Abstract
IgAN is a common form of primary glomerulonephritis and also a disease of tonsillar focal infection. The comprehensive mechanism underlying this disease remains to be defined. To better understand its pathogenesis, we investigated tonsillar CD5+ B cells (B-1 cells) with respect to IgA synthesis. Germinal centre (GC) B cells were isolated from the tonsils of IgAN patients and the number of B-1 cells in the GC determined by flow cytometry. GC B-1 and B-2 (CD5− B) cells were purified by cell sorter, the cells were incubated with agonist anti-CD40 MoAb and the ability for antibody production by B-1 and B-2 cells determined by ELISPOT assay. GC B-1 cells and B-2 cells were incubated with agonist anti-Fas MoAb, and apoptosis in GC B-1 cells and B-2 cells was analysed by flow cytometry. Although B-1 cells do not usually take part in the GC reaction, an increase in B-1 cell numbers was observed in the GC of tonsils from IgAN patients. These B-1 cells were likely IgA1 antibody-producing cells, since the prominent IgA subclass in IgAN is generally considered to be IgA1. Although Fas-dependent apoptosis is essential for the elimination of activated B cells, these B-1 cells showed a reduced susceptibility to Fas-mediated apoptosis. It is conceivable that activated B-1 cells may survive in the GC due to impaired apoptosis and thus produce abnormal antibodies. These findings suggest that the immune responses of B-1 cells in the tonsillar GC could thus have an impact on the pathogenesis of IgAN.
Keywords: IgA nephropathy, tonsillar focal infection, mucosal immunity, B-1 cell, apoptosis
INTRODUCTION
IgAN, which features polymeric IgA-dominant depositions in the mesangium, is the most common form of primary glomerulonephritis world-wide [1,2]. The clinical and pathological features are well documented in several reports [3–5] and yet the comprehensive mechanism underlying induction of the IgA deposition remains to be defined. IgAN patients sometimes complain of gross haematuria during episodes of upper respiratory tract infection, and the tonsils are a frequent site of inflammation during such infections.
The tonsils represent a site of local immunity where immunoglobulin-bearing cells are capable of producing IgA. In the tonsils of IgAN patients demonstrating tonsillitis, the numbers of IgA-positive plasma cells increase [6], IgA-positive cells accumulate in the subepithelial sinusoids [7], IgA1-positive cells enter the germinal centre [8], and the production of polymeric IgA increases [9]. In addition, several clinical studies have demonstrated that the clinical manifestations of IgAN improved after tonsillectomy in IgAN patients [10–13]. Tonsillectomy is well known as an effective treatment for IgAN, particularly in Japan [10–13]. Several reports have shown that the level of serum IgA decreased in IgAN patients after tonsillectomy [13–15]. The tonsils have been considered the foci of abnormal IgA production in IgAN [14,15]; thus, a relationship is suggested between the tonsillar immune response and the pathogenesis of IgAN [16,17]. IgAN, then, is considered a tonsillar focal infection [10–16]. However, several clinical studies have also reported patients who failed to improve after tonsillectomy [10–13]. In such cases, the mechanism of IgA production is still unclear; it may be that the tonsils have no relation to the pathogenesis of IgAN, and the foci of abnormal IgA production may be elsewhere. The findings from these reports suggest that not all IgAN is related to the tonsils.
The germinal centre (GC) is a histologically important area in the tonsils and is mostly comprised of proliferating B cells, along with follicular dendritic cells (FDC), tingle body macrophages, and a small number of T cells [18,19]. Antigen-specific resting B cells are activated in T cell-rich areas outside the GC of secondary lymphoid organs [20,21] and give rise to IgM-secreting plasma cells and B cell blasts that colonize the primary follicles [22,23]. The subsequent GC reaction is initiated by the rapid proliferation of a few B blasts in association with FDC [24], whereas the remaining B cells follow the differentiation pathway from centroblasts to centrocytes and then to either plasma cells or memory B cells [25]. During these processes, somatic hypermutation [26,27], positive selection [28,29], and differentiation of selected GC B cells occur. GCs are thus dynamic microenvironments where not only are antigen-specific B cells selected for high-affinity immunoglobulin but self-reactive and antigen-unresponsive B cells are deleted by apoptosis. Nossal et al. [30] suggested that to avoid autoimmunity, any newly formed self-reactive B cells are eliminated within the GC by apoptosis. Although apoptosis of B cells may be mediated by multiple pathways, Fas-dependent apoptosis is essential for the elimination of activated B cells, including bystander and potentially autoreactive B cells [31]. GC B cells express Fas poorly, but in vitro stimulation with anti-CD40 MoAb up-regulates Fas expression [32]. Members of the bcl-2 gene family have been shown to influence the ability of lymphocytes to undergo apoptosis [33–35]. The bcl-2 gene product could enhance the ability of lymphocytes to survive [36–38].
