Abstract
Lymphoproliferation of healthy donors was tested against mycobacterial antigens (PPD, Ag85, Ag85 peptides). All PPD responders recognized the secretory antigen Ag85 and the peptide specificity for Ag85B was defined. Peptide 91–108 was recognized by 85% of donors. In addition, all CD4 T cell lines generated from 12 donors against PPD or Ag85 responded to 91–108. When this peptide was used to generate T cell lines, the cells responded also to tuberculins from atypical mycobacterial species. Thus the cross-reactive peptide behaved as quasi-universal. The analysis of TCR-BV gene usage by cell lines showed that most Ag85-specific T cells correspond to 91–108-specific clonotypes. Intracytoplasmic staining of cell lines after phorbol myristate acetate stimulation resulted in dominance of interferon-gamma (IFN-γ)-IL-4 double-positive cells, whereas antigen stimulation resulted in production of IFN-γ only. The data show that peptide 91–108 is the major focus of the CD4 response to mycobacterial antigens in peripheral blood mononuclear cells and in T cell lines from PPD responders.
Keywords: mycobacteria, CD4, T cells, Ag85, immunodominant epitope, TCRBV
INTRODUCTION
The need for an improved tuberculosis vaccine is underlined by the statement that bacille Calmette–Guérin (BCG) is ‘at once the least satisfactory and yet the most widely used of all vaccines today’[1]. To design new-generation vaccines more information on the antigenic make-up of mycobacteria [2] must be gained to identify immunodominant proteins and epitopes [3]. This information can be applied to assays of cellular immune response, as an alternative to PPD [4].
The CD4 response is instrumental for protection against mycobacterial infections [5]. This is mediated by mechanisms that have been reviewed [5]. A major fraction of the human CD4 response focuses on the secreted protein family known as Ag85 complex [6,7]. Previous studies have identified immunodominant regions [8,9] and have demonstrated that in mice immunization against Ag85A and Ag85B induces a protective response upon challenge [10,11].
Thus we investigated the CD4 response to Ag85 and to its Ag85B peptides by generating CD4 lines that were analysed for fine specificity, clonal composition, cross-recognition of different species and cytokine synthesis at the single-cell level.
MATERIALS AND METHODS
Media and antigens
RPMI 1640 (Flow, Irvine, UK) with 2-mercaptoethanol 10−5 m, l-glutamine 10 mm, penicillin 100 U/ml, streptomycin 100 μg/ml and 5% autologous plasma was used for cultures.
PPD from Mycobacterium tuberculosis and tuberculin from atypical species (M. avium, M. marinum, M. scrofulaceum, M. intracellulare, M. kansasi, M. fortuitum) (Statens Seruminstitut, Copenhagen, Denmark) were used at 5 μg/ml final concentration. The secretory antigen Ag85 complex (30–32 kD composed of Ag85A, B, C) was purified from M. bovis BCG filtrate as described [12,13]. Ag85 was used at 5 μg/ml. Peptides were synthesized according to the M. bovis Ag85B sequence as 31 18mers overlapping by nine residues [8,13]. Deleted/substituted peptides were synthesized as described [14,15]. Peptides were used at 1 μg/ml final concentration. Human IL-2 (Proleukin) donated by Chiron (Milan, Italy) was used at 30 U/ml.
Peripheral blood mononuclear cell proliferative response
Peripheral blood mononuclear cells (PBMC) were purified on Ficoll–Hypaque gradients. The mononuclear cells were resuspended in medium at 2 × 106 cells/ml. Proliferative response was measured by culturing 0·2 ml cell suspension in microtitre plates (Costar, Cambridge, MA) with or without antigens for 4 days. The wells were pulsed with 0·5 μ Ci 3H-thymidine (25 Ci/mmol; Amersham, Aylesbury, UK) on day 4 and harvested on day 4. The filters were counted in a Matrix 9600 apparatus (Canberra Packard, Meriden, CT). Results are given as stimulation indexes (SI, ratio between ct/min with antigen and ct/min without antigen).
