Abstract
To clarify the role of IP-10 in autoimmune liver diseases, we studied the serum levels of IP-10 in 14 patients with autoimmune hepatitis (AIH), 23 patients with primary biliary cirrhosis (PBC), and 65 patients with chronic viral hepatitis (20 type B and 45 type C). The hepatic expression of IP-10 mRNA and the correlation between the serum levels of IP-10 and clinical parameters were also evaluated. In addition to 20 healthy controls, 16 rheumatoid arthritis (RA) patients were included as an extrahepatic inflammatory disease. The serum level of IP-10 was significantly (P < 0·02) higher in patients with AIH, PBC, and chronic hepatitis B and C than in healthy controls, and it was significantly correlated (P < 0·05) with the serum levels of aspartate aminotransferase and alanine aminotransferase in patients with AIH, PBC, and chronic hepatitis B and C. The serum level of IP-10 was not elevated in RA patients. After successful treatment of AIH and chronic hepatitis C, the serum level of IP-10 decreased to the same level as in healthy volunteers. As we previously showed in cases with chronic hepatitis B or C, in situ hybridization in both AIH and PBC cases demonstrated the expression of IP-10 mRNA in hepatocytes around focal or lobular necrosis surrounded by infiltrating mononuclear cells, whereas IP-10 mRNA was not expressed in areas around the damaged bile ducts in PBC cases. The present results suggest that IP-10 is specifically produced by hepatocytes in inflammatory areas irrespective of the aetiology of hepatitis, and that IP-10 may help to recruit T cells to the hepatic lesions in autoimmune liver diseases as well as in chronic viral hepatitis.
Keywords: chemokine, interferon-inducible protein-10, primary biliary cirrhosis, autoimmune hepatitis
INTRODUCTION
Autoimmune liver diseases are characterized by marked infiltration of mononuclear cells. These mononuclear cells play crucial roles in the pathogenesis of autoimmune liver diseases and chronic viral hepatitis, and T cells are dominant among them [1–3]. Chemokine–chemokine receptor interactions [4,5] and cell adhesion molecules [6] are the two key elements in the T cell recruitment process at the site of inflammation. In a previous report, the importance of cell adhesion molecules in the pathogenesis of hepatic inflammation was demonstrated [7]; however, the specific chemokines involved in the recruitment of T cells to the inflamed liver are not yet well understood.
Chemokines constitute families of low molecular weight proteins that induce chemotaxis of specific subsets of leucocytes. Chemokines are classified into four or more groups based on the relative positions of the first two of the four conserved cysteine residues, and two of these groups, the CC chemokines and CXC chemokines, have been widely investigated [4].
IP-10 was identified by Luster et al. as a 10-kD protein [8] and classified as a member of the CXC chemokine subfamily. While CXC chemokines with an N-terminal ELR (Glu-Leu-Arg) motif, such as IL-8, are specific chemoattractants for neutrophils, those lacking the N-terminal ELR motif, such as IP-10 and monokine induced by interferon-gamma (IFN-γ) (Mig), do not act as chemoattractants for neutrophils [5,9,10], but do act as chemoattractants for activated T cells, similarly to the CC chemokines, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, monocyte chemoattractant protein-1 (MCP-1), and regulated upon activation, normal T cell expressed and presumably secreted (RANTES) [5,11].
We have previously reported that the mRNA of IP-10 was expressed in hepatocytes in chronic hepatitis B (CHB) and C (CHC) patients, as demonstrated by in situ hybridization, and that the serum level of IP-10 was significantly increased in CHC patients [12]. In the present study we measured the serum level of IP-10 in autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC) patients and examined the cell types that expressed IP-10 mRNA in the livers of these patients. In addition, to characterize further the significance of IP-10 in hepatic inflammation, we examined the correlation between the serum level of IP-10 and the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γ-GTP), γ-globulin, total protein (TP), and total bilirubin (T-Bil) in patients with chronic liver diseases. We also examined the serum level of IP-10 after successful treatment of AIH and CHC in order to clarify the correlation between the serum level of IP-10 and hepatic inflammation.
PATIENTS and METHODS
Patients and sera
Thirty-seven patients with autoimmune liver diseases (23 PBC and 14 AIH) and 65 patients with chronic viral hepatitis (20 CHB and 45 CHC) treated at the University Hospital of Kyoto Prefectural University of Medicine between April 1992 and March 1998 were enrolled in this study. Twenty healthy volunteers served as controls. To obtain additional data on an extrahepatic inflammatory disease, 16 patients with rheumatoid arthritis (RA) with normal serum levels of AST and ALT were also included in this study.
