Abstract
Complement and Fcγ receptors are known to mediate the processing of immune complexes (IC), and abnormalities in these mechanisms may predispose to the development of lupus. We explored the processing of IC in mice deficient in complement component C1q. 125I-labelled IC comprising Hepatitis B surface antigen (HBsAg)/human anti-HBsAg (HBsAg/Ab) were injected intravenously and the sites of IC clearance determined by direct counting of organ uptake at various time points. The liver and spleen were the main sites of IC uptake in all mice. The splenic uptake of IC was significantly reduced in the C1q-deficient mice compared with the control mice. C1q-deficient mice also exhibited an initial accelerated hepatic uptake of IC similar to that seen in human subjects with hypocomplementaemia. The hepatic localization of IC at later time points was similar in both groups of mice. These data in mice are consistent with previous observations in humans that confirm that the classical pathway of complement plays an important role in the appropriate processing of IC in vivo.
Keywords: complement, mice, deficiency, monocytes/macrophages, immune complexes
INTRODUCTION
The processing of immune complexes (IC) is mediated by complement and Fcγ receptors. Immune complexes are normally removed effectively by the mononuclear phagocytic system (MPS) and abnormalities in the processing of IC may result in their persistence and deposition outside the MPS, causing inflammation and tissue damage. Abnormal IC clearance has been implicated in the pathogenesis of autoimmune diseases, such as systemic lupus erythematosus [1,2]. However, it remains presently unclear whether patients with SLE have a fundamental abnormality of the mononuclear phagocytic system resulting in defective clearance mediated by Fcγ receptors, or whether the primary problem is one of defective IC delivery to the MPS secondary to hypocomplementaemia.
The role of the complement system in the physiological clearance of IC was first recognized during the 1940s when it was demonstrated that immune precipitates contained more nitrogen when they formed in normal serum compared with heat-treated serum [3]. The interaction of complement with IC has been shown to inhibit precipitation, to promote solubilization and, in primates, to facilitate IC binding to CR1 (CD35) on erythrocytes [4]. By modifying the physiochemical structure of IC, the complement system prevents local accumulation of large IC in tissue outside the MPS and facilitates their disposal. The importance of the complement system in the processing of soluble IC has been demonstrated by human studies that showed abnormal IC clearance in complement-deficient and hypocomplementaemic patients compared to normal healthy volunteers [5–8]. In studies with model soluble IC and immune aggregates, significantly reduced initial splenic localization and subsequent retention of the injected IC (by the liver and spleen) was noted in the patients with SLE, indicating that the uptake of IC in the spleen was complement-dependent [6–8]. Complement proteins have also an important role as accessory molecules in the enhancement of the adaptive immune response, facilitating the localization of antigen to follicular dendritic cells [9], and lowering the threshold for B-cell activation [10]. Failure to localize complexes in the spleen, an important site for antigen presentation, may have important implications for the development of antibody responses, to both exogenous antigens and autoantigens.
The importance of Fcγ receptors in the uptake of IC has been demonstrated by studies of Fcγ receptor blockade in primates [11–12] and mice [13]. Abnormal Fcγ receptor-mediated clearance of IgG-opsonized erythrocytes, a model of haemolytic anaemia, has been demonstrated in SLE [14–16]. Additional evidence that Fcγ receptor function is abnormal in SLE has come from recent studies of functionally important polymorphisms of the Fcγ receptors [17–23].
To explore the role of complement in the clearance and organ localization of IC, we analysed the processing of radiolabelled preformed IC, injected intravenously in mice deficient in the classical pathway of complement caused by a targeted disruption of the C1qA-chain gene [24]. Our study showed that in this murine model the classical pathway of complement affects both the rate of hepatic uptake of IC, and the ability of the spleen to take up and retain the complexes, in a manner analagous to that previously observed in humans.
MATERIALS AND METHODS
Mice
Mice with genetic deletions of the C1qA-chain gene (C1qa -/–) were developed by homologous recombination in embryonic stem cells as previously described [24]. C1qa -/–mice were on a mixed genetic background (129/Ola × C57BL/6). In each experiment mice were between 8 and 14 weeks of age and the control animals were matched for strain, age and sex. Animal care and procedures were conducted according to Institutional guidelines.
Radiolabelling and in vitro characterization of immune complexes
The IC employed in these studies were comprised of hepatitis B surface antigen (HBsAg) (a gift from SmithKline Beecham Pharmaceuticals, Belgium), and a human IgG1 fraction of a polyclonal anti-HBsAg (kindly donated by Dr Peter Spath, Red Cross, Zurich, Switzerland). HBsAg was radiolabelled with 125I by the Bolton and Hunter method [25,26] to an activity of approximately 10 MBq/mg. The IC were prepared in manner similar to that previously described [7]. Briefly, 12·5 µ g HBsAg was mixed with 1 ml anti-HBsAg (equivalent to 50 IU) and incubated for 60 min at 37°C followed by 30 min at 4°C (equivalent to 21-fold antibody excess). Immune complex formation was confirmed by precipitation of the complexes by polyethylene glycol (PEG) and protein A sepharose. Ninety-five percent of IC were confirmed to be more than 45S in size by isopycnic sucrose density gradient centrifugation [27].