Generation of CD4 T cell lines
CD4 T cell lines were prepared as described [16–18]. PBMC were cultured in a 24-well cluster plate (Costar) at 1·5 ml/well in the presence of PPD, Ag85 or peptides for 5 days. IL-2 was added on day 6. The cells were cultured for an additional 5 days and split 1:4 or when needed. Proliferation declined after 3 weeks. The cells were restimulated after 1 month by culturing 4 × 105 T cells with 106 autologous 30 Gy-irradiated PBMC in the presence of antigen and IL-2. Cultures were split after 7 and 14 days. After 3 weeks the cultures were restimulated as described. The T cell lines were close to 100% CD4+ after three cycles [18,19] and used after four cycles. Proliferation assay on T cell lines was performed on 2 × 104 T cells and 105 irradiated PBMC. The plates were pulsed on day 2 and harvested on day 3 as described for PBMC. Results are given as ct/min.
Analysis of TCR-BV gene usage
T cell lines were restimulated and 12 days later 5 × 106 T cells were harvested. After this time interval the irradiated lymphocytes are no longer present and thus do not interfere with TCR-BV analysis, as ascertained in preliminary experiments. The procedures for total RNA extraction, cDNA reverse transcription and TCR-BV gene usage analysis by polymerase chain reaction (PCR) have been reported [20–22].
Intracytoplasmic staining for cytokines in antigen-activated T cells
Staining of PBMC
The procedure described in [23] was followed, but the anti-CD28 MoAb was not used. PBMC (106/ml) were dispensed in Falcon tubes (cat. no. 2052) and cultured overnight with or without antigens. Conditions were the same as for proliferation assays. The following day monensin (Sigma Chemical Co., St Louis, MO) was added at 4 μg/ml. For polyclonal activation, phorbol myristate acetate (PMA; 25 ng/ml), ionomycin 1 μg/ml and monensin 4 μg/ml were added to one tube without antigen. After 4 h incubation, each tube received 20 μl of PerCP-conjugated anti-CD3 or anti-CD4 MoAb (Becton Dickinson, San Josè, CA). After 30 min at 4°C, 2 ml FACS Lysing solution (Becton Dickinson) were added. The tubes were vortexed, left at room temperature for 10 min, and spun for 5 min at 500 g. FACS Permeabilizing solution (500 μl; Becton Dickinson) was added to the pellet. Ten minutes later, the cells were washed with 4 ml PBS, NaN3 0·1% and fetal calf serum (FCS) 0·5%. The tubes were spun as above and 20 μl anti-cytokine antibody (Fastimmune antibodies, Becton Dickinson: anti-Hu IL-4–PE, anti-Hu IL-2–FITC, anti-Hu interferon-gamma (IFN-γ)–PE, anti-Hu tumour necrosis factor-alpha (TNF-α)–FITC) were added. The cells were resuspended, incubated at room temperature for 30 min, washed and resuspended in 500 μl PBS 1% paraformaldehyde.
Staining of T cell lines
Adherent monocytes were prepared by plating PBMC at 5 × 105/well in a flat-bottomed microtitre plate. Five days later, the non-adherent cells were resuspended by washing the wells with medium. The adherent cells were cultured overnight in 100 μl medium. The next day each well received 2 × 105 cells in 100 μl medium from a T cell line restimulated for 3 weeks. After 1 h incubation, the wells received monensin at 4 μg/ml. The co-cultures of T cells and antigen-presenting cells (APC) were incubated for an additional 4 h and stained with MoAbs as described for PBMC. Polyclonally activated T cells were cultured with PMA, ionomycin and monensin as described.
RESULTS
Response to Ag85B peptides
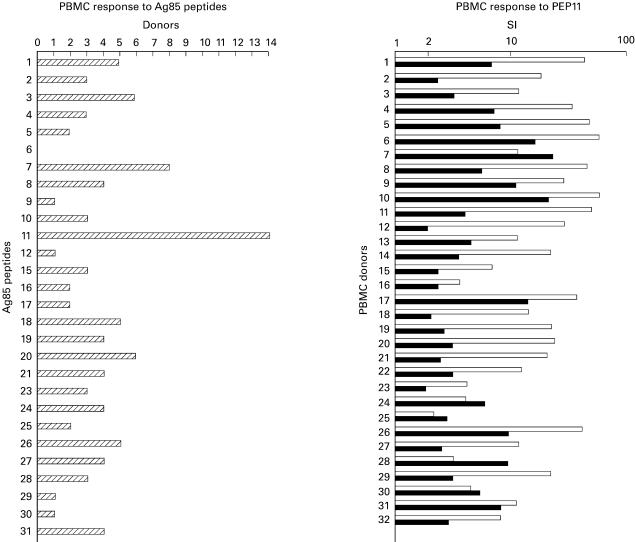
Fourteen PPD-responsive (SI 15–80) normal individuals were tested for response to Ag85. In all cases a positive response was detected with a SI ranging between 6 and 40 (data not shown). The same donors were tested for recognition of Ag85B peptides. A SI >2 was defined as a positive response. Figure 1, left panel, shows that the individuals tested were responsive to peptide 11 (PEP11). Other peptides were recognized by several individuals. To test PEP11 as a candidate universal peptide, 32 additional healthy individuals were examined for PPD and PEP11 response (Fig. 1, right panel). In this group 29 out of 32 (82%) PPD responders recognized PEP11. In a few donors (7–25, 28, 30) the response to PEP11 was higher than the response to PPD.
