Abstract
A variety of studies have stressed the importance of the control of inflammatory cell longevity and the balance of pro-survival and pro-apoptotic signalling. Recently, asthma was found to be associated with reduced apoptosis of inflammatory cells in lung tissue. The aim of the study was to investigate the systemic activation of apoptosis pathways using cDNA array technology in atopy and asthma. Eighteen atopic asthmatics (AA), eight atopic non-asthmatic (AN) and 14 healthy control subjects (C) were included in the study. Peripheral blood mononuclear cells were separated with gradient centrifugation, mRNA purified and the reverse-transcribed probes hybridized to cDNA arrays. The signals were compared by standardizing to the 100 most expressed genes and group differences assessed with the Mann–Whitney U-test. We found a concerted up-regulation of several pro-survival cytokines and growth factors in AN and AA. FAS and FASL were not differentially expressed, but FAST kinase was over-expressed in AN and AA. The tumour necrosis factor pathway was activated in AN and AA with increased cytokine and receptor levels and increased TRAF2, an intracellular signalling product. There were indications of a down-regulated p53 system. In contrast, the Bcl-2 family of genes showed a net pro-apoptotic profile in AN and AA. The group of caspases showed a constant gene expression pattern in all groups. In conclusion, significant differences in the expression of apoptosis-related genes were found in peripheral blood of atopic individuals with and without asthma. cDNA array technology proved to be useful and may be complementary to DNA-based studies in order to analyse interactive and multidimensional pathways as shown here for apoptosis.
Keywords: atopy, asthma, gene expression, cDNA, apoptosis
INTRODUCTION
Airway inflammation is associated with asthma and is more pronounced with increasing asthma severity [1,2]. However, the mechanism leading to the persistent accumulation of inflammatory cells is not fully understood. This could be explained in part by the release of chemotactic cytokines induced by low-grade exposure to allergens with increased cell influx [3]. Alternatively, inflammatory cells resident in the airways of asthmatic individuals could remain in a functional state for a prolonged period [4]. Both mechanisms may result in a net accumulation of inflammatory cells. Apoptosis, a dynamic process involved in the control of the ‘tissue load’ of immune effector cells at inflamed sites, tends to limit inflammatory tissue injury and promote resolution rather than progression of inflammation [5,6]. Recently, Vignola et al. [7] found a significantly higher number of non-apoptotic airway eosinophils and macrophages in subjects with asthma compared with patients with chronic bronchitis. This suggests that these cells can survive longer in the airways of asthmatic subjects. Furthermore, the proportion of non-apoptotic cells correlated with asthma severity. However, it is unclear whether asthmatics have a primary genetic deregulation of apoptosis pathways or if the reduced apoptosis is a secondary phenomenon in the context of a variety of topically released proinflammatory and pro-survival cytokines. We wished to test whether peripheral blood mononuclear cells (PBMC) of atopic individuals showed differences in gene expression associated with reduced apoptosis.
Many different pathways are involved in apoptosis, the main ones being signalling through death receptors (e.g. FAS, TNFR1 [8]), the p53 pathway [9], the Bcl-2 family of genes [10] and the cascade of effector proteases—the caspases [11]. These pathways involve multiple components, which can be pro- and anti-apoptotic. Reduced apoptosis can therefore be the net result of reduced expression of pro-apoptotic factors or increased expression of pro-survival products. The large number of genes, whose differential expression could affect cell survival in particular pathological situations, creates two major problems in attempting to analyse the basis for altered cell survival by measuring gene expression on an individual basis. First of all, it is likely that a small proportion of genes involved will show altered expression, making identification of those genes difficult. Second, and more importantly, the identification of differential expression of a single gene will not necessarily reflect the whole balance of changes in pro-apoptotic and pro-survival gene expression.
