E) a tissue section pretreated with 10 μg/ml RNase A and probed for IL-10 mRNA-expressing cells; and (F) the detection of IL-10 mRNA-expressing cells; mag. ×400.
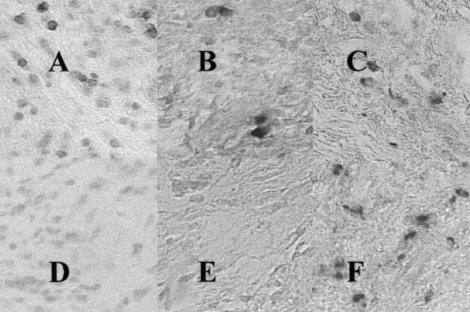
Fig. 2.
Serial sections from the same granulation tissue from the patient with early onset periodontitis (EOP0, as for Fig. 1, showing the corresponding fields and demonstrating: in situ hybridization with specific digoxigenin-labelled oligonucleotide probes for the detection of: (A) IL-2 mRNA; (B) IFN-γ (C) no probe added (negative control); (D) IL-4 mRNA; (E) IL-6 mRNA; (F) IL-10 mRNA; mag. ×400.
When the numbers of positive cells were counted in the sections, several aspects were evident. The polyclonal antibodies were generally more successful at detecting the proteins than the MoAbs in our paraffin-embedded tissue sections. Pre-absorption of the monospecific antibodies with recombinant antigen abrogated the staining in sections to which the absorbed antibodies were applied. We also tested our antibodies on frozen tissue sections (three AP and three EOP) and found similar staining patterns with both MoAbs and polyclonal antisera to those obtained with microwaved paraffin sections (data not shown). However, we were unable to detect mRNA in the frozen tissue sections, probably because the mRNA has been destroyed in the process. There were more cells positively stained by immunohistochemistry than by ISH techniques (Tables 1 and 2). The most widely and most abundantly expressed cytokine in the tissues was IL-10. This was followed by IL-6, IL-4, IFN-γ, then IL-2. The predominant T helper cell subset was Th2, based on the observation that cells which expressed IL-4 or IL-6 were far more numerous (Mann–Whitney U-test: P < 0·01) than cells that expressed either IL-2 or IFN-γ (Table 3).
Table 1.
Number of antigen-expressing cells/field
| EOP-GT | AP-GT | AP-Ging | ||||
|---|---|---|---|---|---|---|
| Antigen | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| CD3 | 138·9 | 35·3 | 81·7 | 26·8 | 91·6 | 22·3 |
| IL-2 | 5·3 | 5·0 | 3·8 | 4·5 | 2·7 | 2·9 |
| IFN-γ | 26·1 | 11·1 | 20·6 | 6·1 | 19·9 | 7·2 |
| IL-4 | 124·1 | 34·4 | 65·1 | 23·4 | 83·1 | 21·2 |
| IL-6 | 133·5 | 40·0 | 115·8 | 30·4 | 105·6 | 41·0 |
| IL-10 | 193·3 | 70·1 | 177·9 | 65·1 | 150·3 | 37·7 |
Each result represents the mean and s.d. of 10 sections.
Twelve fields were counted per section.
Table 2.
Number of mRNA-expressing cells/field
| EOP-GT | AP-GT | AP-Ging | ||||
|---|---|---|---|---|---|---|
| mRNA | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| IL-2 | 1·9 | 2·0 | 1·0 | 1·2 | 1·1 | 1·2 |
| IFN-γ | 19·1 | 8·2 | 15·9 | 5·9 | 14·4 | 5·2 |
| >IL-4 | 84·5 | 32·4 | 53·2 | 12·6 | 50·4 | 23·8 |
| IL-6 | 107·7 | 51·3 | 97·6 | 53·6 | 83·3 | 35·8 |
| IL-10 | 159·8 | 34·9 | 143·1 | 43·5 | 125·8 | 35·8 |
| TNF-α | 22·7 | 12·6 | 16·7 | 7·1 | 16·2 | 5·8 |
| IL-15 | 41·9 | 11·1 | 36·6 | 11·5 | 32·4 | 7·0 |
Each result represents the mean and a.d. of sections.
Twelve fields were couted per section.
Table 3.