Murine complement activation assay
A haemolytic complement consumption assay was employed to determine if the model IC activated mouse complement. The lytic activity of complement was assayed by measuring the release of 51Cr labelled haemoglobin from antibody-sensitized sheep erythrocytes incubated with male mouse serum [28]. Aliquots from a pool of normal male mouse serum were incubated either with or without the immune complexes for 15 min at 37°C to allow complement activation to occur.
In vivo immune complex processing studies
The processing of HBsAg/Ab IC was studied in the gene-targeted mice, in strain-matched controls of mixed genetic background and in both the inbred parental strains (129/Ola and C57BL/6), age-and sex-matched. Immune complexes containing 1·25 µ g of radiolabelled HBsAg at a specific activity of 10 MBq/mg were injected into each mouse via a lateral tail vein in a volume of 200 µ l. The kinetics and sites of IC disposal were determined by direct counting of organ uptake at post mortem of mice killed at various time points from 10 s to 15 h after the injection of 125I-IC. Mice were weighed, killed by exsanguination under general anaesthesia, and the lungs, liver, spleen, kidneys and tail were removed. The samples were counted in a γ-counter and the organs were weighed. The total blood volume of each mouse was calculated based on 0·09 ml per gram of body mass [29]. The precise dose of radioactivity injected per mouse was measured as the number of counts in the injection volume minus the sum of the counts in the needle, syringe and tail. The tail was counted to eliminate any error arising from extravasated radioactivity at the site of injection. Experiments were performed using 51Cr-radiolabelled mouse erythrocytes (51Cr-RBC) as a blood volume marker. Erythrocytes from strain-matched control mice were radiolabelled with 51Cr and resuspended in PBS to a concentration in 100 µ l that gave approximately 10% of the radioactivity of the 125I-IC to be injected. The 51Cr-RBC suspension and 125I-IC were mixed at a 1 : 1 ratio and a total of 200 µ l was injected into each mouse. This enabled us to ascertain that variability of injection quality or difference in the blood pool within each organ were not factors influencing the results.
Control experiments were also performed to study the clearance of HBsAg alone. Mice were injected with noncomplexed radiolabelled HBsAg to determine the percentage uptake of the antigen in the spleen and liver compared with IC uptake. Data were expressed as a percentage of the injected dose of radioactivity present in the organ.
Immune complex cell binding studies
In vivo assays
C1qa -/– and control mice were injected with HBsAg/Ab IC or HAGG. The animals were killed by exsanguination after 30 s and 1 minute. Blood samples were collected into 0·01 m trisodium citrate and then subaliquoted. The radioactivity of a 50-µ l sample of whole blood was counted. A 50-µ l sample of whole blood was mixed with 200 µ l of ice cold PBS and 150 µ l of this mixture layered onto 150 µ l of Di-n-butylphthalate oil (BDH Laboratory Supplies, Poole, UK) and then microfuged at 10 000 × g to separate the cell pellet, including platelets, from the supernatant. This enabled us to calculate the percentage of radioactivity bound to cells. The remaining blood was microfuged to obtain a plasma sample which was counted. A 50-µ l aliquot of plasma was added to an equal volume of 20% trichloroacetic acid (TCA), incubated at room temperature for 15 min and then microfuged at 10 000 × g and the radioactivity bound to the precipitated protein pellet was measured. An equal volume of 8% PEG was added to an aliquot of plasma and processed as described above. The precipitated counts measured the amount of radioactivity present in the form of IC.
In vitro assays
A similar protocol to that described above was employed to study the binding of 125I-HBsAg/Ab IC to cells in vitro. IC in an antigen:antibody ratio of 12·5 µ g Ag: 50 IU Ab in 1 ml as well as an antigen control (12·5 µ g HBsAg: 1 ml HBsAg sero-negative normal human serum) were used. As a platelet-binding positive control a rat IgG2a anti-CD9 was employed (Pharmingen, San Diego, USA). Whole mouse blood in 0·01 m trisodium citrate was diluted 1 : 5 in PBS, and incubated at 37°C for 5 min. A blood sample from a normal human subject was processed at the same time as a further positive control for complement-dependent binding of IC to CR1 on human erythrocytes. Following incubation, 100 µ l duplicates from each reaction mixture, were layered onto Di-n-butylphthalate oil and microfuged at 10 000 × g to separate cells from serum. The percentage radioactivity in the cell pellet was measured.
Whole blood from wild type mice was analysed using an automated cell counter and then layered onto Di-n-butylphthalate oil and microfuged at 10 000 × g to separate cells from serum. The cell pellet was resuspended in PBS and both the plasma and resuspended cell pellet samples were analysed using the automated cell counter to confirm that all the cells from the whole blood samples were contained within the pellet and that the plasma samples were cell free.