With cDNA/oligonucleotide arrays several hundreds to thousands of gene products can be assayed in a single experiment, opening a new dimension to gene expression studies (Fig. 1 [12–15]). With this technique, target mRNA can be copied into radiolabelled cDNA with reverse transcriptase so that the relative abundance of individual mRNAs is reflected in the cDNA product. For gene expression studies single-stranded probe cDNA or oligonucleotides [17,18] derived from sequences of known genes, cDNA libraries or expressed sequence tags (ESTs) can be fixed on filter/glass slide arrays and hybridized with the target cDNA. There are two difficulties in interpreting the results of cDNA/oligonucleotide arrays. First, it is difficult to quantify and compare gene expression between individuals and even within individuals at different time points. Second, the sheer volume of data makes critical analysis and pattern recognition extremely difficult.
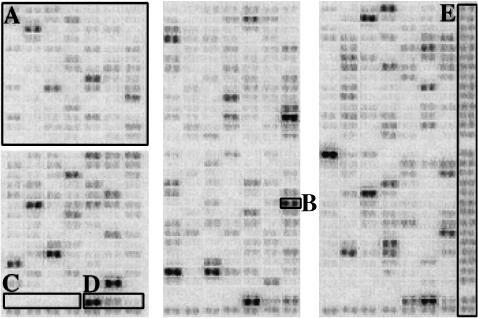
Fig. 1.
Example of a cDNA array after hybridization. With this technique, mRNA can be copied into radiolabelled cDNA with reverse transcriptase so that the relative abundance of individual mRNAs is reflected in the cDNA product. Thus, the intensity of the hybridization signal for a given gene product is a result of its relative abundance in the target sample. This method has proved to provide excellent specificity and reproducibility [14,16,17]. Messenger RNA species comprising 1:10 000–100 000 of the mass of the target poly(A) + RNA, which corresponds to approximately one transcript per 100 000, could readily be detected [15,17,18]. Single-stranded DNA with a length of 200–600 bases of known genes can be fixed to a membrane and probed with the RNA-derived cDNA probe. The intensity of the hybridization signal for a given gene is a result of its relative abundance in the RNA-derived DNA probe. Each gene product is represented in duplicate including positive and negative controls. The complete list of genes is available at: http://www.clontech.com. Square A shows one of six regions of 98 genes each spotted in duplicates (B). The membrane also provides three series of negative (C; plasmid and bacteriophage DNA) and positive controls (D; housekeeping genes). The last column on the right (E) and the bottom row is a control for the hybridization quality and should give an even hybridization signal throughout the membrane.
In the present study we have used cDNA array technology to investigate the expression of 127 genes associated with apoptosis in PBMC of atopic and asthmatic individuals compared with healthy controls.
SUBJECTS AND METHODS
Subjects
Fourteen healthy adults (C; non-atopic; M/F 2/12; age 42 ± 13 years (27–65 years)), eight atopic adults without asthma (AN; M/F 7/1; age 36 ± 9 years (23–49 years)) and 18 atopic asthmatics (AA; M/F 6/12; age 41 ± 14 years (18–66 years)) gave informed consent and were included in the study. Atopy was defined as a weal ≥ 3 mm diameter greater than negative control weal on skin prick test to a range of four common aeroallergens in the UK (Dermatophagoides pteronissinus, cat, dog, mixed grasses; ALK, Hørsholm, Denmark). Atopic asthma was defined as symptomatic bronchial hyperreactivity or reversibility in a sensitized individual. All patients had a physician-diagnosed asthma and received anti-asthma treatment prior to being enrolled in the study. They were taking inhaled corticosteroids (beclomethasone equivalent dose: none, n = 3; 400 μg/day, n = 2; 800 μg/day, n = 1; 1000 μg/day, n = 3; 1500 μg/day, n = 3; 2000 μg/day, n = 6). None had received oral or parenteral corticosteroids, antihistamines, leukotriene antagonists, theophylline-type medications or antibiotics for at least 2 months. The severity of asthma was determined using Aas asthma severity scores [19]. The Aas score is a five-step scale clinical score, which takes into account events occurring during the previous year. Patients with atopic asthma had a range of disease severity (I, n = 2; II, n = 1; III, n = 4; IV, n = 9; V, n = 2).