B cells are classified into two main subpopulations, B-1 and B-2 cells [39–41]. The B-1 subpopulation is derived from precursors of the fetal liver and omentum. The surface phenotypes of B-1 cells are CD19+ and CD5+, which are distinguished from the surface phenotypes of B-2 cells (CD19+ and CD5−). B-2 cells constitute a large part of the B cell population in spleen, lymph nodes, and peripheral blood. B-1 cells, however, are localized in the peritoneal and pleural cavities, and few, if any, are found in spleen and lymph nodes in the normal adult. B-1 cells are maintained by self-renewal in the peritoneum [40] and produce mainly low-affinity IgM class antibodies in a T cell-independent manner [42]. They usually do not take part in the GC reaction. However, in autoimmune-prone individuals, B-1 cells increase in number in the GC and have been suggested to be involved in the generation of self-reactive antibodies [43,44].
It has been recently reported that B-1 cells are the main IgA-producing cells in mucosal tissues [45,46]. We considered the potential contribution of the mucosal immune system to the pathogenesis of IgAN, and we hypothesized that IgAN is the consequence of an altered mucosal immune system, altered tonsillar immunity in particular. We investigated B-1 cells in the tonsillar GC of 12 IgAN patients with chronic tonsillitis to clarify which cellular factors are involved in the pathogenesis of IgAN. We also investigated the improvement in haematuria after tonsillectomy to clarify further the relationship between the number of tonsillar B-1 cells and IgAN, and discuss tonsillectomy as a possible treatment for IgAN.
MATERIALS AND METHODS
Patients
Tonsil tissues obtained from 12 IgAN patients (14–52 years of age, mean 29·6 years; five males and seven females) treated by tonsillectomy in our clinic were used in this study, and tonsil tissues from 20 age-matched patients with chronic tonsillitis without IgAN (IgAN-negative) (17–47 years of age, mean 31·5 years; 10 males and 10 females) were used as controls. All IgAN patients were diagnosed with IgAN by renal biopsy, and all manifested gross haematuria. All patients were under the care of other physicians. We were consulted for tonsillectomies for the treatment of the IgAN in these patients. All patients were administered with steroids before tonsillectomy. These patients did not receive steroid therapy after tonsillectomy in order to evaluate the effect of tonsillectomy on clinical manifestations. Urine samples were monitored both macroscopically and microscopically.
Among these IgAN patients, eight showed improvement in the haematuria within 3 months after surgery. In these eight patients, gross haematuria disappeared and microscopic haematuria was also reduced after tonsillectomy. However, four patients did not show any haematuric improvement. Several clinical studies have demonstrated that the clinical manifestations of IgAN improve after tonsillectomy in IgAN patients; however, other clinical studies have also reported patients who failed to improve after tonsillectomy. Thus, we considered both tonsil-dependent IgAN and tonsil-independent IgAN, and these patients were considered as two groups: haematuria-improved (IgAN-improved) (17–47 years of age, mean 28·7 years; three males and five females) and haematuria-unchanged (IgAN-unchanged) (14–52 years of age, mean 30·4 years; two males and two females). There were no significant differences in age and sex between the two groups.
Reagents
MoAbs used in this study were purchased from PharMingen (San Diego, CA) and included the following: FITC-conjugated anti-CD38 (HIT2), PE-conjugated anti-CD5 (UCHT2), Cy-chrome-conjugated anti-CD19 (HIB19), FITC-conjugated anti-CD95 (anti-Fas; DX2), FITC-conjugated anti-CD40 (5C3), and FITC-conjugated anti-bcl-2 (Bcl-2/100).
Preparation of tonsillar GC cells
Tonsillar B cells were prepared following previously reported methods [47–49]. Briefly, tonsils were immersed in RPMI 1640 (Wako Junyaku Co., Osaka, Japan) containing 2% bovine calf serum (BCS), then pressed first through a sterile stainless steel mesh by gently teasing and then a nylon mesh to obtain a single-cell suspension [47]. GC B cells were obtained following a previously reported method [48–50]. Briefly, tonsillar mononuclear cells were subjected to two rounds of T cell depletion by rosetting with sheep erythrocytes; the resultant cells contained >95% CD19+ B cells, as analysed by FACS Calibur (Becton Dickinson, Sunnyvale, CA). B cells were further separated according to density using a discontinuous Percoll gradient consisting of four layers of 80%, 60%, 50%, and 35% Percoll solutions in a 15-ml conical tube. B cells were laid at the bottom of the gradient and centrifuged at 2000 g for 10 min at 20°C. B cells recovered at the 60–80% Percoll interface were referred to as high-density B cells, and those recovered at 35–50% as low-density B cells. GC B cells were obtained from low-density B cells by depleting IgD+ and CD44+ cells [48–50]. Cells were suspended in complete medium (RPMI 1640 supplemented with 10 ml/l of non-essential amino acids solution, 1 mm HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamycin, and 10% fetal calf serum (FCS)). According to the FACS estimation of CD38+ cells, the purity of GC B cells was >98%. To prevent the death of the GC B cells, all purification steps were performed at 4°C, except centrifugation with Percoll, which was conducted at room temperature.