The diagnoses of chronic liver diseases were based on the appropriate international criteria [13,14]. The patients with RA were diagnosed according to the criteria of the American Rheumatism Association [15]. The AIH and PBC patients were evaluated for liver histology, routine liver tests, and serum levels of IP-10 and serum autoantibodies. The CHB patients were positive for hepatitis B surface antigen (HBsAg) and negative for second- or third-generation anti-hepatitis C virus (HCV) antibody (Ortho-HCV; Ortho Diagnostic Systems, Raritan, NJ). The CHC patients were positive for anti-HCV antibody, positive for serum HCV-RNA detected by reverse transcriptase-nested polymerase chain reaction, and negative for HBsAg. The CHB patients were examined by routine liver tests and evaluated for liver histology and serum levels of IP-10 and HBV-DNA. The CHC patients were evaluated for liver histology, HCV genotype, routine liver tests, and serum levels of IP-10 and HCV-core protein.
HCV genotypes were determined by an ELISA (Kokusai Shiyaku, Kobe, Japan) as previously described [16,17] and the serum level of HCV core protein was quantified using a fluorescent enzyme immunoassay (FEIA; Kokusai Shiyaku, Kobe, Japan) [18]. The detection limit of the HCV core protein assay was 8 pg/ml. Healthy volunteers were verified to have normal liver tests.
All of the 45 CHC patients received IFN-α therapy. The treatment began with intramuscular injection of 6 million units of natural IFN-α (Sumitomo Pharm. Co., Osaka, Japan) or recombinant IFN-α2b (Schering-Plough Co., Osaka, Japan) daily for 2 weeks, followed by three injections weekly for the next 22 weeks. Complete responders (CR) were defined as patients who showed normal serum AST and ALT levels and were negative for serum HCV-RNA (Amplicore test; Nippon Rosche, Tokyo, Japan) for >6 months after IFN therapy. Non-responders (NR) were defined as patients who showed elevated serum AST and ALT levels and failed to clear HCV-RNA in the serum after IFN therapy. Twenty-four patients were CR and 21 were NR. The sera were collected from 24 CHC patients with complete response after IFN therapy, and from 14 AIH patients after successful prednisolone therapy to measure the serum levels of IP-10. Percutaneous liver biopsy was performed in all patients using a Surecut needle (Tochigi Seiko Inc., Tochigi, Japan). Liver specimens were fixed in formalin, stained routinely with haematoxylin and eosin, and with Masson's trichrome for light microscopy. Histological diagnoses were determined according to the criteria of Desmet et al. [19]. Histological activity of the disease was also evaluated by Knodell's histological activity index (HAI) score [20]. The inflammatory score of chronic hepatitis was the sum of the scores for periportal necrosis, portal inflammation, intralobular degeneration and focal necrosis. Parts of the liver specimens of AIH and PBC patients were fixed in 4% buffered paraformaldehyde overnight for use in in situ hybridization. Informed consent was obtained from all patients and from the healthy volunteers, and this study was approved by the Human Research Committee of our Hospital as conforming to the ethical guidelines of the 1975 Declaration of Helsinki.
ELISAs for chemokines
The concentration of serum IP-10 was measured using an ELISA as previously described [12]. In brief, 96-well ELISA plates (Corning Costar Corp., Corning, NY) were coated overnight at 4°C with 50 μl/well of anti-human IP-10 MoAb α hIPb (20 μg/ml in PBS). The antibody-containing solution was removed and unbound sites were blocked with 100 μl of blocking agent (Block-Ace™; Dainippon-Seiyaku, Osaka, Japan) for 2 h at room temperature. After plates were washed with PBS containing 0·05% Tween-20 (Tween–PBS), 50 μl of a standard solution of IP-10 or of a serum sample were added and incubated for 1 h. Next, the plates were washed three times with Tween–PBS and 50 μl of biotinylated anti-human IP-10 MoAb α hIPd (0·3 μg/ml in PBS containing 1% bovine serum albumin (BSA)) were added as the second antibody to detect human IP-10 bound to the first antibody, a hIPb. The plates were incubated for 1 h and washed three times with Tween–PBS, and then 50 μl of streptavidin-βd-galactosidase diluted to 1:1000 was added to each well. After another incubation for 1 h, the plates were washed three times, and then 50 μl of 0·01% 4-methyl-umbelliferyl-βd-galactoside were added, and the plates were shaken for 10 min.
To stop the reaction, 100 μl of 2 m sodium carbonate were added, and the fluorescence intensity was determined at 460 nm using a Fluoroscan II microplate fluorometer (Labosystems, Helsinki, Finland). The samples influenced by the same IgG subclass were excluded from the data. The detection limit of IP-10 was 10 pg/ml in this assay. Intra- and interassay variations were <10%.
The concentrations of MIP-1β and MCP-1 were measured using a commercially available ELISA kit (Quantikine ELISA; R&D Systems, Minneapolis, MN), and that of MIP-1α was measured using a commercially available ELISA kit (Amersham Int. plc, Aylesbury, UK) according to the manufacturer's instructions.
In situ hybridization
The preparation of human IP-10 cDNA [8] by reverse transcriptase-polymerase chain reaction (RT-PCR) and the procedures for in situ hybridization were essentially the same as reported previously [12]. In brief, radiolabelled 35S-cRNA antisense and sense probes for IP-10 were prepared by in vitro transcription from T3 and T7 RNA polymerase promoters in a plasmid that contained human IP-10 cDNA. The full length of the human IP-10 coding sequence was 1172 bp [8] and we used 571 bp nucleotides at positions between 144 and 714 for the IP-10 probes (Fig. 1).