PBMC preparation and cDNA hybridization
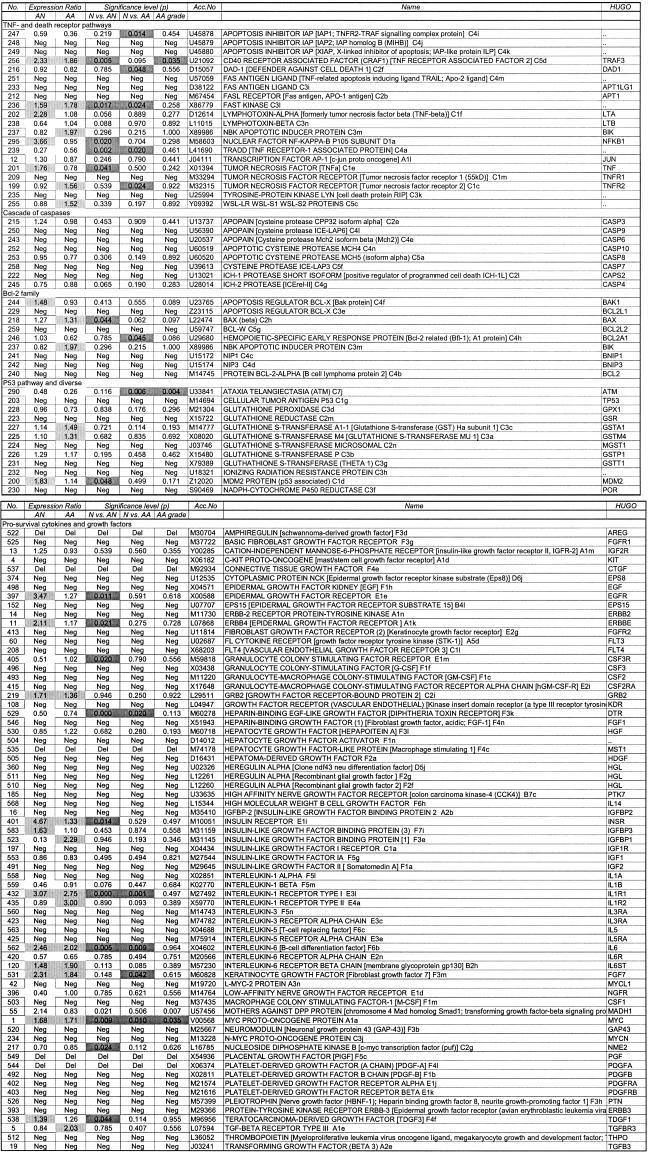
PBMC were separated by gradient centrifugation (Ficoll–Paque 1.077; Life Technologies Ltd, Paisley, UK) and washed in AIM-V serum-free culture medium. The purified mRNA populations (TRIzol™; Life Technologies Ltd, and Oligotex™, Qiagen Ltd, Crawley, UK) were reverse transcribed with oligo-dT primer mix and labelled with α32P-dATP (Amersham Life Science Ltd, Aylesbury, UK). Following an overnight hybridization onto membranes with immobilized probe cDNA for 609 gene products in duplicate (Atlas™; Clontech, Palo Alto, CA; the complete list of genes with accession numbers is published at http://www.clontech.com) the quantification was completed with autoradiography and phosphor imaging. The signal intensity of the different gene products was standardized according to the methods described below. The genes which were subject to analysis in the present study are listed in Table 1.
Table 1.
Relative gene expression in atopic individuals with and without asthma compared with healthy control subjects
Standardization of quantitative hybridization signals
In order to compare the results of different hybridization experiments the data need to be standardized for non-specific variation. Several methods were compared using gene expression results of PBMC from 48 individual hybridization experiments: (i) expressing the results relative to the expression of a single reference/‘housekeeping’ gene (β-actin, glyceraldehyde-3-phosphate dehydrogenase (G3PDH)); (ii) expressing the results relative to the geometric mean of nine ‘housekeeping’ genes (GM9; β-actin, G3PDH, MHC class I HLA-C allele HLA-4, ubiquitin, phospholipase A2, IMP:pyrophosphate phosphoribosyltransferase, α-tubulin, 23-kD highly basic protein, ribosomal protein S9); (iii) expressing the results relative to the geometric mean of the hundred genes with the greatest expression (GM100; the assumption was made that PBMC express similar amounts of mRNAs with comparable overall mean hybridization intensities over the range of 609 gene products); (iv) transforming the data to a standard lognormal distribution (SLD). The standard lognormal signal i for a gene in specimen j (SNij) was calculated as: SNij = ((dij – mi)/SDi), where d is the measured value of the transcript in the specimen, m and SD are, respectively, the mean and the standard deviation of the lognormalized results for a given experiment.