Flow cytometry
To determine the number of B-1 cells in the GC, three-colour flow cytometric analysis was performed. For staining of B-1 and B-2 cells, the GC B cells from IgAN-improved, IgAN-unchanged and IgAN-negative patients were incubated with FITC-conjugated anti-CD38, PE-conjugated anti-CD5, and Cy-chrome-conjugated anti-CD19. The frequencies of B-1 and B-2 cells were analysed by FACS Calibur (Becton Dickinson). For purification of B-1 and B-2 cells, CD5+ and CD5− B cells in the tonsillar GC B cells were sorted with a cell sorter (EPICS Elite; Coulter, Hialeah, FL). To examine the expression of CD40 on B-1 and B-2 cells, the GC B cells were incubated with FITC-conjugated anti-CD40, and PE-conjugated anti-CD5. For intracellular staining to detect the expression of bcl-2 in B-1 and B-2 cells, the GC B cells were surface-stained with PE-conjugated anti-CD5, permeabilized with FACS permeabilizing solution (Becton Dickinson), washed with PBS, and incubated with FITC-conjugated anti-bcl-2 [33]. The samples were analysed by FACS Calibur.
Cell culture
For in vitro activation of B cells, aliquots of 2 × 106 cells in 2 ml of complete medium were cultured in 12-well flat-bottomed plates in the presence of 10 μg/ml of an agonist anti-CD40 MoAb (5C3) at 37°C for 6 days. To examine IgA1 or IgA2 antibody-producing cells, isotype-specific IgA1 and IgA2 antibody-producing cells were measured by ELISPOT assay [47]. Briefly, 96-well filtration plates with a nitrocellulose base (Millititer HA; Millipore Corp., Bedford, MA) were coated with 5 μg/ml of goat anti-human immunoglobulin (H + L) (Southern Biotechnology Associates, Inc. Birmingham, AL) in PBS and incubated overnight at 4°C. Plates were washed three times with PBS and then blocked with complete medium for 1 h. The blocking medium was removed, and the test cells in complete medium were added at varying concentrations and cultured at 37°C (5% CO2 and 95% humidity) for 4 h. After incubation, the plates were thoroughly washed with PBS and then with PBS containing Tween solution (0·05%; PBS–T). For the capture of antibody-producing cells, 1 μg/ml of horseradish peroxidase (HRP)-labelled affinity-purified goat anti-human IgA1 or IgA2 (Southern Biotechnology Associates) in PBS–T was added. After overnight incubation at 4°C, the plates were washed five times with PBS–T; the spots were developed at room temperature with 100 μl of 1·6 mm 3-amino-9-ethylcarbazole (AEC) in 0·1 m sodium acetate buffer pH 5·0 containing 0·05% H2O2 (Moss Inc., Pasadena, MD) after a 30-min incubation. The plates were washed with water and dried, and red–brown-coloured spots were counted as antibody-forming cells with the aid of a stereomicroscope. An increase in antibody-producing cells was determined by comparison with a control culture in the absence of anti-CD40 MoAb.
Induction of apoptosis
For in vitro stimulation of B cells, aliquots of 2 × 106 cells in 2 ml of complete medium were cultured in 12-well flat-bottomed plates in the presence of anti-CD40 MoAb (10 μg/ml) at 37°C for 3 days. B cells were then incubated with 1 μg/ml of an agonist anti-Fas MoAb (DX2) for 24 h, and the percentage of dead cells calculated by dye exclusion after staining with trypan blue solution. For flow cytometric analysis of Fas expression on living cells, B cells were stained with FITC-conjugated anti-Fas MoAb. The cells were then resuspended in medium containing 2 μg/ml propidium iodide (PI; Sigma, St Louis, MO) and 10% FCS to stain dead cells. PI-negative living cells were gated by forward versus side scatter, and the intensity of Fas expression on living cells was examined using FACSCalibur.
Statistical analysis
Student's t-test was used to determine significance of the data. Differences at P < 0·05 were considered significant.
RESULTS
Increase of B-1 cells in the tonsillar GC
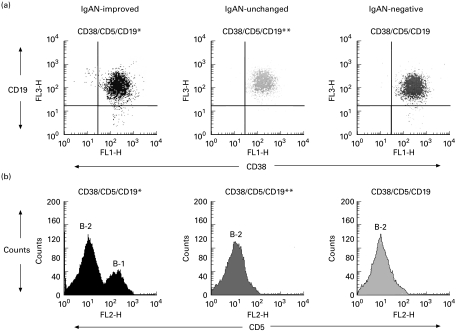
Tonsillar GC B cells from IgAN-improved, IgAN-unchanged and IgAN-negative patients were composed of more than 98% CD38+ and CD19+ B cells (Fig. 1a). In IgAN-improved patients, CD5+ cells were markedly increased in number among the CD38+ and CD19+ B cells, indicating a large number of B-1 cells in the tonsillar GC of IgAN-improved patients (Fig. 1b). IgAN-unchanged and IgAN-negative patients had few B-1 cells in the tonsillar GC. The increase in B-1 cell numbers in the tonsillar GC was shown in all IgAN-improved patients but not in IgAN-unchanged and IgAN-negative patients (Table 1).
Fig. 1.