Fig. 1.
The cDNA sequence of human IP-10. The underlined nucleotides at positions between 144 and 714 were used for the antisense and sense probe (571 bp).
In vitro transcription and labelling of the RNA probes were performed as previously described [21]. Briefly, 62·5 μ Ci of 35S-CTP was added to a mixture containing 40 mm Tris–HCl pH 7·5, 6 mm MgCl2, 2 mm spermidine, 10 mm NaCl, 10 mm DTT, 1 U/μl RNase inhibitor, ATP, UTP, and GTP, each at 0·5 mm, 10 μm unlabelled CTP, linearized template equivalent to 1 μg of whole plasmid, and 40 U of either T3 or T7 RNA polymerase, in a total volume of 20 μl. The mixture was incubated for 3–4 h at 37°C, and then the DNA template was removed by incubation with 1·5 U of RNase-free DNase for 30 min at 37°C. Proteins and free ribonucleotides were removed by phenol-chloroform-isoamyl alcohol extraction followed by ethanol precipitation, and all probes were brought to roughly 350 bases in length by controlled alkaline hydrolysis.
Liver tissues were fixed in 4% buffered paraformaldehyde overnight, and then embedded in paraffin, sectioned, and placed on slides coated with Vectabond (Vecta Labs, Burlingame, CA). After deparaffinization in xylene and hydration through a graded series of ethanol, sections were digested with proteinase K (1 μg/ml) for 30 min at 37°C, washed in distilled water, and acetylated with a freshly prepared solution of acetic anhydride, pH 8·0, for 10 min. After washing in 2 ×SSC, sections were dehydrated through the graded series of ethanol. A hybridization mixture (600 mm NaCl, 10 mm Tris–HCl pH 8·0, 1 mm EDTA, 50% formamide, 1 ×Denhardt's solution, 10% dextran sulphate, 100 μm DTT, 0·2% SDS, and 0·5 mg/ml yeast tRNA) containing the radiolabelled RNA probe was applied to each section and covered with a silanized coverslip.
Hybridization was carried out for 16 h at 45°C in light mineral oil. Excess probe was removed by washing in chloroform followed by 4 ×SSC containing 1 mm DTT for 1 h. After dehydration, the slides were washed at high stringency at 60°C for 15 min in a buffer containing 300 mm NaCl, 10 mm Tris–HCl pH 8·0, 1 mm EDTA, 50% formamide, and 100 μm DTT. Next, after washing the slides briefly in 2 ×SSC, the sections on the slides were digested for 30 min at 37°C with 25 μg/ml RNase A in buffer containing 500 mm NaCl, 10 mm Tris–HCl pH 8·0, and 1 mm EDTA. The sections were then washed for 30 min at 37°C in the same buffer without the enzyme, and were further rinsed in 4 l of 2 ×SSC, washed at high stringency in 0·1 ×SSC at 60°C, dehydrated in a graded series of ethanol containing 300 mm ammonium acetate, and air dried. The slides were dipped into NTB-2 autoradiography emulsion (Eastman Kodak, Rochester, NY) diluted to 1:1 with 600 mm ammonium acetate at 45°C. The slides were dried, and then stored in light-proof boxes containing desiccant and exposed at 4°C for 5 days. The exposed slides were developed in Kodak D19 developer (Eastman Kodak) for 2·5 min, rinsed in 2% acetic acid for 30 s, and fixed in Fixer (Eastman Kodak) for 15 min. The slides were washed in tap water, and then counterstained with haematoxylin.
Statistical analysis
Data are expressed as mean ±s.d. Statistical analyses were performed using Mann–Whitney U-test and Spearman's rank correlation tests. P <0·05 was considered significant.
RESULTS
The serum level of IP-10 in AIH and PBC in comparison with CHB, CHC, RA, and healthy controls
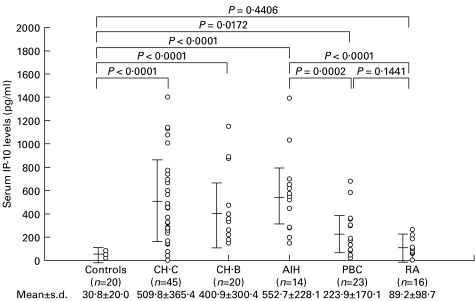
Table 1 summarizes the clinical characteristics of the 102 patients with chronic liver diseases of different aetiologies (AIH, PBC, CHB, and CHC), 16 patients with RA, and 20 healthy volunteers examined in this study. The serum levels of IP-10 in all groups of chronic liver diseases were significantly (P < 0·02) higher than the level in healthy controls (Fig. 2). The serum levels of IP-10 in AIH, CHC, and CHB patients were comparable and significantly (P < 0·05) higher than the level in PBC patients. The serum level of IP-10 in RA patients was not significantly different from that in healthy controls.