Fig. 1.
Left panel: response of 14 normal donors to Ag85B peptides. Responses with stimulation index (SI) >2 were scored as positive. Eleven individuals responded to PEP11. Right panel: response of 32 normal donors to PPD (□) and PEP11 (▪). Results are shown as SI (ratio between ct/min of antigen-stimulated wells and ct/min of control wells).
Peptide specificity of established T cell lines
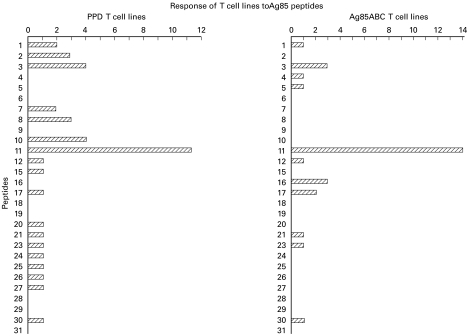
T cell lines were generated from normal donors by stimulation with PPD (12 lines) or with Ag85 (14 lines) (Fig. 2). Peptides 6, 9, 18, 19, 28, 29 and 31 did not stimulate any lines and other peptides stimulated few lines. Only PEP11 stimulated 11 out of 12 PPD-reactive lines (left panel) and 14 out of 14 Ag85-reactive lines (right panel).
Fig. 2.
Peptide specificity of T cell lines. Left panel: 12 T cell lines generated by stimulation with PPD were tested with Ag85B peptides; 11/12 exhibited a stimulation index (SI) >2 with PEP11. Right panel: 14 T cell lines generated by stimulation with Ag85 were tested with the panel of peptides; 14/14 exhibited an SI >2 with PEP11.
Deleted peptides for the definition of the minimal universal peptide
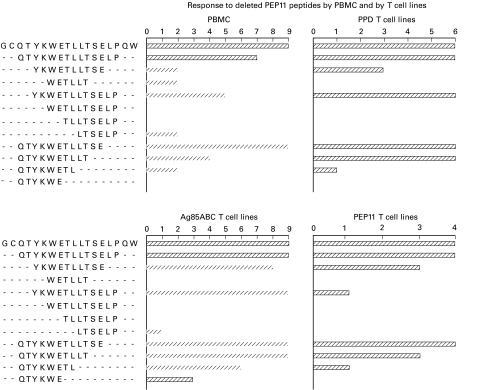
Amino and carboxy deleted PEP11 peptides were screened for stimulatory activity on PBMC and on T cell lines produced by stimulation with PPD, Ag85 and PEP11. The panels in Fig. 3 demonstrate that only peptide QTYKWETLLTSE (aa 93–104) exhibited the same stimulatory profile of PEP11, even though other peptides with more deletions were recognized by some but not all of the PBMC or T cell lines examined.
Fig. 3.
Response to deleted peptides. Amino and carboxy deleted peptides derived from the sequence of PEP11 were tested on peripheral blood mononuclear cells (PBMC) (upper left panel), PPD-specific (upper right panel), Ag85-generated (lower left panel) and PEP11-generated T cell lines (lower right panel). The shortest peptide that retained the stimulatory ability of PEP11 with all of the T cells shown in the four panels was QTYKWETLLTSE (aa 93–104).
In addition, the minimal peptide was synthesized with one, two or three phenylalanine (F) residues at the amino or carboxy terminus, or both. All modified peptides, and in particular the insoluble ones (two or more F residues), maintained the same antigenicity as the unmodified parental peptide (data not shown).