Immune complex complement fixation assay
To confirm that the HbsAg/Ab IC were opsonized by the fixation of complement in the form of C3b an immunoabsorbant assay was employed. Duplicate aliquots of goat polyclonal antimouse C3 antibody (Cedarlane Ltd, USA) were used to coat an ELISA plate at concentrations ranging from 20 µ g/ml to 0·15 µ g/ml diluted in PBS. PBS alone was used as a nonspecific binding control. The plate was incubated at 4°C overnight and then washed three times in PBS. The 125I-HbsAg/Ab IC were prepared as described earlier, diluted 1/10 v/v in PBS and then incubated at 37°C for 20 min with an equal volume of wild type mouse serum diluted to a concentration of 1/2·5 v/v in PBS for opsonization of the IC to occur. The negative controls employed were IC incubated with PBS alone, IC incubated with heat-inactivated serum (wild-type mouse serum heated at 56°C for 30 min) and 125I-HBsAg alone incubated with wild type mouse serum. Fifty microlitre aliquots of these IC/serum mixtures were added to the ELISA plate and incubated for 60 min at 37°C and then for 30 min at 4°C for the IC to bind to the surface bound antimouse C3b via the complement component C3b bound to the surface of the HBsAg/Ab IC. The ELISA plate was washed three times in PBS to remove any unbound IC and then the plate was cut into individual wells and the radioactivity of each well determined by measurement in a gamma-counter. The radioactivity of the corresponding nonspecific binding wells was subtracted from the radioactivity in the wells coated with antimouse C3b to give a measure of IC binding via fixed complement. All samples were processed in duplicate.
Statistical analysis
For statistical evaluation of the differences in the organ uptake of the IC the t-test was used. Data are expressed as means (± SEM). Statistics were calculated using GraphPad Prism™ version 2·0 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
HBsAg/Ab IC activate murine complement and fix mouse C3b
Activation of complement in the fluid phase by HBsAg/Ab was confirmed in the haemolytic assay
HBsAg/Ab IC, prepared as described above by the addition of antigen to polyclonal anti-HBsAg (Ag:Ab ratio of 1 : 21), were incubated with fresh mouse serum at various dilutions in PBS from 1 : 5 to 1 : 40 v/v. HBsAg/Ab IC incubated with serum at a 1 : 5 v/v ratio resulted in depletion of haemolytic complement activity by 63·95% ± 4·16%; (mean ±SD of 3 samples); 1 : 40 v/v ratio: 29·65% ± 7·13% depletion. HBsAg or Ab alone did not significantly activate complement (< 5% depletion).
The ability of the HBsAg/Ab IC to fix murine complement in the form of C3b was demonstrated by an immunoabsorbance assay
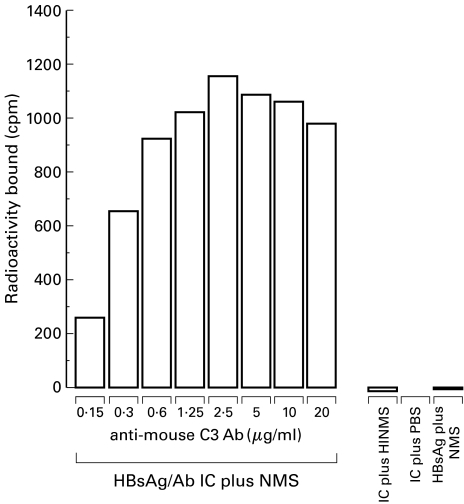
This assay demonstrated binding of the radiolabelled IC incubated with normal mouse serum to an ELISA plate via a surface bound polyclonal antimouse C3b antibody. No significant binding of the HBsAg/Ab IC incubated with heat inactivated serum, PBS, or HBsAg alone, was demonstrable. Maximum binding of HBsAg/Ab IC opsonized in mouse serum occurred at a concentration of 2·5 µ g/ml of the solid phase ligand (antimouse C3b antibody) (1160 cpm) and then declined, in a dose dependent manner, to 257 cpm at an anti-C3b concentration of 0·15 µ g/ml. For the IC incubated with heat-inactivated serum, PBS, or HBsAg alone incubated with normal serum the radioactivity bound was less than 20 cpm at all concentrations of the surface bound antimouse C3b. (Fig. 1). The radioactivity bound was calculated as the counts in the sample well minus the counts from the corresponding nonspecific binding well. All samples were processed in duplicate.
Fig. 1.
Fixation of murine complement by HBsAg/Ab IC. Graph demonstrating binding of the opsonized HBsAg/Ab IC to the solid phase ligand (polyclonal antimouse C3) via C3b. There is negligible binding of the HBsAg/Ab IC when treated with heat inactivated normal mouse serum (HINMS) or PBS rather than normal mouse serum (NMS). HbSAg alone treated with NMS does not bind significantly. This demonstrates that only the HBsAg/Ab IC was able to fix complement in the form of C3b.
Kinetics of HBsAg/Ab immune complex processing in the gene-targeted mice
The initial kinetics of HBsAg/Ab complex clearance from the circulation were studied by direct organ and blood counting post mortem.