Verification experiments of cDNA array data
Several identified differentially expressed genes have been validated on a mRNA level by reverse transcriptase-polymerase chain reaction (RT-PCR) (e.g. IL4, IL6, IFNG, VEGF, TFCP2), a protein level by ELISA (e.g. TGFB1–3) and a receptor level by double-stained FACS analysis (e.g. ITGB4, IL2RA). The results of these validation experiments are available upon request.
Statistical analysis
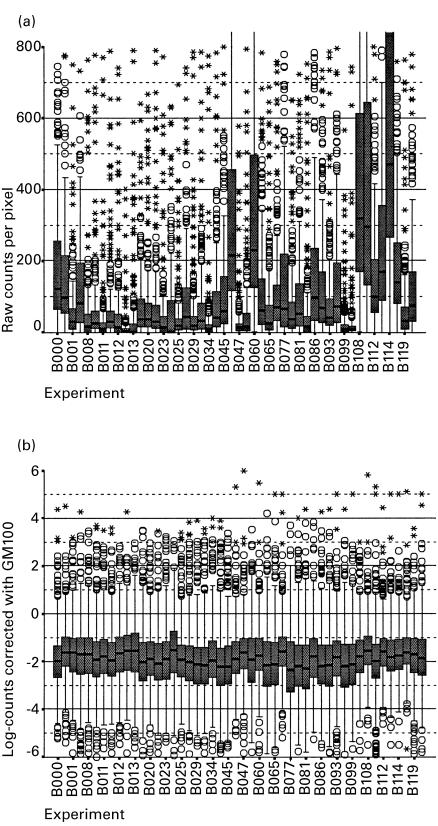
Data were analysed using SPSS for Windows 7.5.1 (SPSS Inc., Chicago, IL) statistical package. Multiple Mann–Whitney U-and Kruskal–Wallis tests were used to screen for differential gene expression after standardization with the GM100 method as appropriate. Expression ratios (ER) were calculated by dividing the geometric mean of the expression of a particular gene in AN or AA by the geometric mean of the expression in C. In a screening approach to identify group differences between the phenotypes, a graphical approach was taken in addition to statistical significance testing. Figure 2 shows a dot plot comparing log-transformed and standardized gene expression in healthy controls and atopic asthmatics. The significance level was taken at a conventional 0·05.
Fig. 2.
Effect of the standardization procedure against the 100 most expressed genes (GM100) on the hybridization results of 48 different experiments. The figure shows box plots of the distribution of the results in each experiment before (a) and after (b) this procedure, which enables direct comparison of results. Each experiment is represented with one box. The shaded boxes cover 25–75% of data with the median value marked as a line. The hinges indicate 10 and 90%, circles and asterixes represent outliers and extreme values, respectively. Over the range of 609 different gene products the assumption has been made that peripheral blood mononuclear cells (PBMC) express similar amounts of mRNAs with comparable overall mean hybridization intensities. It can therefore be expected that the distribution of the signals resulting from the different experiments must be similar. Ideally, a standardization method would be able to produce a unique distribution for all experiments. The figure shows the efficacy of the GM100 method for this standardization process, e.g. experiment B000, B060, B108, B114 had a tendency to higher values before the application of GM100, which is corrected to a large extent after standardization.