Recognition of PEP11 across different mycobacterial species
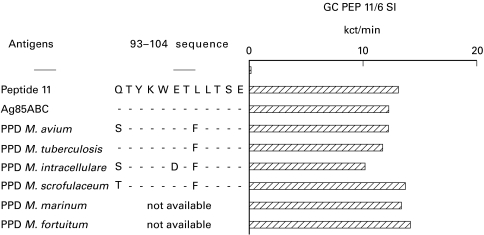
A T cell line was generated from donor GC by repeated stimulation in vitro with PEP11. The line was stimulated with control antigens (PEP11 and Ag85) and with tuberculins derived from different mycobacterial species. In all cases proliferative responses were similar (Fig. 4). The same results were obtained with three additional lines specific for PEP11 from other donors (data not shown), suggesting that the few amino acid substitutions, as shown in the figure, are irrelevant for CD4 T cell recognition.
Fig. 4.
Recognition of PEP11 across different mycobacterial species. A T cell line induced by in vitro stimulation with PEP11 was tested with a panel of tuberculin preparations obtained from different species. The 93–104 sequence in the different species is shown with the corresponding substitutions. Proliferative responses comparable to those obtained with PEP11 and Ag85 were seen with all mycobacterial species tested here.
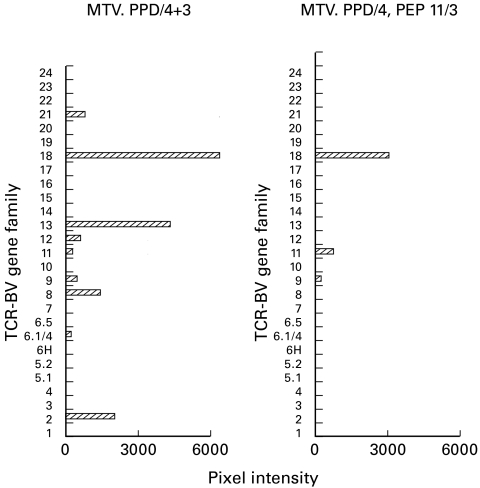
TCR-BV gene usage by PPD-specific T cell lines
In order to estimate clonal heterogeneity in the PPD-specific and in the PEP11-specific components of a T cell line, TCR-BV gene usage was analysed on two lines generated from the same donor. One line (Fig. 5, left panel) was generated by seven cycles with PPD. In addition to a dominant BV 18 gene usage, also BV 21, 13, 12, 11, 9, 8, 6.1/4, 2 were expressed. When the same line was split after four cycles with PPD and restimulated with PEP11 three times, BV 18 (and to a lesser extent BV 11 and 9) was preserved (Fig. 5, right panel), suggesting that the dominant BV 18 component accounts for most PEP11-specific clones.
Fig. 5.
TCR-BV gene usage by PPD-specific lines. A PPD-specific line split and restimulated with PPD or with PEP11 for three cycles. The analysis of TCR-BV gene family usage showed that BV 18 was well represented, but flanked by other BV genes, in the PPD-restimulated line. In contrast, BV 18 was the dominant family in the PEP11-selected T cell line. Results are shown as pixel intensity of the ethidium bromide-stained bands of polymerase chain reaction products run on an agarose gel.
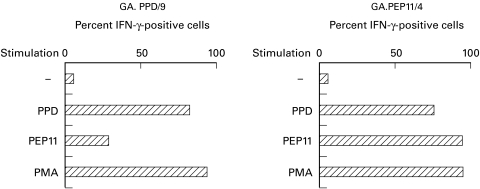
Intracytoplasmic cytokine staining of antigen-specific CD4 T cells
Within the PBMC from four PPD responders, CD4+ T cells can be induced to synthesize IFN-γ upon antigen stimulation. The frequency of PPD-specific cells according to IFN-γ synthesis ranged between 2000 and 20 000/106 CD4 cells, corresponding to 0·2–2% positive cells (data not shown). When established T cell lines were stimulated with adherent monocytes and antigen, most cells were antigen-responsive, as expected. Figure 6, left panel, shows that within a PPD-responsive line >85% of the cells were activated by antigen to become IFN-γ-positive, while PMA activated up to 95% of cells. Stimulation with PEP11 activated >30% of the cells, and this can be taken as an indication of the frequency of PEP11-reactive cells versus PPD-reactive cells. The right panel shows that a T cell line obtained by stimulation with PEP11 contained >95% cells that were induced by PEP11 to synthesize IFN-γ, with a lower frequency of PPD-responsive cells (80%). Similar figures were obtained for TNF-α, but in all cases IL-4-positive cells were undetectable whereas PMA stimulation resulted in >60% IFN-γ and IL-4 double-positive cells (data not shown).