Tonsillar germinal centre (GC) B cells were isolated from IgAN-improved, IgAN-unchanged, and IgAN-negative patients and labelled with FITC-conjugated anti-CD38, PE-conjugated anti-CD5, and Cy-chrome-conjugated anti-CD19. Tonsillar GC B cells from these patients were composed of >98% CD38+ and CD19+ B cells (a). In IgAN-improved patients, CD5+ cells were markedly increased among CD38+ and CD19+ B cells, indicating a large number of B-1 cells in the tonsillar GC of IgAN-improved patients. IgAN-unchanged and IgAN-negative patients had few B-1 cells in the tonsillar GC (b).
Table 1.
Frequency of B-1 and B-2 cells in tonsillar germinal centre (GC)
| Patients | Number | B-1 cells† (%) | B-2 cells‡ (%) |
|---|---|---|---|
| IgAN-improved§ | 8 | 35·2 ± 6·8 | 64·8 ± 6·8 |
| IgAN-unchanged¶ | 4 | 2·8 ± 1·8 | 97·2 ± 1·8 |
| IgAN-negative†† | 20 | 2·3 ± 1·4 | 97·7 ± 1·4 |
Percentage of CD5 + B cells among CD38+ and CD19+ B cells.
Percentage of CD5 − B cells among CD38+ and CD19+ B cells.
Haematuria-improved IgAN patients after tonsillectomy.
Haematuria-unchanged IgAN patients after tonsillectomy.
Chronic tonsillitis patients without IgAN.
Each value is expressed as the mean ± s.e.m.
P < 0·01.
IgAN-unchanged and IgAN-negative patients had few B-1 cells in the tonsillar GC. The increase of B-1 cells in the tonsillar GC was shown in all IgAN-improved patients (P < 0·01) but not in IgAN-unchanged and IgAN-negative patients.
B-1 cells in the GC are probably IgA1 antibody-producing cells
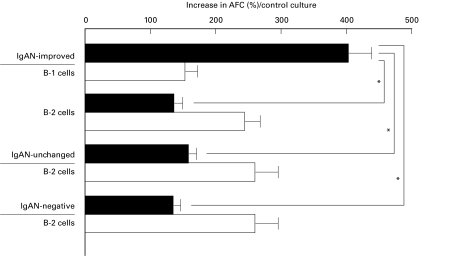
To investigate the ability for antibody production of B-1 and B-2 cells in the GC, sorted B-1 or B-2 cells from tonsils of IgAN-improved patients were examined. In IgAN-unchanged and IgAN-negative patients, B-2 cells but not B-1 cells were examined since there were too few tonsillar GC B-1 cells from these patients. B-1 cells in the tonsillar GC from IgAN-improved patients were able to produce IgA1 predominantly. B-2 cells from IgAN-improved, IgAN-unchanged, and IgAN-negative patients showed predominant differentiation to IgA2 antibody-producing cells (Fig. 2).
Fig. 2.
The ability for antibody production of B-1 and B-2 cells in the germinal centre (GC). Sorted B-1 or B-2 cells from tonsils of IgAN-improved, IgAN-unchanged, and IgAN-negative patients were stimulated with anti-CD40 MoAb for 6 days. B-1 cells in the tonsillar GC from IgAN-improved patients were able to produce IgA1 (▪) predominantly (P < 0·01). B-2 cells from IgAN-improved, IgAN-unchanged, and IgAN-negative patients showed predominant differentiation to IgA2 (□) antibody-producing cells. These results are expressed as the mean ±s.e.m. AFC, Antibody-forming cells. *P < 0·01.
Fas-mediated apoptosis-resistant B-1 cells
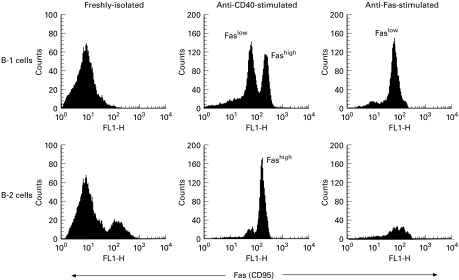
B-1 cells do not usually establish in the GC. However, in the case of IgAN-improved patients, the number of B-1 cells was expanded in the tonsillar GC. To investigate the exact mechanism of B-1 cell expansion, we explored the levels of apoptosis of tonsillar GC B-1 cells, since abnormal B cells are eliminated within the GC by apoptosis to prevent immune dysfunction. To determine the sensitivity of tonsillar GC B cells to Fas-mediated apoptosis, such cells from IgAN-improved, IgAN-unchanged and IgAN-negative patients were stimulated with anti-CD40 MoAb for 3 days followed by incubation with anti-Fas MoAb for 24 h. The proportion of live cells remaining from IgAN-improved patients was greater than that from IgAN-unchanged and IgAN-negative patients (data not shown). We then compared the Fas-mediated apoptosis sensitivity of sorted B-1 and B-2 cells from the tonsillar GC of IgAN-improved patients. Prior to culture, tonsillar GC B-1 and B-2 cells expressed low levels of Fas (Fig. 3). After 3 days of culture in the presence of anti-CD40 MoAb, Fas expression differed between B-1 and B-2 cells. B-2 cells showed high Fas levels with a single peak in the FACS profile, whereas the profile of B-1 cells was biphasic, with low (Faslow) and high (Fashigh) Fas-expressing subpopulations (Fig. 3). The difference appeared not to be due to differing levels of CD40 expression, since B-1 and B-2 cells showed almost the same levels of CD40 expression, with a single peak in the FACS profile in both cases (data not shown).