RESULTS
Standardization of hybridization results
Raw data obtained from individual hybridization experiments are expressed in counts per pixel (Fig. 1). However, because of the variation in efficiency of mRNA purification, hybridization, reverse transcription and 32P-dATP activity, different experiments produce a systematic difference in signal intensity (Fig. 2a). It is therefore necessary to standardize the results so that gene expression can be compared between experiments. Standard methods of mRNA quantification, e.g. RT-PCR, traditionally express the results of individual genes relative to that of a housekeeping gene, e.g. G3PDH or β-actin. This approach was tested with our gene array approach by comparing the expression of all individual genes with each individual housekeeping gene. Expressing the results relative to any single housekeeping reference gene resulted in very large inter-experimental variation with all reference genes showing an inter-individual variation of at least two-to-four-fold (data available on request).
This approach was extended to compare the expression of individual genes with multiple housekeeping genes (GM9) and with the hundred most expressed genes (GM100). Applying the GM9 and especially the GM100 method produced much improved standardization. Figure 2b shows the efficacy of the GM100 method for this standardization process, e.g. experiment B000, B060, B108, B114 had a tendency to higher values before the application of GM100, which is corrected, to a large extent after standardization. The GM100 method eliminated the non-specific variation seen with single reference genes resulting in reproducible and comparable measures of gene expression. Such transformed data were appropriate for non-parametric group comparisons, cluster analysis and machine-based learning procedures.
The GM100 method proved to be easily applicable, suitable for automated data analyses, robust, reproducible and superior to the other methods tested. Therefore, in the present study we standardized the hybridization results after correcting for background by expressing the results relative to the geometric mean of the hundred most expressed genes.
Apoptosis-related gene expression in atopy and asthma compared with normal
We were able to identify significantly altered expression of several of 127 apoptosis-related genes in atopy and asthma compared with the healthy subjects (Table 1). The alteration in gene expression followed one of three patterns: genes whose expression was altered in both AN and AA (n = 6), genes with altered expression in AN but not AA (n = 11) and genes with altered expression in AA only (n = 6). These genes fell into different pathways, which are presented below.
Pro-survival cytokine and growth factor genes
There were 71 gene products related to pro-survival cytokines, growth factors and their signalling, of which 12 were differentially regulated in AN and/or AA compared with C.
Two growth factor genes, IL6 and VEGF, were significantly up-regulated in both AN and AA, and heparin-binding epidermal growth factor-like growth factor was significantly down-regulated in AN and AA. Expression of the gene for teratocarcinoma-derived growth factor was increased in AN and that for FGF7 increased in AA.
The stimulating type I receptor for IL1 was up-regulated in both AN and AA. Several genes for growth factor receptors and associated signalling molecules showed increased expression in AN; these were EGFR and ERBBE, MADH1 and the insulin receptor α-chain. By contrast, the CSF3R gene was down-regulated in AN.
The proto-oncogene MYC was up-regulated in AA and AN. Conversely the c-myc transcription factor NME2 was down-regulated in AN. Subjects with severe asthma (Aas score >3) had significantly higher expression of MYC and MADH1 compared with subjects with a milder form of asthma (Aas score ≤3).
TNF and death receptor pathways
A number of genes in the TNF signalling pathways were altered in AN and/or AA. The TNF gene itself was up-regulated in AN along with the TNF receptor-associated factor, TRAF3. The gene for the TNF receptor-associated protein TRADD was down-regulated in both AA and AN. The family of TNF-related genes showed the most altered gene expression in AA subjects alone. These individuals showed increased TNF receptor 2 gene expression but decreased expression of the apoptosis inhibitor IAP (IAP1; TNFR2-TRAF signalling complex protein) and defender against cell death 1 (DAD1). The TNF-inducible transcription factor NFKB1 was up-regulated in AN only.
FAS and both FAS antigen ligands (TRAIL and APT1LG) had non-measurable expression levels in all groups, but FAST kinase, a receptor kinase activated upon FAS ligation, was over-expressed in AN and AA.
Cascade of caspases
The group of eight caspase genes that were measured did not show differential expression in AA or AN compared with controls.
Bcl-2 family
We measured nine members of the Bcl-2 family. Most pro-survival Bcl-2 family members were not expressed, although one, BCL2A1, showed expression that was down-regulated in AA. One pro-apoptotic member of the Bcl-2 family, BAX, was significantly up-regulated in AN and showed a similar trend in AA that was just short of statistical significance (P = 0·062).