Kinetics of organ uptake in C1q-deficient mice determined by direct organ counting
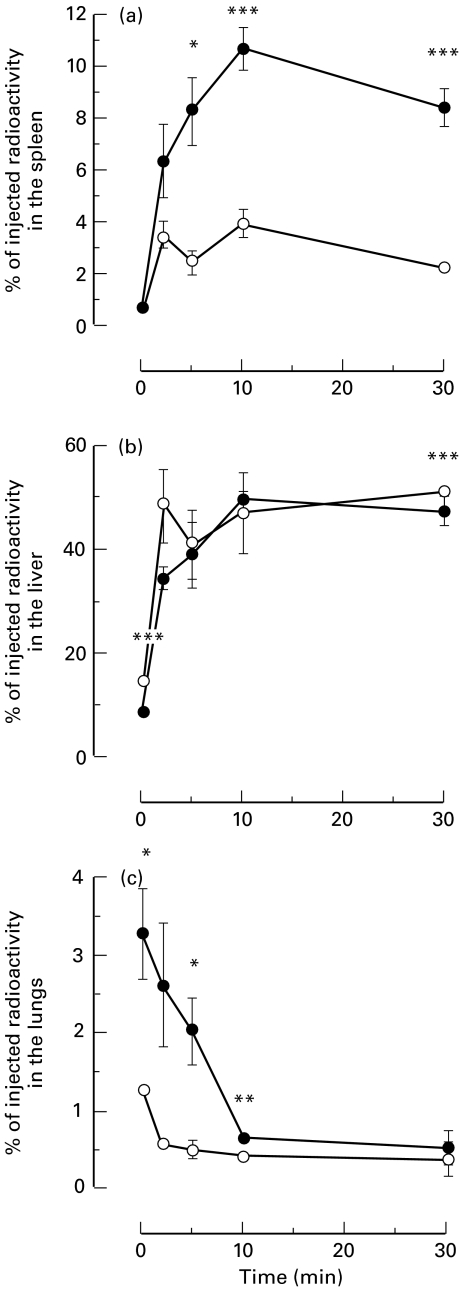
C1q-deficient and normal control mice were injected with HBsAg/Ab IC and sacrificed at different times ranging from 10 s to 30 min. The organ counts showed a similar rapid uptake of the majority of the IC to the spleen and the liver. The kinetics of splenic uptake of IC are shown in Fig. 2(a). The immediate splenic uptake of IC was not detectably different between the two groups of animals, but by 5 min the mean splenic uptake of IC in 3 wild-type mice was 8·28% (± 1·34) compared with 2·42% (± 0·49) in 3 C1q-deficient mice; P = 0·0148. By 10 min the mean splenic uptake of IC in 4 wild-type mice had reached a maximum of 10·72% (± 0·82) compared with 3·89% (± 0·55) in the C1q-deficient animals; P = 0·0002. After 30 min the percentage of injected dose dropped to 8·39% (± 0·73) and 2·21% (± 0·19) in 7 wild type and C1q-deficient animals, respectively; P < 0·0001.
Fig. 2.
Kinetics of HBsAg/Ab IC processing in C1q-deficient mice. Graphs showing the kinetics of organ uptake of HBsAg/Ab IC, expressed as percentage of injected radioactivity, in C1qa -/– (○) and C1qa+/+ (•) mice. (a) The splenic uptake of IC was significantly reduced in the C1qa -/–mice by five minutes after injection and remained so throughout the time course. (b) The hepatic uptake of IC was initially accelerated (at 10 s) in the C1q-deficient mice but became not detectably different from the wild‐type controls at the following time points. (c) The lung showed an increased uptake of IC during the first 10 min in the C1qa+/+ mice compared with the C1qa -/–mice. Data are expressed as means ± standard error of the mean. *P < 0·05, **P < 0·01, ***P < 0·001
In the wild-type mice the initial hepatic uptake was rapid, reaching 50·02% (± 1·43) at 10 min. Ten seconds after injection of the IC, the mean hepatic uptake in 3 C1q-deficient animals was significantly greater than that seen in 3 wild‐type mice (14·20% ± 0·11 and 8·36% ± 0·25, respectively; P < 0·0001). After this initial time point the kinetics of hepatic uptake were similar in both the experimental groups (Fig. 2b). This observation is in agreement with previous studies in humans with acquired or genetically determined hypocomplementaemia [5,7,8].
The kinetics of HBsAg/Ab IC uptake in the lungs and kidneys were also studied during the first 30 min postinjection. By 10 s 3·32% (± 0·60) of the IC were localized in the lungs of the wild-type mice compared to only 1·26% (± 0·03) in the C1q‐deficient mice (P = 0·0263) (Fig. 2c). Although the percentage of the injected radioactivity present in the lungs decreased with time, it was still significantly greater in the wild type mice after 10 min (0·66% ± 0·04 compared to 0·44% ± 0·02; P = 0·0019). The proportion of injected IC present in the lungs 30 min after injection showed no difference between the two groups. Clearance of the IC from the kidneys was not substantially different between the two experimental groups (data not shown).
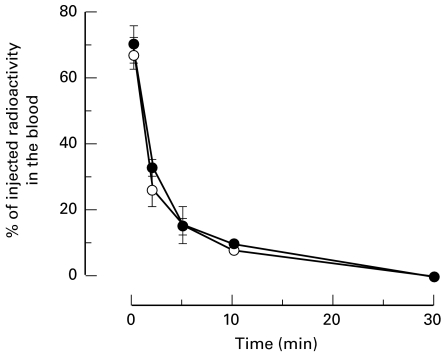
Blood clearance kinetics in C1q-deficient mice
In contrast to the observations made in hypocomplementaemic patients (7), no obviously detectable difference in clearance of the HBsAg/Ab IC from the circulation was observed between C1qa−/−and C1qa+/+ mice (Fig. 3). PEG and TCA precipitation analysis of fresh plasma showed that the majority of protein bound radioactivity was initially in the form of IC with a progressive decline with time. No significant differences between C1q-deficient and wild-type mice were observed (data not shown).