Fig. 6.
Intracytoplasmic staining of antigen-specific CD4 T cells. PPD-generated (left panel) and PEP11-generated (right panel) T cell lines were stimulated with PPD or PEP11 (or with phorbol myristate acetate (PMA) as a positive control) and stained for intracytoplasmic IFN-γ synthesis. Results are shown as percentage of positively stained T cells over total CD4 lymphocytes.
DISCUSSION
Mycobacterial secretory antigens can induce protective immunity [10,24,25]. These antigens trigger an immune response similar to that induced by natural infection [24,25].
The M. bovis BCG Ag85 antigen complex has been identified in culture supernatants of all mycobacterial species [12]. It binds fibronectin and facilitates cell adhesion on macrophages. In fact fibronectin binding may contribute to efficient phagocytosis, initiating an immune response if the bacteria are taken up by phagocytes with APC function [2].
The Ag85 complex induces a proliferative response of IFN-γ-producing CD4 cells as well as cytotoxic T lymphocyte (CTL) activation [8,13]. The relevance of IFN-γ in mycobacterial infections [5] is supported by its increase during activation of an immune response [22]. In addition to a protective role, IFN-γ production that follows antigen stimulation has been proposed as a complementary assay for detection of mycobacterial infection [26]
For these reasons it is important to investigate the response to Ag85. It has been observed that regions of Ag85 are the focus of the proliferative response of PBMC from PPD responders. In two reports [7,13] similar peptide pools (20mers overlapping by 10 residues) were used to screen PBMC from healthy controls and from patients, with mapping results that match only partially. More recently [9] mapping with 15mers overlapping by 10 residues identified seven regions within Ag85 that were recognized by at least 4/12 (33%) of the normal, PPD-responsive subjects. None of the peptides, even though defined as immunodominant, was recognized by more than 7/12 subjects. This corresponds to the peptide that contains the same PEP11 sequence we have defined as universal. Thus our findings are in good agreement with this report [9], whereas discrepancies may be found when the other stimulatory peptides are defined. We have defined the sequence QTYKWETLLTSE (aa 93–104) as universal, as it is recognized by 41 out of 46 (89%) normal, PPD-responsive subjects if the data from the two panels of Fig. 1 are taken together. This percentage largely exceeds the one observed in [9], that corresponds to seven responders to this sequence out of 12 subjects (58%). The discrepancy could be attributed to different sample sizes (12 versus 46 subjects) rather than to recurrent HLA class II alleles that may skew the profile of dominant epitopes in the population. In fact, even though our population was not HLA typed, it was representative of the highly outbred population that lives in Northern Italy. An additional reason that may account for different percentages of responders to the relevant sequence is that different criteria were used to define positivity, such as counts 3 s.d. above background in [9], SI > 4 in [7], SI > 5 in [13]. Finally, discrepancies may also be attributed to the different peptide concentrations used in the in vitro assays, that ranged from 1 μg/ml in this study, to 10 μg/ml in [7], to 20 μg/ml in [13] and up to 250 μg/ml in [9].
We have analysed several parameters of cellular immune responses in PBMC and in specific CD4 lines. T cell lines allow a careful characterization of the fine specificity and of the clonal heterogeneity of specific CD4 cells. We have already used PPD-specific lines and clones from peripheral blood and from pleural exudates [18] to study APC requirements and processing requirements [27].
The data we present show that within the population of PPD-responsive lymphocytes, the majority is specific for Ag85. PEP11 is the focus of the CD4 response in most individuals and in all T cell lines. PPD-specific CD4 T cell lines contain clonotypes that use several TCR-BV gene families [22]. We have shown that a representative T cell line generated by PPD stimulation exhibits a limited TCR-BV gene usage following selection with PEP11. This was the case with different donors and thus a large fraction of PPD-responsive cells are PEP11-specific. The same dominant TCR-BV genes were used by T cell lines from the same donors at different times, as observed previously for other recall and primary antigens [22]. A limited clonal heterogeneity estimated by using a different approach has been described in CD4 T cell lines and clones derived from pleural exudates [17], while functional heterogeneity has been reported [28].