Fig. 3.
Comparisons of Fas expression levels and sensitivity to Fas-mediated apoptosis between tonsillar germinal centre (GC) B-1 and B-2 cells from IgAN-improved patients. The figure shows Fas expression on living cells. Propidium iodide (PI)-positive cells (dead cells) were deleted by flow cytometric analysis. Freshly isolated B-1 and B-2 cells expressed Fas poorly. After 3 days of culture with anti-CD40 MoAb, B-2 cells showed a high level of Fas (Fashigh) with a single peak in the FACS profile, whereas the profile of B-1 cells was biphasic, with low (Faslow) and high (Fashigh) Fas-expressing subpopulations. When anti-CD40-stimulated B cell populations were cultured with anti-Fas MoAb for 24 h, the proportion of dead cells in the B-1 and B-2 populations was 50% and 99%, respectively, which corresponded to the proportion of Fashigh cells in each population. The remaining living cells belonged to the Faslow population, indicating that only the Fashigh population underwent Fas-mediated apoptosis.
We then asked if this difference in Fas expression affected Fas-mediated apoptosis. When anti-CD40-stimulated B cell populations were cultured with anti-Fas MoAb for 24 h, the proportions of dead cells in the B-1 and B-2 populations were 50% and 99%, respectively, which corresponded to the proportion of Fashigh cells in each population. The remaining living cells belonged to the Faslow population (Fig. 3), indicating that only the Fashigh population underwent Fas-mediated apoptosis. This was confirmed by experiments in which sorted Faslow and Fashigh B-1 cells were cultured in vitro in the presence of anti-Fas MoAb. Under these conditions, only Fashigh B-1 cells underwent apoptosis (data not shown). Fas ligation, thus, induced apoptosis in Fashigh B-1 and B-2 cells but not in Faslow B-1 cells.
Expression of bcl-2 protein in B-1 and B-2 cells
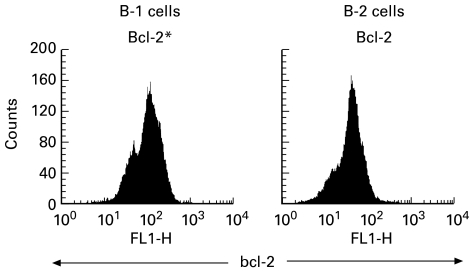
The bcl-2 gene product has been identified as a molecule that can enhance the intrinsic ability of lymphocytes to survive. To explore this possibility, the expression of bcl-2 was compared in the tonsillar GC B-1 and B-2 cells from IgAN-improved patients. Intracellular bcl-2 protein was measured by flow cytometry. Expression of bcl-2 was higher in the B-1 cells in comparison with that in the B-2 cells (Fig. 4).
Fig. 4.
Comparison of bcl-2 expression levels in tonsillar germinal centre (GC) B-1 and B-2 cells from IgAN-improved patients. The GC B cells were surface-stained with PE-conjugated anti-CD5, and intracellular stained with FITC-conjugated anti-bcl-2. bcl-2 expression was higher in B-1 cells in comparison with that in B-2 cells.
DISCUSSION
This study shows a relationship between the tonsillar immune response and the pathogenesis of IgAN. We demonstrated an increase in B-1 cell numbers in tonsillar GC from IgAN patients who showed clinical improvement after tonsillectomy. Tonsillectomy is considered an effective treatment for IgAN [10–15], but several clinical studies have reported patients who failed to improve after tonsillectomy [10–15]. In this study, the increase in B-1 cells in the tonsillar GC was not observed in patients who failed to improve after tonsillectomy. There were no differences in the number of B-1 cells between these patients and those with chronic tonsillitis without IgAN. Thus, it may be that the tonsils had no relation to the pathogenesis of IgAN in these patients. In such cases, the mechanism of IgA production is still unclear, and the foci of abnormal IgA production may be elsewhere. With respect to serum IgA titres and IgA-producing cells in tonsils, there were no differences between IgAN-improved, IgAN-unchanged, and IgAN-negative patients (data not shown).
In IgAN, the mechanism of the induction of IgA deposition remains to be defined. Several alterations related to IgA class switching in IgAN have been reported: namely, elevation of serum IgA [51–53], increases in circulating IgA immune complexes [54], increases in IgA-bearing cells in peripheral blood [55], and enhancement of in vitro IgA production by peripheral blood mononuclear cells [56–59]. It is believed that there is a hyperproduction of IgA in IgAN, and potential mechanisms have been studied by many investigators [56–59]. Reports have been intriguing concerning the prospective mechanisms of altered IgA production in IgAN. However, there are critical discrepancies that make it difficult to understand the meaning of the polymorphism in IgA synthesis. The IgA subclass prominent in IgAN is generally considered to be IgA1 [7,8,53,60]. This implies the presence of abnormalities in IgA1 production with IgAN. Considering the characteristic alterations in IgAN patients, elucidating the mechanism of IgA1 production would be a significant step towards understanding the pathogenesis of IgAN. In this study, we demonstrated that tonsillar GC B-1 cells in IgAN patients were able to produce IgA1 predominantly. IgA1-positive cells have been reported in the tonsillar GC [8]; our results suggest B-1 cells to be a major source of IgA1.