Table 1.
Clinical characteristics of the patients
| Healthy control n = 20 | CHC n = 45 | CHB n = 20 | AIH n = 14 | PBC n = 23 | RA n = 16 | |
|---|---|---|---|---|---|---|
| Gender (M:F) | 10:10 | 22:23 | 13:7 | 1:13 | 3:20 | 1:15 |
| Age (years) | 46·4 ± 14·4 | 52·3 ± 10·3 | 41·9 ± 11·5 | 54·6 ± 8·8 | 57·8 ± 11·5 | 63·8 ± 7·7 |
| AST (U/l) | 16·7 ± 3·2 | 69·0 ± 46·8 | 117·2 ± 109·0 | 202·0 ± 208·7 | 59·4 ± 36·3 | 18·0 ± 5·5 |
| ALT (U/l) | 9·7 ± 4·1 | 100·9 ± 78·1 | 210·1 ± 202·7 | 237·7 ± 184·8 | 54·7 ± 42·3 | 13·6 ± 6·7 |
| γ-GTP (U/l) | 10·0 ± 3·97 | 42·0 ± 36·4 | 57·3 ± 47·8 | 75·8 ± 33·3 | 271·5 ± 251·3 | 18·9 ± 6·3 |
| T-Bil (mg/ml) | 0·49 ± 0·14 | 0·83 ± 0·29 | 0·94 ± 0·56 | 1·10 ± 0·61 | 1·74 ± 2·44 | 0·43 ± 0·1 |
| γ-globulin (g/dl) | 1·15 ± 0·17 | 1·57 ± 0·40 | 1·52 ± 0·48 | 2·55 ± 0·98 | 2·04 ± 0·81 | 2·25 ± 0·78 |
| HAI score | ND | 8·02 ± 3·63 | 8·75 ± 3·48 | 13·1 ± 4·4 | ND | ND |
CHC, Chronic hepatitis C; CHB, chronic hepatitis B; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; RA, rheumatoid arthritis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ glutamyl transpeptidase; T-Bil, total bilirubin; HAI score, histological activity index score as determined by Knodell's scoring system; ND, not determined. Data are expressed as means ± s.d.
Fig. 2.
The serum levels of IP-10 in healthy controls, Chronic hepatitis C (CHC). Chronic hepatitis B (CHB), autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and rheumatoid arthritis (RA) patients. The bars show mean ±s.d. in each group.
Correlation between the serum level of IP-10 and clinical parameters in chronic liver diseases
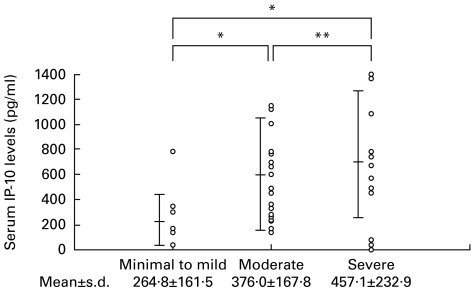
The serum levels of IP-10 were compared among the 45 CHC patients on the basis of their histological diagnoses (Fig. 3). The serum level of IP-10 was significantly lower in patients with minimal to mild hepatitis (264·8 ± 161·5 pg/ml) than in patients with moderate hepatitis (376·0 ± 167·8 pg/ml; P = 0·0321) and severe hepatitis (457·1 ± 232·9 pg/ml; P = 0·0010). The serum level of MIP-1α was below the limit of detection and the serum levels of MIP-1β and MCP-1 were not significantly different depending on the histological diagnoses of these patients (data not shown).
Fig. 3.
The serum levels of IP-10 were compared in 45 chronic hepatitis C (CHC) patients divided into three groups classified by the method of Desmet et al. [19]. Minimal to mild = minimal hepatitis or mild hepatitis; Moderate = moderate hepatitis; Severe = severe hepatitis. The bars show mean ±s.d. in each group. *P < 0·05; **not significant.
Table 2 shows the correlations between the serum level of IP-10 and the serum levels of AST, ALT, γ-GTP, γ-globulin, TP, and T-Bil. The serum level of IP-10 was significantly (P < 0·05) correlated with the serum levels of ALT and AST in PBC, AIH, CHC, and CHB patients. The serum level of IP-10 was also significantly correlated with the inflammatory scores in AIH (r = 0·808, P = 0·005, n = 14), CHC (r = 0·369, P = 0·0143, n = 45), and CHB (r = 0·613, P = 0·0075, n = 20). In PBC, the serum level of IP-10 was not significantly correlated with the staging of Scheuer [1] (data not shown).
Table 2.