P53 pathway
There was no evidence of increased responses either to DNA damage or to oxidative stress in atopy or asthma. The ataxia telangiectasia gene ATM, induced by DNA damage, showed markedly reduced expression in AA (ER = 0·26) and a significant association of reduced expression with increased asthma severity. The gene for the p53-neutralizing protein MDM2 was up-regulated in AN. The glutathione reductase and NADH-cytochrome P450 systems were not differentially expressed in AN and AA.
DISCUSSION
Atopy and asthma arise through a combination of genetic and environmental factors. These factors may act systemically in inducing the IgE response that is characteristic of atopy or, in the case of asthma, locally in influencing events within the airways. Recently, a role for reduced apoptosis has been postulated as contributing towards the accumulation of inflammatory cells in the lungs of asthmatics [4,7–20]. In studies on bronchial biopsy material, Vignola et al. [7] showed reduced levels of p53, together with increased amounts of granulocyte-macrophage colony-stimulating factor (GM-CSF) and Bcl-2, consistent with the reduced number of apoptotic events seen in the biopsies. From the studies of biopsy material it is not possible to determine whether local factors or systemic factors are responsible for the altered gene expression. In order to pool cDNA array data from different individuals a suitable standardization procedure needs to be applied to correct for experimental variation of signal intensities. As shown in this study, the GM100 method eliminated the non-specific variation seen with single reference genes, resulting in reproducible and comparable measures of gene expression.
We have demonstrated that gene array techniques can be used to detect alterations in the expression of several genes associated with cell survival and/or apoptosis in PBMC of asthmatic and non-asthmatic atopic individuals compared with controls. These alterations in gene expression could be due to genetic (i.e. hereditary) or environmental factors. The expression of many genes was altered in AN only and was normal in AA. For some of these genes the difference in AA and AN may be due to systemic effects of inhaled corticosteroids taken by the AA, but not the AN group. For example, steroids are known to inhibit the expression of NFKB1 [21], one of the genes that was highly up-regulated in AN (ER = 3·66), but showed normal expression in AA (ER = 0·95). Corticosteroids are also known to suppress TNF, which was only found to be up-regulated in our untreated AN and not in AA [22–24].
The pattern of expression of growth factors and pro-survival related genes was generally balanced towards higher expression of pro-survival genes in AN and, to a lesser extent, in AA. A number of different growth factor pathways showed up-regulated gene expression. Along these lines, IL6 and the stimulatory IL1 receptor, IL1R1, were up-regulated in AN and AA. Previous studies have found up-regulation of IL1 and IL6 in blood monocytes and alveolar macrophages during or following acute asthmatic attacks [25]. The present results demonstrate that PBMC showed altered expression of these gene pathways in atopic subjects without clinical disease, and in individuals with stable asthma state of the disease, as well as in atopic subjects without clinical disease.
VEGF, which can be induced by TNFA, IL1 and members of the fibroblast growth factor family [26], was up-regulated in both AN and AA, although the degree of up-regulation was less in AA (ER = 2·02 versus 3·56 in AN). VEGF can be inhibited by high doses of dexamethasone [27] and the reduced expression in AA compared with AN may represent a steroid effect. VEGF, expressed by a variety of cells including endothelial cells, is crucial for fibroblast chemotaxis and angioneogenesis. VEGF has a potential to be involved in processes involved in remodelling of lung tissue, as can be seen in patients with long-standing chronic asthma and chronic airflow limitation. In addition, FGF7, which is involved in epithelial repair processes [28], was up-regulated in the AA group.
Although EGF expression could not be detected in our samples, two EGF receptor genes, EGFR and ERBBE, were up-regulated in AN, but not AA. This may have functional significance if the cells expressing EGF binding receptors migrate to a site where EGF is present, i.e. the lungs. Another protein capable of binding to the EGF receptors, DTR (also called HBEGF) was down-regulated in both AN and AA. Also consistent with increased survival signals was the observation that MYC, a potent inducer of proliferation with pro-survival properties, was up-regulated in both AA and AN.