The B-1 cell population is comprised of cells that produce low-affinity, poly- or autoreactive antibodies [32,41–44]. B-1 cells do not usually participate in T cell-dependent immune reactions and hence, do not establish in GC. In rare cases, however, B-1 cells may be drawn into a GC reaction and, within this microenvironment, undergo somatic hypermutation. Through this process, low-affinity autoantibodies may give rise to high-affinity, pathogenic autoantibodies, as detected in patients suffering from autoimmune diseases [41,43]. The vigorous clonal expansion of GC B cells and the processes of somatic hypermutation and class switch recombination may subject the long-lived B-1 cells to an increased risk of malignant transformation, since these activities may increase the likelihood of transforming events. No information regarding the antigenic stimulus for B-1 cells has been provided by this study. However, several bacteria have been considered antigens in the pathogenesis of IgAN [61,62], and B-1 cells are characteristically high responders to bacterial antigens [40]. Thus, B-1 cells only proliferate and mutate within a GC in rare instances and, if they do, are at an increased risk of becoming involved in immune dysfunction [44]. The possibility of somatic hypermutation and class switch recombination of immunoglobulin in B-1 cells from tonsillar GC with IgAN, in addition to an affinity to or immune complex formation of antibodies produced by B-1 cells, is a fertile field for future investigations.
In the present study, we demonstrated reduced susceptibility to Fas-mediated apoptosis in B-1 cells from the tonsillar GC of IgAN-improved patients. This finding suggested that Fas-mediated apoptosis-resistant B-1 cells in the tonsillar GC might contribute to IgAN pathogenesis. Although apoptosis of B cells may be mediated by multiple pathways, Fas-dependent apoptosis is essential for the elimination of activated B cells, including bystander and potentially autoreactive B cells [31]. The data obtained in the present study demonstrate that B-1 cells are not as sensitive as B-2 cells to Fas-mediated apoptosis, raising the possibility that activated B-1 cells, compared with their B-2 counterparts, may not be as efficiently eliminated. We also demonstrated a higher level of bcl-2 expression in B-1 than B-2 cells. This result supports the reduced susceptibility of B-1 cells to apoptosis. Members of the bcl-2 gene family have been shown to influence the ability of lymphocytes to undergo apoptosis [33–35] and the bcl-2 gene product can enhance the ability of lymphocytes to survive [36–38]. The high level of bcl-2 expression in B-1 cells may protect these cells from undergoing apoptosis and allow them to expand clonally in vivo. It is conceivable that activated B-1 cells may survive longer than B-2 cells due to impaired apoptosis and may thus produce various antibodies, including immune complex-forming antibodies. Defects in the regulation of tonsillar GC B-1 cells would therefore have a major impact on the pathogenesis of IgAN. Understanding the mechanisms up-regulating bcl-2 expression or rendering B-1 cells susceptible to apoptosis might lead to novel therapeutic strategies in the management of IgAN. The present findings might be helpful in the development of practical laboratory and clinical strategies.
Acknowledgments
This work was supported by Grant-in-Aid for Basic Scientific Reseach (A) from the Ministry of Education, Science, Sports and Culture of Japan (10307040).
REFERENCES
- 1.D'Amico G. Idiopathic IgA mesangial nephropathy. Nephron. 1985;41:1–8. doi: 10.1159/000183538. [DOI] [PubMed] [Google Scholar]
- 2.Emancipator SN, Lamm ME. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989;60:168–76. [PubMed] [Google Scholar]
- 3.Galla JH. IgA nephropathy. Kidney Int. 1995;47:377–87. doi: 10.1038/ki.1995.50. [DOI] [PubMed] [Google Scholar]
- 4.Emancipator SN, Rao CS, Amore A, et al. Macromolecular properties that promote mesangial binding and mesangiopathic nephritis. J Am Soc Nephrol. 1992;2:S149–58. doi: 10.1681/ASN.V210s149. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka K, Maki S. Human IgA nephritis: immunocytochemical evidence of a chronic inflammatory proliferative disorder. Histol Histopathol. 1995;10:203–12. [PubMed] [Google Scholar]
- 6.Bene MC, Faure G, de Hurault Lingny B, et al. Immunoglobulin A nephropathy. Quantitative immunohistomorphometry of the tonsillar plasma cells evidences an inversion of the immunoglobulin A versus immunoglobulin G secreting cell balance. J Clin Invest. 1983;71:1342–7. doi: 10.1172/JCI110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terasawa K, Osakada M, Takahashi Y, et al. Concentration of IgA1-positive cells on the subepithelial sinusoid of the palatine tonsils in IgA nephritis. Jpn J Tonsil. 1991;31:41–45. [Google Scholar]
- 8.Kusakari C, Takasaka T, Nose M, et al. Immunohistochemical study in tonsil of IgA nephropathy. JJIAO. 1991;9:58–59. [Google Scholar]