Fig. 3.
Kinetics of clearance of HBsAg/Ab IC from the bloodstream of C1qa -/– (○) and C1qa+/+ (•) demonstrating no significant difference in the clearance of the IC from the blood between the C1q-deficient mice and their wild type controls.
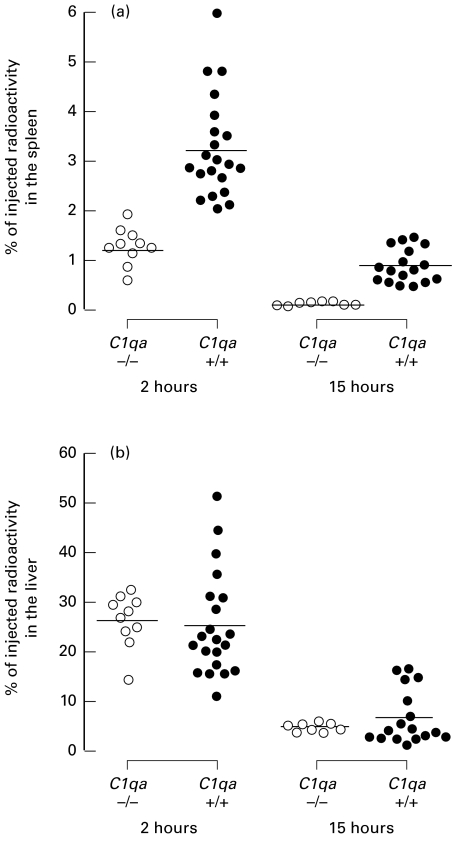
HBsAg/Ab IC organ retention studies in C1q-deficient mice
Further analysis of the splenic and hepatic retention of the HBsAg/Ab IC was carried out in larger groups of animals at 2 and 15 h post injection. At both these time points the splenic uptake of IC was substantially reduced in the C1q-deficient animals compared with control animals (Fig. 4a). After 2 h the mean splenic uptake was 1·29% (± 0·12) in 10 C1qa -/–mice compared to 3·25% (± 0·22) in 21 C1qa+/+ mice; P < 0·0001. By 15 h the percentage of IC retained in the spleen in 8 C1q-deficient animals had fallen to 0·12% (± 0·01) but remained higher in 17 control mice, 0·90% (± 0·08); P < 0·0001. In contrast, the hepatic uptake and retention were similar in both groups with a hepatic uptake of approximately 25% at 2 h and 5% at 15 h post injection (Fig. 4b).
Fig. 4.
HBsAg/Ab IC organ retention. Splenic and hepatic uptake and retention of HBsAg/Ab IC at 2 and 15 h in C1qa -/–(○) and C1qa+/+(•) mice. Horizontal bars denote means. (a) A highly significant reduction in the splenic uptake of IC in the C1q-deficient mice compared with the controls was observed at both 2 and 15 h postinjection (P < 0·0001 for both time-points). (b) No difference was found in the liver at these times.
The splenic uptake of HBsAg alone at the 2 h time point was similar between C1qa -/–and control mice. The splenic uptake of HBsAg accounted for only 0·37% ± 0·02 (n = 9) of the radioactivity injected.
Binding of immune complexes to cells
In primates soluble IC are delivered to the MPS bound on erythrocyte CR1, while in rodents platelets have been shown to bind circulating IC, albeit transiently [30]. In our study there was no binding of the HBsAg/Ab or HAGG IC to blood cells at 30 s or 1 minute after injection in either the C1q-deficient or control mice, as demonstrated by negligible radioactivity in the cell pellet after centrifugation through oil. Less than 1% of the radioactivity from the injected IC was present in the cell pellet at 30 s post injection in controls and C1q-deficient mice. More than 95% of the radioactivity in the plasma was precipitated by TCA which demonstrated that the immune complexes were free in the plasma rather than bound to circulating blood cells (data not shown).
Similar findings were confirmed by in vitro assays. The HBsAg/Ab IC, opsonized with murine complement, did indeed bind to human erythrocytes under the same conditions and the binding of the anti-CD9 monoclonal antibody provided strong evidence for the presence of mouse platelets in the cell pellet preparations (data not shown). The study utilizing an automated cell counter to determine the distribution and concentration of blood cells within the plasma and cell pellet fractions of the preparations of murine blood spun through Di-n-butylphthalate oil confirmed that the serum fractions were cell free and that all the platelets present in the whole blood were present in the resuspended cell pellet following microfugation (data not shown).
DISCUSSION
Interactions between immune complexes and the fixed macrophage system in vivo are not fully understood. Fcγ receptors and complement are both known to be involved in the clearance of IC, but the relative importance of these systems is not known. The study of the organ localization of model soluble IC in gene-targeted mice deficient in the classical complement pathway component C1q provided a unique opportunity to explore the contribution of the classical complement pathway in the processing of IC in a murine model.