Somewhat surprisingly, one of the genes that showed the highest up-regulation in AN was the α-chain of the insulin receptor (ER = 4·67), although expression was normal in AA, again potentially due to the systemic effects of inhaled corticosteroids. Steroids have been shown to affect insulin receptor expression in normal rats [29] and chickens [30,31], although the effects were variable. The effects of steroids on abnormally increased INSR have not been studied and therefore it is not possible to conclude whether the normalization of INSR expression in AA subjects represents a systemic corticosteroid effect.
Although the alterations of the expression of growth factor-related genes showed a concerted pro-survival pattern, the situation regarding apoptosis-related genes was less clear-cut. If PBMC reflected the decreased apoptosis reported in lung tissue of asthmatics [4,7–19], it would have been expected that pathways involved in apoptosis would show similar alterations in gene expression reflective of decreased apoptosis. However, gene expression in the different apoptotic pathways examined showed variably altered patterns of expression, with some suggestive of increased apoptosis and others of decreased apoptosis.
Tumour necrosis factor can either induce cell activation in a NFKB-dependent manner or apoptosis signalling through a cascade of TNF receptor type I, TRADD and the caspases. TNF receptor type II is not involved in the latter. The increased expression of TNFR2 and reduced expression of TRADD in AA is compatible with increased TNF signalling in favour of cell activation rather than apoptosis. The significance of the reduced expression of the inhibitor of apoptosis IAP1 is unclear, since IAP1/2 inhibit apoptosis mediated through TNFR1, which was not expressed in any of the groups.
In contrast to the pro-survival pattern of the TNF pathway in AN and AA, both the FAS and Bcl-2 family of genes showed alterations compatible with increased apoptosis. FAST kinase, a receptor kinase activated upon FAS ligation, was over-expressed in AN and AA. This finding would be compatible with increased signalling through FAS and therefore increased apoptosis; although FAS and both FAS antigen ligands (TRAIL and APT1LG) had non-measurable expression levels in all groups. BAX, a pro-apoptotic gene of the Bcl-2 family, was increased in AN, and BCL2A1, a pro-survival gene, was decreased in AA. Both these changes indicate a change towards facilitated apoptosis.
The increased expression of p53-neutralizing protein MDM2 and the four-fold reduced ataxia telangiectasia gene ATM, which is induced by DNA damage upon p53 activation, are indicative of an overall reduced net p53 activity at a transcriptional level in AN and AA. These findings are compatible with reduced induction of apoptosis via the p53 pathway.
The finding that the expression of apoptosis effector proteases, the caspases, was not altered in AA or AN is perhaps not surprising, given that they are produced in excess well in advance of an apoptotic event and when activated induce apoptosis within minutes without the need for any transcriptional activity.
In conclusion, using cDNA technology it was possible to demonstrate that PBMC of asthmatic and non-asthmatic atopic individuals showed differences in expression of a number of genes associated with cell survival and/or apoptosis. The pattern of altered gene expression did not show a definite pattern that was suggestive of pro-survival or pro-apoptosis. However the number of genes whose altered expression would be predicted to favour increased survival exceeded that of genes likely to reduce survival. This suggests that the altered gene expression seen in bronchial biopsies [7] may be determined, at least in part, by systemic factors so that cells are programmed for increased survival before entry into the inflammatory site where local factors could enhance their survival further.
Acknowledgments
We thank Professor Tony Hegarty and Dr Jacky Ohanian for the permission to use their phosphor imager. Many thanks as well to Frazer Smillie for technical advice. I.C.B. was supported by the Uarda Frutiger Foundation, the Swiss National Foundation and the Novartis Jubilee Foundation of Switzerland.