- 9.Egido J, Blasco R, Lozano R, et al. Immunological abnormalities in tonsils of patients with IgA nephropathy: inversion in the percentage of IgA versus IgG-bearing lymphocytes and increased polymeric IgA synthesis. Clin Exp Immunol. 1984;57:101–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Iino Y, Ambe K, Kato Y, et al. Chronic tonsillitis and IgA nephropathy. Clinical study of patients with without tonsillectomy. Acta Otolaryngol (Stockh) Suppl. 1993;508:29–35. doi: 10.3109/00016489309130263. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama N, Shimizu J, Nakamura M, et al. Clinicopathological study of the effectiveness of tonsillectomy in IgA nephropathy accompanied by chronic tonsillitis. Acta Otolaryngol (Stockh) Suppl. 1993;508:43–48. doi: 10.3109/00016489309130265. [DOI] [PubMed] [Google Scholar]
- 12.Sanai A, Kudoh F. Effects of tonsillectomy in children with IgA nephropathy, purpura nephritis, or other chronic glomerulonephritides. Acta Otolaryngol (Stockh) Suppl. 1996;523:172–4. [PubMed] [Google Scholar]
- 13.Tomioka S, Miyoshi K, Tabata K, et al. Clinical study of chronic tonsillitis with IgA nephropathy treated by tonsillectomy. Acta Otolaryngol (Stockh) Suppl. 1996;523:175–7. [PubMed] [Google Scholar]
- 14.Masuda Y, Terazawa K, Kawakami S, et al. Clinical and immunological study of IgA nephropathy before and after tonsillectomy. Acta Otolaryngol (Stockh) Suppl. 1988;454:248–55. doi: 10.3109/00016488809125036. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama N, Masuda Y. Relationship between IgA nephropathy and tonsillectomy. Clinical and immunological study. Jpn J Tonsil. 1985;24:237–44. [Google Scholar]
- 16.Tokuda M, Shimizu J, Sugiyama N, et al. Direct evidence of the production of IgA by tonsillar lymphocytes and the binding of IgA to the glomerular mesangium of IgA nephropathy patients. Acta Otolaryngol (Stockh) Suppl. 1996;523:182–4. [PubMed] [Google Scholar]
- 17.Tomino Y, Sakai H, Endo M, et al. Cross-reactivity of IgA antibodies between renal mesangial area and nuclei of tonsillar cell in patients with IgA nephropathy. Clin Exp Immunol. 1983;51:605–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Nieuwenhuis P, Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984;170:421–7. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- 19.Koopman G, Pals ST. Cellular interactions in the germinal center: role of adhesion receptors and significance for the pathogenesis of AIDS and malignant lymphoma. Immunol Rev. 1992;126:21–26. doi: 10.1111/j.1600-065x.1992.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 20.Gray D. Recruitment of virgin B cells into an immune response is restricted to activation outside of follicles. Immunology. 1988;65:73–78. [PMC free article] [PubMed] [Google Scholar]
- 21.Kelsoe G. B cell diversification and differentiation in the periphery. J Exp Med. 1994;180:5–11. doi: 10.1084/jem.180.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–72. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YJ, Zhang J, Lane PJ, et al. Site of specific B cell activation in primary and secondary responses to T-cell dependent and T-cell independent antigens. Eur J Immunol. 1991;21:2951–8. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 24.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:2951–9. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 25.Liu YJ, Johnson GD, Gordon J. Germinal centers in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–22. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 26.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–86. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berek C, Berger A, Apel M. Maturation of the immune responses in germinal centers. Cell. 1991;67:1121–7. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 28.Leanderson T, Kallberg E, Gray D. Expansion, selection and mutation of antigen-specific B cells in germinal centers. Immunol Rev. 1992;126:47–54. doi: 10.1111/j.1600-065x.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 29.MacLennan ICM, Liu YJ, Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992;126:143–8. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 30.Nossal GJV, Karvelas M, Pulendran B. Soluble antigen profoundly reduces memory B-cell numbers even when given after challenge immunization. Proc Natl Acad Sci USA. 1993;90:3088–94. doi: 10.1073/pnas.90.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choe J, Kim HS, Zhang X, et al. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. J Immunol. 1996;157:1006–16. [PubMed] [Google Scholar]
- 32.Hirose S, Yan K, Abe M, et al. Precursor B cells for autoantibody production in genomically Fas-intact autoimmune disease are not subject to Fas-mediated immune elimination. Proc Natl Acad Sci USA. 1997;94:9291–5. doi: 10.1073/pnas.94.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmer M, Vallejo AN, Weyand CM, et al. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+ CD28− T cells from rheumatoid arthritis patients. J Immunol. 1998;161:1018–25. [PubMed] [Google Scholar]
- 34.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–91. [PubMed] [Google Scholar]