The main sites of uptake of the HBsAg/Ab and HAGG IC were the liver and spleen and this is consistent with previous reports on IC processing in mice [30–37], and in primates [4,38]. No difference was observed in the immediate kinetics of IC clearance from the circulation between the C1q-deficient and control mice. However, by five minutes the splenic uptake of the HBsAg/Ab IC in the complement deficient mice was significantly lower. This highly significant reduction was also present at all subsequent time points analysed. Previous studies in a C2-deficient human [8] and in mice deficient of complement components factor B and C2 [39] suggested that C3 activation is the predominant requirement for the appropriate splenic localization of IC.
In the liver, there were no differences in the uptake of the model immune complexes between the gene-targeted strains and normal control mice apart from an initial accelerated hepatic uptake in the C1q-deficient mice, which is in agreement with the previous observations in humans [5,7,8]. Previous studies have emphasized the importance of the liver in the clearance of both particulate and soluble IC in animals [40,41], and of the spleen in the clearance of both IgG-coated erythrocytes [42] and soluble IC in humans [5,7,8]. Our data are compatible with these findings of earlier studies in that the major organ for IC uptake was the liver in both normal and gene-targeted mice. It is interesting to note that the consistent reduction in splenic uptake of IC observed in the C1q knockout mice was not detected in earlier studies using complement-depleted animals [43].
The basis for this defective splenic localization of injected IC is not yet clear. One possibility is that it is only IC which are able to bind efficiently to complement and Fcγ receptors on fixed macrophages, with subsequent internalization and processing, that are retained effectively by the spleen. Immune complexes bearing little or no C3, as would be found in association with defective classical pathway activation, may only bind to the low affinity receptors, Fcγ RII and Fcγ RIII and failure of uptake and retention may occur. In contrast as the percentage hepatic localization of the injected HBsAg/Ab and HAGG was similar in all mice studied it may be that either complement or Fcγ receptors alone are sufficient for normal hepatic uptake and retention, or that hepatic uptake of IC is mediated largely by receptor-based pinocytic mechanisms.
The initial clearance of IC from the blood and their processing in the lungs and kidneys were also studied in the normal and C1q-deficient mice. In contrast to studies in humans that showed an accelerated blood clearance in complement-deficient patients [7,8], there was no accelerated clearance in the C1q-deficient mice. The lungs constitute a major site for the initial uptake of IC in pigs, but had not been considered to be a significant site for immune complex clearance in primates and mice [44]. In our experiments approximately 3% of the IC injected localized rapidly to the lungs in the wild‐type mice and then declined quickly. This IC trapping in the lungs was significant reduced in the complement-deficient mice. This result suggests a role for complement receptors in the clearance of IC in the lungs of mice, although we cannot exclude the possibility that this observation may be due to C1q-mediated aggregation of the soluble IC. Whether or not there is a very small population of murine pulmonary intravascular macrophages that might mediate this effect is controversial [45].
As a control for the studies of the HBsAg/Ab we examined the clearance of HBsAg alone. We noted in our studies that only approximately 10% of the HBsAg/Ab IC uptake in the spleen occurred with HBsAg alone. The results with HBsAg suggest that there is a highly specific uptake mechanism for IC in the spleen, as would be expected for an organ with such a crucial role in antigen processing and presentation.
Other groups have previously shown that IC injected into lagomorphs and rodents bound quickly, albeit transiently, to platelets [30,46]. This was not the finding in our studies, neither in vivo nor in vitro, with the model IC studied. A number of variables (antigen, IC size, antibody species, and ability to activate complement) may explain the different data in the literature. The protein responsible for the immune adherence reaction in mice is still unidentified. The mouse complement receptors MCR1 and MCR2 are coded by alternatively spliced Cr2 gene transcripts and are found on B lymphocytes and phagocytes but not on platelets or erythrocytes [47]. Immune adherence receptors that bind C3b are present on mouse platelets and unstimulated neutrophils but are not MCR1 or MCR2. Recently, a 190-kD membrane protein capable of binding C3b has been identified on mouse neutrophils and has been designated C3bR-190 [48]. This protein is also present on mouse platelets but in reduced amounts. Two further C3d-binding proteins of 125- and 150-kD, respectively, have also been identified on mouse platelets [48]. However, the physiological role, if any, of immune complex binding to cellular components in the blood in mice remains still unresolved. In the present study our failure to demonstrate binding of C3 opsonized IC to platelets might have reflected a relatively inefficient opsonization of the IC by C3.
The observations in the C1q-deficient mice of markedly reduced splenic IC uptake is analogous to previous findings of in vivo IC processing in SLE patients [6–8]. In primates, the mode of delivery of IC to the liver and spleen is fundamentally different to that in mice. In primates, IC which fix complement are transported in the blood bound to erythrocytes via CR1. It has previously been suggested that the defective splenic uptake of IC observed in humans in the presence of hypocomplementaemia may be related primarily to a failure of binding to erythrocytes, and abnormal delivery of IC to the splenic vascular bed may occur [7]. From the present study we can conclude that it is likely that hypocomplementaemia also has a much more fundamental role in influencing IC processing at a cellular level within the spleen, independent of the mode of their delivery to this organ.
Acknowledgments
This work was supported by the Arthritis Research Campaign (U.K.) and the Wellcome Trust (grant 054838). J.T.N. was a recipient of a Medical Research Council (U.K.) Clinical Training Fellowship.