REFERENCES
- 1.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;23:1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 2.Vignola AM, Campbell AM, Chanez P. HLA-DR and ICAM-1 expression on bronchial epithelial cells in asthma and chronic bronchitis. Am Rev Respir Dis. 1993;148:689–94. doi: 10.1164/ajrccm/148.3.689. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Asthma: past, present and future. Eur Respir J. 1993;6:1507–20. [PubMed] [Google Scholar]
- 4.Simon HU, Blaser K. Inhibition of programmed eosinophil death: a key pathogenic event for eosinophilia? Immunol Today. 1995;16:53–55. doi: 10.1016/0167-5699(95)80086-7. [DOI] [PubMed] [Google Scholar]
- 5.Haslett C, Savill JS, Whyte MK, et al. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc Lond B Biol Sci. 1994;345:327–33. doi: 10.1098/rstb.1994.0113. [DOI] [PubMed] [Google Scholar]
- 6.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Vignola AM, Chanez P, Chiappara G, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–73. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Dixit VM. Death receptors: signalling and modulation. Science. 1999;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 9.Evan G, Littlewood TA. Matter of life and cell death. Science. 1999;281:1317–22. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 10.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1999;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 11.Thornberry NA, Lazebnik YA. Caspases: enemies within. Science. 1999;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 12.Chalifour LE, Fahmy R, Holder EL, et al. Method for analysis of gene expression patterns. Anal Biochem. 1994;216:299–304. doi: 10.1006/abio.1994.1045. [DOI] [PubMed] [Google Scholar]
- 13.Liew JC, Hwang DM, Fung YW, et al. A catalogue of genes in the cardiovascular system as identified by expressed sequence tags. Proc Natl Acad Sci USA. 1994;91:10645–9. doi: 10.1073/pnas.91.22.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schena M, Shalon D, Heller R, et al. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614–9. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggan DJ, Bittner M, Chen Y, et al. Expression profiling using cDNA microarrays. Nature Genet. 1999;21(Suppl. 1):10–14. doi: 10.1038/4434. [DOI] [PubMed] [Google Scholar]
- 16.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–70. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 17.DeRisi JL, Penland L, Brown PO, et al. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nature Genet. 1996;14:457–60. doi: 10.1038/ng1296-457. [DOI] [PubMed] [Google Scholar]
- 18.DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–6. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 19.Aas K. Heterogeneity of bronchial asthma. Allergy. 1981;36:3–14. doi: 10.1111/j.1398-9995.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 20.Druilhe A, Wallaert B, Tsicopoulos A, et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–57. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- 21.Unlap T, Jope RS. Inhibition of NFkB DNA binding activity by glucocorticoids in rat brain. Neurosci Lett. 1995;198:41–44. doi: 10.1016/0304-3940(95)11963-w. [DOI] [PubMed] [Google Scholar]
- 22.le Contel C, Parant F, Parant M. Indirect and selective down-regulation of serum tumor necrosis factor-alpha release by interleukin-1 beta. Immunobiology. 1992;186:199–213. doi: 10.1016/s0171-2985(11)80250-0. [DOI] [PubMed] [Google Scholar]
- 23.Wandinger KP, Wessel K, Trillenberg P, et al. Effect of high-dose methylprednisolone administration on immune functions in multiple sclerosis patients. Acta Neurol Scand. 1998;97:359–65. doi: 10.1111/j.1600-0404.1998.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 24.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 25.Tillie-Leblond I, Pugin J, Marquette CH, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–94. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 26.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–8. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 27.Tsai JC, Hsiao YY, Teng TJ, et al. Regulation of vascular endothelial growth factor secretion in human meningioma cells. J Formos Med Assoc. 1999;98:111–7. [PubMed] [Google Scholar]
- 28.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–65. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 29.Macho L, Fickova M. In vivo role of corticosterone in regulation of insulin receptors in rat adipocytes during hypokinesia. Endocr Regul. 1992;26:183–7. [PubMed] [Google Scholar]
- 30.Taouis M, Derouet M, Chevalier B, Simon A. Corticosterone effect on insulin receptor number and kinase activity in chicken muscle and liver. J Gen Comp Endocrinol. 1993;89:167–75. doi: 10.1006/gcen.1993.1020. [DOI] [PubMed] [Google Scholar]
- 31.Bisbis S, Taouis M, Derouet M, et al. Corticosterone-induced insulin resistance is not associated with alterations of insulin receptor number and kinase activity in chicken kidney. J Gen Comp Endocrinol. 1994;96:370–7. doi: 10.1006/gcen.1994.1192. [DOI] [PubMed] [Google Scholar]