Correlations between serum IP-10 and clinical parameters
| CHC n = 45 | CHB n = 20 | AIH n = 14 | PBC n = 23 | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| AST | 0·590 | 0·0001 | 0·704 | 0·0005 | 0·889 | 0·0014 | 0·604 | 0·0023 |
| ALT | 0·497 | 0·0009 | 0·565 | 0·0094 | 0·846 | 0·0040 | 0·456 | 0·0288 |
| γ-GTP | 0·447 | 0·0021 | 0·190 | 0·4214 NS | 0·232 | 0·5476 NS | 0·291 | 0·1781 NS |
| γ-globulin | 0·022 | 0·8840 NS | 0·457 | 0·0429 | 0·001 | 0·9990 NS | 0·109 | 0·6202 NS |
| TP | 0·014 | 0·9280 NS | 0·438 | 0·0533 NS | 0·086 | 0·8391 NS | 0·174 | 0·4270 NS |
| T-Bil | 0·197 | 0·1936 NS | 0·114 | 0·6315 NS | 0·530 | 0·1425 NS | 0·410 | 0·0522 NS |
CHC, Chronic hepatitis C; CHB, chronic hepatitis B; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ glutamyl transpeptidase; TP, total protein; T-Bil, total bilirubin. Data were analysed by Spearman's correlation test. NS, Not significant.
In CHC, the serum levels of IP-10 did not significantly differ between the different HCV genotypes (data not shown), and was not significantly correlated with the serum level of HCV core protein (r = 0·280, P = 0·0653, n = 45). In CHB, the serum levels of IP-10 were not significantly correlated with the level of HBV-DNA (r = 0·255, P = 0·2662, n = 20).
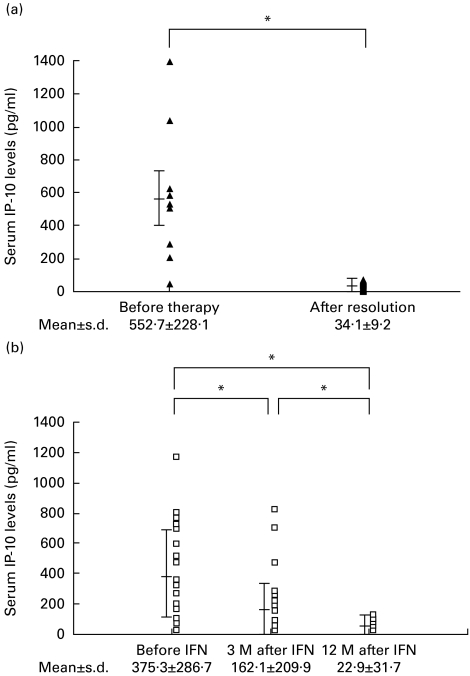
The serum level of IP-10 after successful treatment of AIH and CHC
In 14 AIH patients, the serum level of IP-10 was measured before and after successful prednisolone therapy (Fig. 4a). The serum level of IP-10 before steroid therapy (552·7 ± 228·1 pg/ml) was significantly (P < 0·0001) higher than that after resolution of the hepatitis (34·1 ± 9·2 pg/ml), when the serum levels of ALT and AST were within the normal ranges.
Fig. 4.
(a) Changes in the serum levels of IP-10 in autoimmune hepatitis (AIH) patients who received prednisolone therapy (n = 14). Before therapy, before prednisolone therapy; after resolution, after successful prednisolone therapy. (b) Changes in the serum levels of IP-10 in chronic hepatitis C (CHC) patients who showed complete response to the IFN therapy (n = 24). Before IFN, before IFN therapy; 3 M after IFN, 3 months after the completion of IFN therapy; 12 M after IFN, 12 months after the completion of IFN therapy. *P < 0·0001.
The serum level of IP-10 was measured in 24 CR patients with CHC prior to IFN therapy, and at 3 months and 12 months after the completion of the therapy (Fig. 4b). The serum level of IP-10 before IFN therapy (375·3 ± 286·7 pg/ml) was significantly (P = 0·0056 and P < 0·0001, respectively) higher than the levels at 3 months after IFN therapy (162·1 ± 209·9 pg/ml) and 12 months after the therapy (22·9 ± 31·7 pg/ml). The serum level of IP-10 at 12 months after therapy was not significantly different from the level in healthy volunteers.
The serum levels of the other T cell chemokines, MIP-1β and MCP-1, were not significantly related to the histological activity of the hepatitis, nor did they significantly change after the IFN therapy in these CR patients (data not shown). In NR patients with CHC, the serum level of IP-10 after IFN therapy was not significantly different from the level before the therapy (data not shown).