REFERENCES
- 1.Atkinson JP. Complement activation and complement receptors in systemic lupus erythematosus. Springer Semin. Immunopathol. 1986;9:179–94. doi: 10.1007/BF02099021. [DOI] [PubMed] [Google Scholar]
- 2.Lachman PJ, Walport MJ. Deficiency of the effector mechanisms of the immune response and autoimmunity. Ciba Found Symposium. 1987;129:149–71. doi: 10.1002/9780470513484.ch11. [DOI] [PubMed] [Google Scholar]
- 3.Heidelberger M. Quantitative chemical studies on complement or alexin I A method. J Exp Med. 1941;73:691–4. doi: 10.1084/jem.73.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornacoff JB, Hebert LA, Smead WL, et al. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schifferli JA, Ng YC, Estreicher J, et al. The clearance of tetanus toxoid/anti-tetanus toxoid immune complexes from the circulation of humans. Complement- and erythrocyte complement receptor 1- dependent mechanisms. J Immunol. 1988;140:899–904. [PubMed] [Google Scholar]
- 6.Halma C, Daha MR, Camps JA, et al. Deficiency of complement component C3 is associated with accelerated removal of soluble 123I-labelled aggregates of IgG from the circulation. Clin Exp Immunol. 1992;90:394–400. doi: 10.1111/j.1365-2249.1992.tb05857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies KA, Peters AM, Beynon HLC, et al. Immune complex processing in patients with systemic lupus erythematosis –In vivo imaging and clearance studies. J Clin Invest. 1992;90:2075–83. doi: 10.1172/JCI116090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies KA, Erlendsson K, Beynon HLC, et al. Splenic uptake of immune complexes in man is complement dependent. J Immunol. 1993;151:3866–73. [PubMed] [Google Scholar]
- 9.Papamichail M, Gutierrez C, Embling P, et al. Complement dependence of localisation of aggregated IgG in germinal centres. Scand J Immunol. 1975;4:343–7. doi: 10.1111/j.1365-3083.1975.tb02635.x. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey PW, Allison ME, Akkaraju S, et al. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson SB, Kimberly RP, Valinsky JE, et al. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med. 1986;164:474–89. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimberly RP, Edberg JC, Merriam LT, et al. The in vivo handling of soluble complement fixing Ab/dsDNA immune complexes in chimpanzees. J Clin Invest. 1989;84:962–70. doi: 10.1172/JCI114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984;133:855–62. [PubMed] [Google Scholar]
- 14.Frank MM, Hamburger MI, Lawley TJ, et al. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979;300:518–23. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- 15.Parris TM, Kimberly RP, Inman RD, et al. Defective Fc receptor mediated function of the mononuclear phagocyte system in lupus nephritis. Ann Intern Med. 1982;97:526–32. doi: 10.7326/0003-4819-97-4-526. [DOI] [PubMed] [Google Scholar]
- 16.Hamburger MI, Lawley TJ, Kimberley RP, et al. A serial study of splenic reticuloendothelial system Fc receptor functional activity in systemic lupus erythematosus. Arthritis Rheum. 1982;25:44–54. doi: 10.1002/art.1780250108. [DOI] [PubMed] [Google Scholar]
- 17.Salmon JE, Edberg JC, Brogle NL, et al. Allelic polymorphisms of human Fcγ receptor IIA and Fcγ receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992;89:1274–8. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parren PWHI, Warmerdam PAM, Boeije LCM, et al. On the interaction of IgG subclasses with low-affinity Fcγ RIIa (CD32) on human monocytes, neutrophils, and platelets. J Clin Invest. 1992;90:1537–46. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmon JE, Millard S, Schacter LA, et al. Fcγ RIIa alleles are heritable risk factors of lupus nephritis in African Americans. J Clin Invest. 1996;97:1348–54. doi: 10.1172/JCI118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duits AJ, Bootsma H, Derksen RHWM, et al. Skewed distribution of IgG Fcγ receptor IIa (CD32) is associated with renal disease in systemic lupus erythematosus patients. Arthritis Rheum. 1995;39:1832–6. doi: 10.1002/art.1780381217. [DOI] [PubMed] [Google Scholar]
- 21.Norsworthy P, Theodoridis E, Botto M, et al. Overrepresentation of the Fcgamma receptor type IIA R131/R131 genotype in caucasoid systemic lupus erythematosus patients with autoantibodies to C1q and glomerulonephritis. Arthritis Rheumatism. 1999;42:1828–32. doi: 10.1002/1529-0131(199909)42:9<1828::AID-ANR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Manger K, Repp R, Spriewald BM, et al. Fcγ receptor IIa polymorphism in caucasian patients with systemic lupus erythematosus. Association with clinical symptoms. Arthritis Rheum. 1998;41:1181–9. doi: 10.1002/1529-0131(199807)41:7<1181::AID-ART6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Edberg JC, Redecha PB, et al. A novel polymorphism of Fcγ RIII (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 25.Bolton AE, Hunter W. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973;133:529–39. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraker PJ, Speck JC. Protein and cell membrane iodinations with a sparingly soluble chloramide, 1,3,4,6-tetrachloro-3a, 6a–diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 27.Johns P, Stanworth DR. A simple numerical method for the construction of isokinetic sucrose density gradients, and their application to the characterisation of immunoglobulin complexes. J Immunol Methods. 1976;10:231–52. doi: 10.1016/0022-1759(76)90174-5. [DOI] [PubMed] [Google Scholar]