The identification of immunodominant and universal peptides [29,30] applies to vaccine design and has diagnostic relevance. Assays for cellular immunity to mycobacteria are based on poorly defined PPD preparations. Replacing PPD with defined peptides is attractive. In intradermal assays, the water-soluble peptides are diluted in the biological fluids and this may prevent uptake by dermal APC. Insoluble peptides can now be tested in intradermal assays, since their antigenicity is not affected by hydrophobic residues.
Immunodominant epitopes are of special interest if conserved among mycobacterial species. PEP11 is in fact conserved in atypical strains, since a PEP11-specific line was equally stimulated by tuberculins from different species. This was consistent with the highly conserved sequence of Ag85 and in the 93–104 region in particular [31]. T cell lines generated and maintained with PEP11 were used in order to make sure that cross-recognition of interspecies PEP11 sequences was not due to recognition of Ag85 shared epitopes other than PEP11 itself. It is evident that the amino acid substitutions are irrelevant for recognition by CD4 T cells. The possibility still exists that T cell lines with the same PEP11 specificity, but from other individuals, may discriminate among species because of different T cell receptors (specific for the same epitope, but differing in the CDR3 sequence, or specific for different epitopes within the same peptide). On the other side, discrimination between BCG immunization and mycobacterial infection may be possible considering antigens encoded by genes deleted in BCG, such as ESAT-6 [30], since BCG and M. tuberculosis are quasi-identical according to sequence analysis [31].
Intracytoplasmic staining on PBMC estimated the frequency of antigen-responding cells in healthy donors. Our figures from several experiments were of the same magnitude as those obtained for cytomegalovirus (CMV)-specific CD4 cells [23]. The PPD-responsive cells were defined according to IFN-γ, TNF-α and IL-2 production, confirming the paradigm that Th1 cells, that mediate protection, are dominant in these responses [5,32].
More information on the frequency of Ag85- and PEP11-specific CD4 cells versus PPD-specific CD4 cells was obtained with T cell lines. In these populations a high percentage of PPD-responsive cells is PEP11-specific and most, but not all, PEP11-responding cells are PPD-specific. This can be explained by the fact that CD4 cells carried over in the PPD selected line recognize PEP11 epitopes that are inefficiently produced upon processing of PPD. This discrepancy between recognition of an epitope in the context of a peptide or in the context of a protein has been described using retroviral antigens [15]. This may impose limitations upon the use of peptides removed from their original context for immunization and must be considered when peptides are inserted in recombinant carriers [20].
Acknowledgments
We thank Dr E. Saman (Innogenetics, Ghent, Belgium) for help with the synthesis of Ag85B peptides and Mr Lorenzo Guzzinati and Ms Silvia Nicora for valuable secretarial work. This work was supported by a grant from the National Health Institute, Rome (Tuberculosis National Project, no. 93-99/D/T11) to F.M. and a grant FWO-Vlaanderen (G.0355.97) to K.H.