In situ hybridization of IP-10 mRNA in AIH and PBC
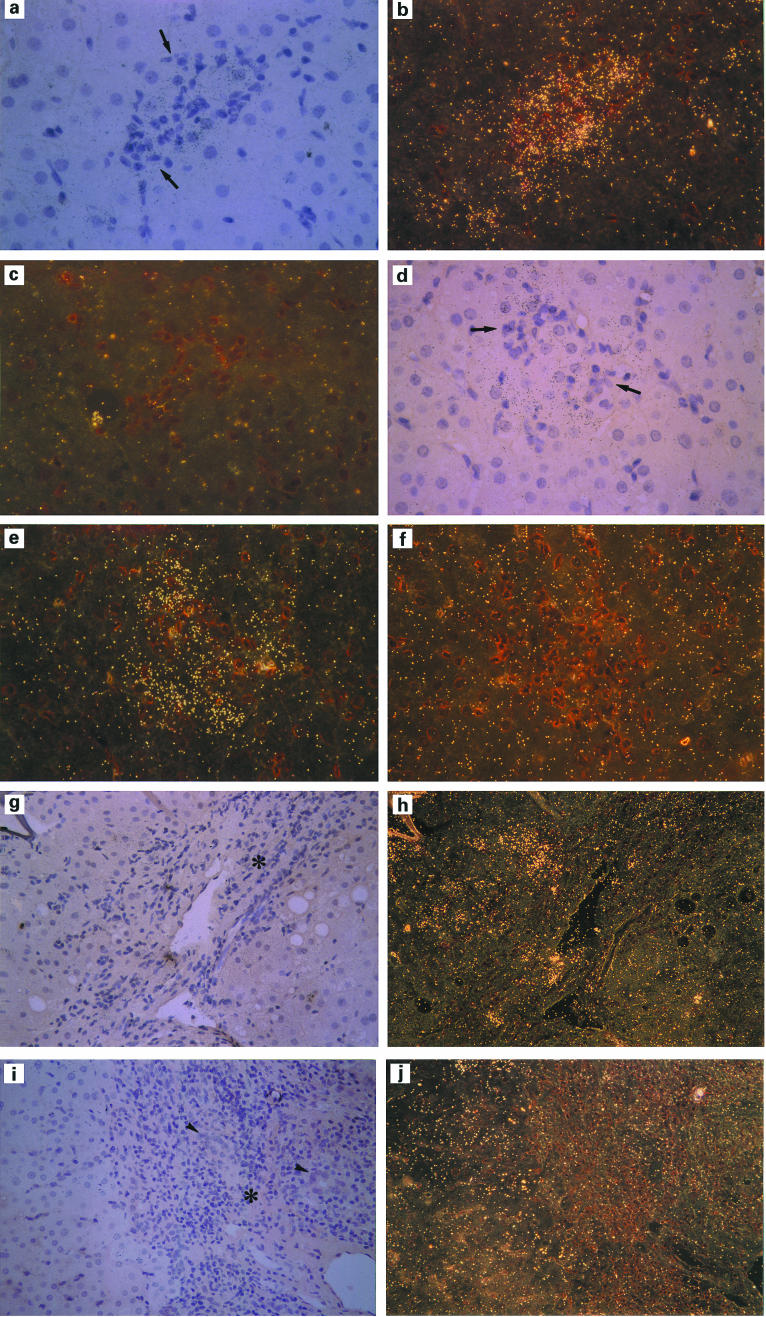
We investigated IP-10 mRNA expression in the liver tissues of patients with AIH and PBC using in situ hybridization (Fig. 5). Hybridization with the sense probe of IP-10 was poor in all sections, which we considered as control backgrounds (Fig. 5c,f). Hybridization of the antisense IP-10 probe demonstrated that the expression of IP-10 mRNA was localized in hepatocytes around intralobular focal necrosis or lobular necrosis (Fig. 5a,b) and poorly detected in portal areas (Fig. 5g,h) in the liver sections of AIH patients, which is similar to the results in chronic viral hepatitis B and C [12]. In PBC patients, the expression of IP-10 mRNA was also localized mainly in hepatocytes in areas of focal or lobular necrosis (Fig. 5d,e); however, mRNA expression of IP-10 was not detected in portal areas, including the damaged bile ducts (Fig. 5i,j).
Fig. 5.
Representative results of in situ hybridization analysis of IP-10 mRNA expression in autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC) patients. A liver section of AIH, around the intralobular necrosis, hybridized with antisense probe (a,b) and sense probe (c) for IP-10. (× 400, original mag.) A liver section of PBC, around the intralobular necrosis, hybridized with antisense probe (d,e) and sense probe (f) for IP-10 (× 400, original mag.). A liver section of AIH, portal and periportal area, hybridized with antisense probe (g,h) (× 200, original mag.). A liver section of PBC, portal and periportal area, hybridized with antisense probe (i,j) (× 200, original mag.). Light field microscopy is shown in (a,d,g,i); dark field microscopy is shown in (b,c,e,f,h,j). The arrows indicate intralobular infiltration of mononuclear cells, the arrowheads show damaged bile ducts, and the asterisks show portal areas.
DISCUSSION
We have previously reported that the mRNA of IP-10 was expressed in hepatocytes around the necrotic areas surrounded by infiltrating mononuclear cells in CHC and CHB patients using in situ hybridization, and that the serum level of IP-10 was elevated in CHC patients, and that it decreased in CR patients after IFN therapy [12]. To clarify whether or not the production of IP-10 is related to the hepatic inflammation in autoimmune liver diseases, we studied the serum levels of IP-10 in AIH and PBC patients, and assessed the cell type-specific expression of IP-10 mRNA in their liver tissues.