Ratio of Th1:Th2 and percentage of CD3+ expressing IL-2 or IL-4
| EOP-GT | AP-GT | AP-Ging | ||||
|---|---|---|---|---|---|---|
| Ratio | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| IL-4/IL-2 | ||||||
| Antigen | 23·3 | 7·1 | 17·0 | 12·2 | 30·8 | 8·7 |
| mRNA | 44·5 | 11·6 | 53·2 | 12·8 | 45·8 | 13·2 |
| Percent CD3 | ||||||
| IL-2+ | 3·8 | 3·9 | 4·7 | 4·9 | 2·9 | 3·1 |
| IL-4+ | 89·3 | 13·9 | 79·7 | 18·2 | 90·7 | 14·2 |
Each result represents the mean and s.d. of 10 sections.
Twelve fields were counted per section.
We observed a greater number of IL-4-expressing cells (P < 0·02) in the EOP patient tissue sections compared with the AP sections. Overall, this was attributable to a greater number of T cells, confirmed by utilizing the pan T cell marker, CD3 MoAb, because the ratios of IL-4 to CD3+ cells were no different. We did not see significant differences in the expression of the other cytokines between the EOP and AP tissue sections. We did observe a slight, but non-significant, increase in the numbers of cytokine-expressing cells in the AP granulation tissue when compared with the gingival tissue sections, although the cytokine profile was virtually identical (Tables 1–3).
DISCUSSION
As reviewed in the Introduction, a number of studies have looked at the relative contribution of Th1 and Th2 cell types in periodontal disease [6–10], sometimes with conflicting results. Some workers argue for a predominance of ‘cell-mediated immunity’ for the progression of the disease [1,18], while others point out that the humoral response, mediated by Th2 cells, plays a protective role [22]. The results of this study indicate that the disease is complex. The dominance of a humoral type of response is clear in the chronic lesion, but there is room to speculate that in the early stages of the disease cell-mediated reactions are involved in disease progression. We have not only demonstrated the expression of the cytokines by leucocytes in periodontal tissue by immunochemical methods, i.e. the protein is present, but our ISH technique clearly demonstrates that the cells express the mRNAs that encode each of the proteins. This refutes arguments that the cells had merely taken up the proteins by pinocytosis and furthermore the presence of the cytokine mRNA in discrete cells indicates local production is occurring. We cannot be entirely certain that cells expressing the mRNA are actively secreting the cytokines, but when greater numbers of cells are labelled by immunohistochemistry the corresponding ISH experiment shows an identical trend.
It is well recognized that the nature of any immune response is determined by the activity of particular T cell subsets. The duration and intensity of the immune response is facilitated or helped by CD4-expressing cells, while the CD8-expressing cells have an inhibitory or suppressor function. However, in many circumstances we do not require the action of specialized suppressor cells because Th cells with distinct cytokine-producing phenotypes such as Th1 and Th2 inhibit the actions of each other. As part of their arsenal, T cells synthesize and secrete an array of pleotrophic cytokines [23]. These can be split principally into two categories, the cytokines that augment cytotoxic T cell functions, i.e. IL-2, and IFN-γ (secreted by Th1 cells); and those that elicit a humoral type of response, i.e. IL-4, IL-5 and IL-6 (secreted by Th2 lymphocytes). The latter cells also tend to secrete the anti-inflammatory cytokine IL-10 [24–27]. On the other hand, Th0 cells are capable of secreting IFN-γ and IL-4 and these cells represent a population of CD4+ cells that have not differentiated to become either Th1 or Th2. Th1-type cells are particularly effective in promoting microbial killing because they produce IFN-γ. By contrast, Th2 cells are less effective in this role because IL-4 and IL-10, products of these cells, actually inhibit these activities by inhibiting the secretion of IFN-γ. Thus, a Th2-dominated response actually reduces cellular immunity in the presence of certain bacterial infections, particularly of organisms that evade the immune response by invading host cells, and may be associated with failure to control the infection. Th2 cell activity tends to promote and maintain a humoral immune response.
The products of other cell types impinge upon, as well as mediate, T cell activity. In the periodontitis tissue sections, TNF-α mRNA-expressing cells were also detected. IFN-γ is known to play an important role in the early stages of host defence against infection. This cytokine is known to stimulate IFN-γ production, for example, and both IFN-γ and TNF-α are then largely responsible for the activation of mononuclear phagocytes, and an increase in killing of microorganisms. Apart from inhibiting Th1 cell functions, IL-10 also reduces the secretion of TNF-α by a number of cell types [28]. The prevalence of IL-10 in periodontitis lesions may have an important bearing on microbial killing because of its potential role in diminishing TNF-α- and IFN-γ-mediated responses.