REFERENCES
- 1.Fine PEM. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989;11:S353–9. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- 2.Young DB, Kaufmann SHE, Hermans PWM, Thole JER. Mycobacterial protein antigens: a compilation. Molec Microbiol. 1992;6:133–45. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 3.Lamb JR, Lathigra R, Rothbard JB, Sweetser D, Young RA, Ivanyi J, Young DB. Identification of mycobacterial antigens recognized by T lymphocytes. Rev Infect Dis. 1989;11:S443–7. doi: 10.1093/clinids/11.supplement_2.s443. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz MA. A new TB vaccine. Immunologist. 1997;5:15–20. [Google Scholar]
- 5.Kauffmann S. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 6.Peake P, Gooley A, Britton WJ. Mechanism of interaction of the Ag85B secreted protein of Mycobacterium bovis with fibronectin. Infect Immun. 1993;61:4828–34. doi: 10.1128/iai.61.11.4828-4834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche PW, Peake PW, Billman-Jacobe H, Doran T, Britton W. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–26. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huygen K, Lozes E, Gilles B, et al. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–70. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver RF, Wallis RS, Ellner JJ. Mapping of T cell epitopes of the 30-kDa α antigen of Mycobacterium bovis strain Bacillus Calmette–Guérin in purified protein derivative (PPD)-positive individuals. J Immunol. 1995;154:4665–74. [PubMed] [Google Scholar]
- 10.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Med. 1996;2:893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 11.Kamath AT, Feng CG, MacDonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of M. tuberculosis. Infect Immun. 1999;67:1702–7. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bruyn J, Huygen K, Bosmans R, et al. Purification, characterization and identification of a 32 kDa protein antigen of M. bovis BCG. Microb Pathog. 1987;2:351–66. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 13.Launois P, DeLeys R, Niang M'B N'D, et al. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–87. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manca F, Habeshaw J, Dalgleish AG, Fenoglio D, Li Pira G, Sercarz E. Role of flanking variable sequences in antigenicity of consensus regions of HIV gp120 for recognition by specific human T helper clones. Eur J Immunol. 1993;23:269–74. doi: 10.1002/eji.1830230142. [DOI] [PubMed] [Google Scholar]
- 15.Manca F, Fenoglio D, Valle M, et al. Human CD4+ T cells can discriminate the molecular and structural context of T epitopes of HIV gp120 and HIV p66. J AIDS. 1995;9:227–37. [PubMed] [Google Scholar]
- 16.Manca F, Fenoglio D, Valle M, et al. Human T helper cells specific for HIV reverse transcriptase: possible role in intrastructural help for HIV envelope specific antibodies. Eur J Immunol. 1995;25:1217–23. doi: 10.1002/eji.1830250513. [DOI] [PubMed] [Google Scholar]
- 17.Manca F, Rossi G, Valle M, Lantero S, Damiani G, Li Pira G, Celada F. Limited clonal heterogeneity of antigen specific T cells focussing in the pleural space during mycobacterial infection. Infect Immun. 1991;59:503–13. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manca F, Fenoglio D, Li Pira G, Kunkl A, Celada F. Effect of antigen antibody ratio on macrophage uptake, processing and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med. 1991;173:37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca F, Habeshaw JA, Dalgleish AG. HIV envelope glycoprotein, antigen specific T cell response and soluble CD4. The Lancet. 1990;335:811–5. doi: 10.1016/0140-6736(90)90935-x. [DOI] [PubMed] [Google Scholar]
- 20.Manca F, Li Pira G, Fenoglio D, et al. Recognition of human T cell leukaemia virus (HTLV-1) envelope by human CD4 T cell lines and clones from seronegative individuals: specificity and clonal heterogeneity. Blood. 1995;85:1547–54. [PubMed] [Google Scholar]
- 21.Manca F, De Berardinis PG, Fenoglio D, et al. Antigenicity of HIV-derived T helper determinants in the context of carrier recombinant proteins: effects on T helper repertoire selection. Eur J Immunol. 1996;26:2461–9. doi: 10.1002/eji.1830261029. [DOI] [PubMed] [Google Scholar]
- 22.Li Pira G, Oppezzi L, Seri M, et al. Repertoire breadth of human CD4 cells specific for primary (HIV gp120 and p66) and for secondary (PPD and tetanus toxoid) antigens. Hum Immunol. 1998;59:137–48. doi: 10.1016/s0198-8859(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 23.Komanduri KW, Viswanathan MN, Wieder ED, Schmidt DK, Bredt BM, Jacobson MA, McCune JM. Restoration of cytomegalovirus specific CD4 T-lymphocyte responses after gancyclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nature Med. 1998;4:953–9. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 24.Andersen P. Effective vaccination of mice against M. tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz MA, Lee B, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of M. tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–4. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M, Converse PJ, Astemborski J, et al. Comparison between a whole blood interferon gamma release assay and tuberculin skin testing for the detection of tuberculosis infection among patients at risk for tuberculosis exposure. J Infect Dis. 1999;179:1297–300. doi: 10.1086/314707. [DOI] [PubMed] [Google Scholar]
- 27.Manca F, Valle MT, Megiovanni AM, et al. Requirement for different presenting cells and for different processing mechanisms by human CD4 T helper clones specific for M. tuberculosis antigens. Hum Immunol. 1998;59:265–74. doi: 10.1016/s0198-8859(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 28.Boom WH, Wallis RJ, Chervenak KA. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–43. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes; promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–41. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 30.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of ESAT-6 protein in Mycobacterium tuberculosis and virulent M. bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 32.McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ counts have impaired Th1 responses. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]