The serum level of IP-10 in AIH and PBC patients was significantly elevated to a level similar to that in CHC or CHB patients (Fig. 2), indicating that the mechanism up-regulating the serum IP-10 was not dependent on the aetiology of hepatitis. The increased serum level of IP-10 was not a common feature in inflammatory diseases of other organs, since the serum level of IP-10 in RA patients was similar to that in healthy controls (Fig. 2).
The serum level of IP-10 increased in proportion to the degree of histological activity (Fig. 2) and was significantly correlated with the serum levels of ALT and AST in chronic liver diseases (Table 2). In addition, the serum level of IP-10 was significantly (P < 0·001) decreased in CR patients with CHC after IFN therapy and in AIH patients after successful prednisolone therapy (Fig. 4). This specific correlation between the serum level of IP-10 and hepatic inflammation in chronic liver diseases stands to reason if IP-10 is induced in the liver by IFN-γ or tumour necrosis factor-alpha (TNF-α) secreted by liver-infiltrating mononuclear cells, as we hypothesize [12]. In fact, the administration of IFN-γ or TNF-α was shown to induce the expression of IP-10 mRNA in the liver [22,23].
We do not argue that IP-10 is involved exclusively in the pathogenesis of inflammatory liver diseases, since we know that it may also play an important role in inflammatory skin or bowel diseases [24,25]. However, we suppose that IP-10 is not likely to be induced in extrahepatic organs in patients with chronic liver diseases, since the serum concentrations of IFN-γ, TNF-α and other proinflammatory cytokines that may be spilt from the inflamed liver are low in chronic liver diseases [26,27].
The serum level of IP-10 was not correlated with the serum level of HCV-core protein or HBV-DNA. However, IP-10 can be directly induced by viruses in rat astrocytes and microglias [28] and in mouse liver tissues [29], so we cannot discount the possibility of direct induction of IP-10 by HBV or HCV.
To clarify the cell type-specific expression patterns of IP-10 mRNA in AIH, in situ hybridization was performed in the liver tissues of AIH patients, and the results revealed that the expression of IP-10 was mainly induced in hepatocytes (Fig. 5a,b,c). This finding is corroborated by the recent detection of IP-10 mRNA in primary cultured hepatocytes [30]. In the PBC patients of the present study, the IP-10 mRNA was not expressed in the areas of damaged bile ducts but was expressed in the hepatocytes around the focal necrotic areas surrounded by infiltrating mononuclear cells (Fig. 5d,e,f,j,i). These results indicate that the IP-10 in PBC and AIH patients derives not from the biliary epithelial cells but from the hepatocytes, as we found in our previous investigation of CHC and CHB patients [12]. Using immunohistochemistry, Haycock et al. [31] reported that MIP-1α, MIP-1β, and MCP-1 were localized in the biliary epithelium or infiltrating mononuclear cells, whereas IP-10 was localized in the portal tract or periportal hepatocytes in chronic liver diseases. In human liver allografts, in situ hybridization and immunohistochemistry showed that MIP-1α and MIP-1β were localized in sinusoidal endothelial cells in the early phase after transplantation [32]. These previous reports suggest that the expression of chemokines in the inflamed human liver is cell type- and disease-specific.
We have shown in the present study that IP-10 is specifically induced in hepatocytes in patients with AIH and PBC. Recently, the receptor of IP-10 was identified on the cell surface of type 1 T helper cells (Th1 cells) and termed CXC chemokine receptor 3 (CXCR3) [33,34]. This receptor is important for the chemotaxis of Th1 cells in conjunction with another chemokine receptor, CC chemokine receptor 5 (CCR5) [35]. We have shown here that IP-10, a chemokine that reflects necroinflammation of hepatocytes, was produced by hepatocytes around the focal necrosis irrespective of the aetiology of hepatitis. Therefore, we suggest that the IP-10 produced by hepatocytes may be one of the key components leading to the chemotaxis of activated T cells, including Th1 cells, to the liver in chronic liver diseases of various aetiologies, including autoimmune liver diseases such as AIH and PBC.
Acknowledgments
We are grateful to Dr Masahiro Tamaru (Japan Tobacco Central Pharmaceutical Research Institute, Kanagawa, Japan) for excellent technical assistance, and to Dr Naoto Kageyama and Dr Shigeru Kawano (Akashi Municipal Hospital, Hyogo, Japan) for providing serum of patients with RA.