Secondary aims of this study were to investigate the possibility that the early onset form of periodontitis had a different T cell profile from that of AP and that the T cell population might differ in the superficial layers of the periodontal tissue when compared with the deep connective/granulation tissue. No difference was seen in the relative proportions of the Th subsets in the present study, nor did we see any differences in the CD4 to CD8 ratios in our earlier study [21]. The more rapid progression of EOP compared with AP could not be explained by a predominance of one particular T cell subset over the other. We could not measure the cytokine profile in normal periodontal tissues, principally because obtaining ethical approval for the collection of sufficient samples presented an obstacle, but with the few normal tissues that were obtained it can be clearly shown that the inflammatory infiltrate was very low, varying from sparse to non-existent.
There was no detectable difference in Th1 cytokine-expressing cells, perhaps because their numbers were low. Nor did we see a significant difference in IL-10 expression, but this cytokine was also expressed by B cells, macrophages and other cell types. Perhaps if it were possible to discriminate and count only IL-10-expressing T cells the trend we did see (i.e. an increased number in EOP patients) would reach significance.
In a number of chronic inflammatory conditions such as rheumatoid arthritis it has been suggested that IL-2 can be replaced by IL-15 [29], since both type-1 cytokines share the same receptor transducer component [30,31]. In periodontal granulation tissue we were able to demonstrate the presence of IL-15 mRNA in more T cells than we could detect IL-2 or IL-2 mRNA, suggesting cytokine replacement in this tissue.
The results clearly show that the Th2 cell is more abundant than Th1 in severe periodontal disease. This result is consistent with a large number of studies [7–10], and reviews [1,18]. We have demonstrated for the first time the simultaneous presence of both the antigens and the mRNA for many of the important mediators of the immune and inflammatory response, providing strong evidence that the inflammatory cells are actively engaged in the modulation of disease activity by secretory mechanisms. The majority of the indicators show a marked anti-inflammatory component in the disease process and the predominance of a humoral type of response. Some of our earlier work on the characterization of plasma cells might support this supposition [32,33], but we do not yet know against which antigens the B cells are targeted. The question remains: how do these features relate to the continuance of the destructive processes of the disease? Although we note the presence of the anti-inflammatory mechanisms we do not know whether the destructive processes are effectively under control. Tissue destruction (connective tissue and bone loss) might be episodic in nature and it has not been possible to elucidate the mechanisms involved because the predominant disease state is one of quiescence.
REFERENCES
- 1.Zadeh HH, Nichols FC, Miyasaki KT. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol 2000. 1999;20:239–88. doi: 10.1111/j.1600-0757.1999.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 2.Kinane DF, Mooney J, Ebersole JL. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker JJ, Tondreau SP. The stimulation of human peripheral blood lymphocytes by oral bacteria: macrophage and T-cell dependence. J Dent Res. 1985;64:906–12. doi: 10.1177/00220345850640061001. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Ito H, Harada Y. T-cell dependence for establishment of the IgG-dominant B-cell lesion in periodontitis. J Periodont Res. 1987;22:187–9. doi: 10.1111/j.1600-0765.1987.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Harada Y, Matsuo T, et al. Possible role of T cells in the establishment of IgG plasma cell-rich periodontal lesion—augmentation of IgG synthesis in the polyclonal B cell activation response by autoreactive T cells. J Periodont Res. 1988;23:39–45. doi: 10.1111/j.1600-0765.1988.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 6.Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. Selected Th-1 and Th-2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin Exp Immunol. 1996;103:422–8. doi: 10.1111/j.1365-2249.1996.tb08297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol. 1995;99:384–91. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyagi T, Sugawara-Aoyagi M, Yamazaki K, Hara K. Interleukin 4 (IL-4) and IL-6-producing memory T-cells in peripheral blood and gingival tissue in periodontitis patients with high serum antibody titres to Porphyromonas gingivalis. Oral Micrbiol Immunol. 1995;10:304–10. doi: 10.1111/j.1399-302x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 9.Manhart SS, Reinhardt RA, Payne JB, Seymour GJ, Gemmell E, Dyer JK, Petro TM. Gingival cell IL-2 and IL-4 in early onset periodontitis. J Periodontol. 1994;144:662—6. doi: 10.1902/jop.1994.65.9.807. [DOI] [PubMed] [Google Scholar]