- 35.Ohta K, Iwai K, Kasahara Y, et al. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol. 1995;7:1817–22. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- 36.Boise LH, Gonzalez-Garcia M, Postema CE, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–605. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 37.Nunez G, London L, Hockenbery D, et al. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–8. [PubMed] [Google Scholar]
- 38.Akbar AN, Borthwick N, Salmon M, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections: the role of apoptosis in T cell memory. J Exp Med. 1993;178:427–35. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzenberg LA, Stall AM, Lalor PA, et al. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 40.Kipps TJ. The CD5 B cell. Adv Immunol. 1989;47:117–85. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- 41.Herzenberg LA, Haughton G, Rajewsky K. CD5 B cells in development and disease. Ann NY Acad Sci. 1992;651:591–601. [Google Scholar]
- 42.Hayakawa K, Hardy RR, Honda M, et al. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–8. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16:450–6. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 44.Fischer M, Klein U, Kuppers R. Molecular single-cell analysis reveals that CD5-positive peripheral blood B cells in healthy humans are characterized by rearranged Vκ genes lacking somatic mutation. J Clin Invest. 1997;100:1667–76. doi: 10.1172/JCI119691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiroi T, Yanagita M, Iijima H, et al. Deficiency of IL-5 receptor α-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999;162:821–8. [PubMed] [Google Scholar]
- 46.Arita M, Kodama S, Suzuki M, et al. Regulatory and protective role of adenoid B-1 cells in nasopharyngeal immunity. IV International Symposium on Tonsils and Adenoids, Abstract Book, Ghent, 2–5. November 1999:p. 19. [Google Scholar]
- 47.Kerakawauchi H, Kurono Y, Mogi G. Immune responses against Streptococcus pyogenes in human tonsils. Laryngoscope. 1997;107:634–9. doi: 10.1097/00005537-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Pascual V, Liu YJ, Magalski A, et al. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lebecque S, de Bouteiller O, Arpin C, et al. Germinal center founder cells display propensity for apoptosis before onset of somatic mutation. J Exp Med. 1997;185:563–71. doi: 10.1084/jem.185.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choe J, Kim HS, Armitage RJ, et al. The functional role of B cell antigen receptor stimulation and IL-4 in the generation of human memory B cells from germinal center B cells. J Immunol. 1997;159:3757–66. [PubMed] [Google Scholar]
- 51.Clarkson AR, Seymour AE, Thompson AJ, et al. IgA nephropathy: a syndrome of uniform morphology, diverse clinical features and uncertain prognosis. Clin Nephrol. 1977;8:459–67. [PubMed] [Google Scholar]
- 52.Lai KN, Chui SH, Lai FM, et al. Predominant synthesis of IgA with r light chain in IgA nephropathy. Kidney Int. 1988;33:584–91. doi: 10.1038/ki.1988.37. [DOI] [PubMed] [Google Scholar]
- 53.van den Wall Bake AW, Daha MR, van der Ark A, et al. Serum levels and in vitro production of IgA subclasses in patients with primary IgA nephropathy. Clin Exp Immunol. 1988;74:115–22. [PMC free article] [PubMed] [Google Scholar]
- 54.Yagame M, Tomino Y, Miura M, et al. Detection of IgA-class circulating immune complexes (CIC) in sera from patients with IgA nephropathy using a solid-phase anti-C3 Facb enzyme immunoassay (EIA) Clin Exp Immunol. 1987;67:270–7. [PMC free article] [PubMed] [Google Scholar]
- 55.Nomoto Y, Sakai H, Arimori S. Increase of IgA-bearing lymphocytes in peripheral blood from patient with IgA nephropathy. Am J Clin Pathol. 1979;71:158–66. doi: 10.1093/ajcp/71.2.158. [DOI] [PubMed] [Google Scholar]
- 56.Hale GM, Macintosh SL, Hiki Y, et al. Evidence for IgA-specific B cell hyperactivity in patients with IgA nephropathy. Kidney Int. 1986;29:718–26. doi: 10.1038/ki.1986.57. [DOI] [PubMed] [Google Scholar]
- 57.Waldo FB, Beischel L, West CD. IgA synthesis by lymphocytes from patients with IgA nephropathy and their relatives. Kidney Int. 1986;29:1229–35. doi: 10.1038/ki.1986.132. [DOI] [PubMed] [Google Scholar]
- 58.Yano N, Endoh M, Miyazaki M, et al. Altered production of IgE and IgA induced by IL-4 in peripheral blood mononuclear cells from patients with IgA nephropathy. Clin Exp Immunol. 1992;88:295–301. doi: 10.1111/j.1365-2249.1992.tb03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scivittaro V, Ranieri E, DiCillo M, et al. In vitro immunoglobulin production in relatives of patients with IgA nephropathy. Clin Nephrol. 1994;42:1–9. [PubMed] [Google Scholar]
- 60.Yana N, Asakura K, Endoh M, et al. Polymorphism in the Iα1 germ-line transcript regulatory region and IgA productivity in patients with IgA nephropathy. J Immunol. 1998;160:4936–42. [PubMed] [Google Scholar]
- 61.Kukuminato Y, Hamamoto M, Kataura A. Role of serum antibodies to Streptococci in patients with IgA nephropathy. Acta Otolaryngol (Stockh) Suppl. 1993;508:6–10. doi: 10.3109/00016489309130259. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki S. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet. 1994;343:12–16. doi: 10.1016/s0140-6736(94)90875-3. [DOI] [PubMed] [Google Scholar]