REFERENCES
- 1.Scheuer PJ. Viral hepatitis. In: MacSween RNM, Anthony PP, Scheuer PJ, editors. Pathology of the liver. Edinburgh: Chuchill Livingstone; 1987. pp. 202–23. [Google Scholar]
- 2.Dienes HP, Hijtteroth T, Hess G, Meuer SC. Immunoelectron microscopic observations on the inflammatory infiltrates and HLA antigens in hepatitis B and non-A, non-B. Hepatology. 1987;6:1317–25. doi: 10.1002/hep.1840070623. [DOI] [PubMed] [Google Scholar]
- 3.Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572–7. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 5.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990;12:59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- 8.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 9.Dewald B, Moser B, Barella L, Schumacher C, Baggiolini M, Clark-Lewis I. IP-10, a γ-interferon-inducible protein related to interleukin-8, lacks neutrophil activating properties. Immunol Letters. 1992;32:81–84. doi: 10.1016/0165-2478(92)90203-z. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP-10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taub DD, Lloyd AR, Conlon K, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narumi S, Tominaga Y, Tamaru M, Shimai S, Okamura H, Nishioji K, Itoh Y, Okanoue T. Expression of interferon-inducible protein-10 (IP-10) in chronic hepatitis. J Immunol. 1997;158:5536–44. [PubMed] [Google Scholar]
- 13.Leevy CM, Tygstrup N. Basel: S Karger AG; 1976. Standardization of nomenclature diagnostic criteria and diagnostic methodology for diseases of the liver and biliary tract. [Google Scholar]
- 14.Johnson PJ, McFarlane IG. Meeting report: International autoimmune hepatitis group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Tukiyama-Kohara K, Yamaguchi K, Maki N, Ohta Y, Miki K, Mizokami M, Kohara M. Antigenicities of group 1 and 2 hepatitis C virus polypeptides: molecular basis of diagnosis. Virology. 1993;192:430–7. doi: 10.1006/viro.1993.1058. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Tukiyama-Kohara K, Yamaguchi K, Yagi S, Tanaka S, Hasegawa A, Ohta Y. Significance of specific antibody assay for genotyping of hepatitis C virus. Hepatology. 1994;19:1347–53. [PubMed] [Google Scholar]
- 18.Tanaka T, Johnson YNL, Mizokami M, et al. Simple fluorescent enzyme immunoassay for detection and quantification of hepatitis C viremia. J Hepatol. 1995;23:742–5. doi: 10.1016/0168-8278(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 19.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 20.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:432–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 21.Angerer LM, Stoler MH, Angerer RC. In: In situ hybridization with RNA probe: an annotated recipe. Valentino KL, Eberwine JH, Barchas JD, editors. New York: Oxford University Press; 1987. pp. 42–70. [Google Scholar]
- 22.Narumi S, Wyner LM, Stoler MH, Tannenbaum CS, Hamilton TA. Tissue-specific expression of murine IP-10 mRNA following systemic treatment with interferon γ. J Leukocyte Biol. 1992;52:27–33. doi: 10.1002/jlb.52.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Ohmori Y, Wyner LM, Narumi S, Armstrong D, Stoler MH, Hamilton TA. Tumor necrosis factor-α induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am J Pathol. 1993;142:861–70. [PMC free article] [PubMed] [Google Scholar]
- 24.Flier J, Boorsma DM, Bruynzeel DP, van Beek PJ, Stoof TJ, Scheper RJ, Willemze R, Tensen CP. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113:574–8. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 25.Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331–6. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh Y, Okanoue T, Enjyo F, Morimoto M, Takeuchi T, Kagawa K, Kashima K. Elevated interleukin-6 and γ-globulin during interferon therapy of hepatitis B. Am J Gastroenterol. 1992;87:1485–7. [PubMed] [Google Scholar]
- 27.Yoshioka K, Kakumu S, Arao M, Tsutsumi Y, Inoue M. Tumor necrosis factor alpha production by peripheral blood mononuclear cells of patients with chronic liver disease. Hepatology. 1989;10:769–73. doi: 10.1002/hep.1840100504. [DOI] [PubMed] [Google Scholar]
- 28.Vangri P, Farber JM. IFN and virus-inducible expression of an immediate early gene, crg-2/IP-10, and a delayed gene, I-Aα, in astrocytes and microglia. J Immunol. 1994;153:1411–8. [PubMed] [Google Scholar]
- 29.Amichay D, Gazzinelli RT, Karupiah G, Moench TR, Sher A, Farber JM. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-γ with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–20. [PubMed] [Google Scholar]
- 30.Wang H, Gao X, Fukumoto S, Tademoto S, Sato K, Hirai K. Differential expression and regulation of chemokines JE, KC, and IP-10 gene in primary cultured murine hepatocytes. J Cell Physiol. 1999;181:361–70. doi: 10.1002/(SICI)1097-4652(199911)181:2<361::AID-JCP18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Haycock C, Millward-Sadler H, Mackay C, Sheron N. Expression patterns of MIP-1α, MIP-1β, MCP-1, MCP-2, MCP-3 and IP-10 in human inflammatory liver disease. Hepatology. 1996;24:522A(Abstr.). [Google Scholar]
- 32.Adams DH, Hubscher S, Fear J, Jhonston J, Shaw S, Afford S. Hepatic expression of macrophage inflammatory protein-1α and macrophage inflammatory protein-1β after liver transplantation. Transplantation. 1996;61:817–25. doi: 10.1097/00007890-199603150-00024. [DOI] [PubMed] [Google Scholar]
- 33.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of Type 1 helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark- Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–72. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]