- 10.Gemmell E, Seymour GJ. Cytokine profiles of cells extracted from humans with periodontal diseases. J Dent Res. 1998;77:16–26. doi: 10.1177/00220345980770010101. [DOI] [PubMed] [Google Scholar]
- 11.Gemmell E, Seymour GJ. γδ T lymphocytes in human periodontal disease tissue. J Periodontol. 1995;66:780–5. doi: 10.1902/jop.1995.66.9.780. [DOI] [PubMed] [Google Scholar]
- 12.Nagai A, Takahashi K, Sato N, et al. Abnormal proportion of γδ T cells in peripheral blood is frequently detected in patients with periodontal disease. J Periodontol. 1993;64:963–7. doi: 10.1902/jop.1993.64.10.963. [DOI] [PubMed] [Google Scholar]
- 13.Kinane DF, Goudie RB, Karim SN, Garioch JJ, Moughal NA, Al Badri A. Heterogeneity and selective localisation of T cell clones in human skin and gingival mucosa. J Periodont Res. 1993;28:497–9. doi: 10.1111/j.1600-0765.1993.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Lappin D, Kinane DF. In situ localization of cell synthesis and proliferation in periodontitis gingiva and tonsillar tissue. Oral Dis. 1996;2:210–6. doi: 10.1111/j.1601-0825.1996.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 15.Koulouri O, Lappin DF, Radvar M, Kinane DF. Cell division synthetic capacity and apoptosis in periodontal lesions analysed by in situ hybridization and immunohistochemistry. J Clin Perio. 1999;26:183–9. doi: 10.1034/j.1600-051x.1999.260810.x. [DOI] [PubMed] [Google Scholar]
- 16.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–74. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng HX, Zheng LF. T cells and T-cell subsets in periodontal diseases. J Periodont Res. 1989;24:121–6. doi: 10.1111/j.1600-0765.1989.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 18.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal diseases. Periodontol 2000. 1997;14:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 19.Hart TC, Marazita ML, Schenkein HA, Brooks CN, Gunsolley JG, Diehl SR. No female preponderance in juvenile periodontitis after correction for ascertainment bias. J Periodontol. 1991;62:745–9. doi: 10.1902/jop.1991.62.12.745. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Poole I, Kinane DF. Detection of interleukin-1β mRNA-expressing cells in gingival crevicular fluid by in situ hybridization. Arch Oral Biol. 1995;40:941–7. doi: 10.1016/0003-9969(95)00057-v. [DOI] [PubMed] [Google Scholar]
- 21.Lappin DF, Koulouri O, Radvar M, Hodge P, Kinane DF. Relative proportions of mononuclear cell types in periodontal lesions analyzed by immunohistochemistry. J Clin Periodontol. 1999;26:183–9. doi: 10.1034/j.1600-051x.1999.260309.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebersole JL, Taubman MA. The protective role of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–41. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 23.Paliard X, de Waal Malefijt R, Yssel H, et al. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988;141:849–55. [PubMed] [Google Scholar]
- 24.Bucy RP, Panoskaltsis-Mortari A, Huang GQ, et al. Heterogeneity of single cell cytokine gene expression in clonal T cell populations. J Exp Med. 1994;180:1251–62. doi: 10.1084/jem.180.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelso A, Groves P, Troutt AB, Francis K. Evidence for the stochastic acquisition of cytokine profile by CD4+ T cells activated in a T helper type 2-like response in vivo. Eur J Immunol. 1995;25:1168–75. doi: 10.1002/eji.1830250506. [DOI] [PubMed] [Google Scholar]
- 26.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–9. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Sad S. The expanding universe of T cell subsets—Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 28.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 29.McInnes IB, al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nature Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 30.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–9. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–400. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinane DF, Lappin DF, Koulouri O, Buckley A. Humoral immune responses in periodontal disease may have mucosal and systemic immune features. Clin Exp Immunol. 1999;115:534–41. doi: 10.1046/j.1365-2249.1999.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinane DF, Takahashi K, Mooney J. Crevicular fluid and serum IgG subclasses and corresponding mRNA expressing plasma cells in periodontitis lesions. J Periodontol Res. 1997;32:176–8. doi: 10.1111/j.1600-0765.1997.tb01401.x. [DOI] [PubMed] [Google Scholar]