- 28.Andrews BS, Theofilopoulos AN. A microassay for the determination of hemolytic complement activity in mouse serum. J Immunol Methods. 1978;22:273–81. doi: 10.1016/0022-1759(78)90035-2. [DOI] [PubMed] [Google Scholar]
- 29.Skogh T. Tissue distribution of intravenously injected dinitrophenylated human serum albumin. Effect Specific IgG IgA Antibodies. Scand J Immunol. 1982;16:465–75. doi: 10.1111/j.1365-3083.1982.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 30.Edberg JC, Tosic L, Taylor RP. Immune adherence and the processing of soluble complement-fixing antibody/DNA immune complexes in mice. Clin Immunol Immunopathol. 1989;51:118–32. doi: 10.1016/0090-1229(89)90212-2. [DOI] [PubMed] [Google Scholar]
- 31.Finbloom DS, Plotz PH. Studies of reticuloendothelial function in the mouse with model immune complexes: II. Serum clearance, tissue uptake, and reticuloendothelial saturation in NZB/W mice. J Immunol. 1979;123:1600–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Finbloom DS, Plotz PH. Studies of reticuloendothelial function in the mouse with model immune complexes: I. Serum clearance and tissue uptake in normal C3H mice. J Immunol. 1979;123:1594–9. [PubMed] [Google Scholar]
- 33.Emlen W, Mannik M. Clearance of circulating DNA-anti-DNA immune complexes in mice. J Exp Med. 1982;155:1210–5. doi: 10.1084/jem.155.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finbloom DS, Abeles D, Rifai A, et al. The specificity of uptake of model immune complexes and other protein aggregates by the murine reticuloendothelial system. J Immunol. 1980;125:1060–5. [PubMed] [Google Scholar]
- 35.Cosio FG, Hebert LA, Birmingham DJ, et al. Clearance of human antibody/DNA immune complexes and free DNA from the circulation of the non human primate. Clin Immunol Immunopathol. 1987;42:1–9. doi: 10.1016/0090-1229(87)90167-x. [DOI] [PubMed] [Google Scholar]
- 36.Emlen W, Burdick G. Clearance and organ localisation of small DNA anti-DNA immune complexes in mice. J Immunol. 1988;140:1816–22. [PubMed] [Google Scholar]
- 37.Aguado MT, Mannik M. Clearance kinetics and organ uptake of complement solubilised immune complexes in mice. Immunology. 1987;60:225–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Waxman ME, Hebert LA, Cornacoff JB, et al. Complement depletion accelerates the clearance of immune complexes from the circulation of primates. J Clin Invest. 1984;74:1329–40. doi: 10.1172/JCI111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor PR, Nash JT, Theodoridis E, et al. A targeted disruption of the murine complement factor B gene resulting in loss of expression of three genes in close proximity, factor B, C2 and D17H6S45. J Biol Chem. 1998;273:1699–704. doi: 10.1074/jbc.273.3.1699. [DOI] [PubMed] [Google Scholar]
- 40.Haakenstad AO, Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974;112:1939–48. [PubMed] [Google Scholar]
- 41.Veerhuis R, Krol MC, Van Es LA, et al. Difference in clearance kinetics of particulate immune complexes and soluble aggregates of IgG in vivo. Clin Immunol Immunopathol. 1986;41:379–91. doi: 10.1016/0090-1229(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 42.Frank MM, Lawley TJ, Hamburger MI, et al. Immunoglobulin G Fc receptor-mediated clearance in autoimmune diseases. Ann Intern Med. 1983;98:206–18. doi: 10.7326/0003-4819-98-2-218. [DOI] [PubMed] [Google Scholar]
- 43.Bockow B, Mannik M. Clearance and tissue uptake of immune complexes in complement depleted and control mice. Immunology. 1981;42:497–504. [PMC free article] [PubMed] [Google Scholar]
- 44.Davies KA, Chapman PT, Norsworthy PJ, et al. Clearance pathways of soluble immune complexes in the pig. Insights into the adaptive nature of antigen clearance in humans. J Immunol. 1995;155:5760–8. [PubMed] [Google Scholar]
- 45.Staub NC. Pulmonary intravascular macrophages. Annu Rev Physiol. 1994;56:47–67. doi: 10.1146/annurev.ph.56.030194.000403. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RP, Kujala G, Wilson K, et al. In vivo and in vitro studies of the binding of antibody/ds DNA immune complexes to rabbit and guinea-pig platelets. J Immunol. 1985;134:2550–8. [PubMed] [Google Scholar]
- 47.Kinoshita T, Takeda J, Hong K, et al. Monoclonal antibodies to mouse complement receptor type 1 (CR1) their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140:3066–72. [PubMed] [Google Scholar]
- 48.Quigg RJ, Alexander JJ, Lo CF, et al. Characterisation of C3-binding proteins on mouse neutrophils and platelets. J Immunol. 1997;159:2438–44. [PubMed] [Google Scholar]