Abstract
The relationship between activation of NADPH-oxidase, alterations in membrane potential and triggering of Ca2+ fluxes in human phagocytes has been investigated using neutrophils from four subjects with chronic granulomatous disease (CGD). Cytosolic Ca2+ and membrane potential were measured by spectrofluorimetry, and net efflux and influx of Ca2+ by radiometric procedures. Exposure of normal neutrophils to the chemotactic tripeptide, N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm) was accompanied by an abrupt increase in cytosolic Ca2+ coincident with membrane depolarization and efflux of the cation. These events terminated at around 30 s after the addition of FMLP and were followed by membrane repolarization and store-operated influx of Ca2+, both of which were superimposable and complete after about 5 min. Activation of CGD neutrophils was also accompanied by an increase in cytosolic Ca2+, which, in spite of an efficient efflux response, was prolonged in relation to that observed in normal cells. This prolonged increase in cytosolic Ca2+ in activated CGD neutrophils occurred in the setting of trivial membrane depolarization and accelerated influx of Ca2+, and was associated with hyperactivity of the cells according to excessive release of elastase and increased activity of phospholipase A2. Treatment of CGD neutrophils with the type 4 phosphodiesterase inhibitor, rolipram (1 μm) restored Ca2+ homeostasis and attenuated the increase in elastase release. These findings support the involvement of NADPH-oxidase in regulating membrane potential and Ca2+ influx in activated neutrophils, and may explain the disordered inflammatory responses and granuloma formation which are characteristic of CGD.
Keywords: calcium, chronic granulomatous disease, elastase, neutrophils
INTRODUCTION
Chronic granulomatous disease (CGD) is an inherited phagocyte dysfunction syndrome, which results in predisposition, usually from early childhood, to recurrent and often life-threatening bacterial and fungal infections, as well as a granulomatous response in affected tissues [1,2]. On the molecular/biochemical level, CGD is a heterogeneous group of disorders resulting from point mutations in genes which encode a series of translocatable cytosolic and membrane-associated polypeptides which are required for activation, assembly and function of NADPH-oxidase [1–3]. The absence of NADPH-oxidase activity in neutrophils and other phagocytes results in failure of these cells to reduce molecular oxygen to superoxide which is the precursor of antimicrobial oxidants such as hydrogen peroxide, hypochlorous acid, singlet oxygen and hydroxyl radical [1–3].
Interestingly, the alterations in membrane potential which accompany activation of neutrophils by receptor- and non-receptor-mediated stimuli of membrane-associated oxidative metabolism are almost completely absent in CGD [4–6]. Membrane depolarization is coincident with activation of NADPH-oxidase, underscoring the apparent mechanistic relationship between these events [4–6]. Until fairly recently the functional implications of the attenuated membrane depolarization response in CGD phagocytes were incompletely understood. However, it is now accepted that the membrane depolarization which accompanies activation of various immune and inflammatory cell types, including neutrophils, limits calcium influx, thereby preventing hyperactivation [7–9]. This suggests that dysregulation of Ca2+ homeostasis may represent an additional abnormality of CGD phagocytes, albeit of undetermined functional and clinical significance [10].
In the current study we have investigated the relationship between NADPH-oxidase, membrane potential, Ca2+ fluxes and proinflammatory activities of neutrophils using cells from four patients with CGD, three of whom were assessed on several occasions, and healthy control subjects. Our data suggest that accelerated Ca2+ influx resulting in hyperactivation of several proinflammatory activities is consequent to the absence of NADPH-oxidase activity and attenuated membrane depolarization in CGD neutrophils.
PATIENTS AND METHODS
Patients
Two related patients (first cousins) aged 12 (JS) and 15 years (DT) at the time of the first visit, respectively, with X-linked CGD (C-688→ T mutation resulting in an Arg-226-stop in gp91phox) were each investigated on three different occasions over a 16-month period. In the case of patient 1 the values for serum C-reactive protein (CRP) on the three consecutive visits were 8, 7·5 and 11·7 μg/ml, while for patient 2 these were 40, <3 and <3 μg/ml. Two additional patients, brother (DE) and sister (RS) aged 24 and 28 years, respectively, with the autosomal recessive form of CGD (deficiency of p47phox GT deletion in exon 2) were investigated on one and four occasions, respectively, on each of which the CRP values were <3 μg/ml.
Materials and methods
Unless indicated all chemicals and reagents were purchased from the Sigma Chemical Co. (St Louis, MO).
Neutrophils
Purified neutrophils were prepared from heparinized (5 U of preservative-free heparin/ml) venous blood of CGD subjects and healthy adult volunteers and separated from mononuclear leucocytes by centrifugation on Histopaque-1077 (Sigma Diagnostics) cushions at 400 g for 25 min at room temperature. The resultant pellet was suspended in PBS (0·15 m, pH 7·4) and sedimented with 3% gelatin to remove most of the erythrocytes. After centrifugation, erythrocytes were removed by selective lysis with 0·84% ammonium chloride at 4°C for 10 min. The neutrophils, which were routinely of high purity (> 90%) and viability (> 95%), were resuspended to 1 × 107/ml in PBS and held on ice until use.
Superoxide production
This was measured only on the initial visit of each CGD patient using lucigenin (bis-N-methylacridinium nitrate) chemiluminescence (LECL) [11]. Neutrophils (1 × 106) were preincubated for 15 min at 37°C in 900 μl of indicator-free Hanks' balanced salt solution (HBSS, pH 7·4, 1·25 mm CaCl2) containing 0·2 mm lucigenin, after which they were activated with the synthetic chemotactic tripeptide, N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm final) or phorbol 12-myristate 13-acetate (PMA; 20 ng/ml final). Spontaneous and stimulated LECL responses were then monitored with an LKB Wallac 1251 chemiluminometer (Turku, Finland) after the addition of the stimulant (100 μl). LECL readings were integrated for 5-s intervals and recorded as mV/s.
Membrane potential
The potential sensitive fluorescent dye dipentyloxacarbocyanine (di-O-C5(3)) was used to measure changes in membrane potential in activated neutrophils [4]. The cells (1 × 106/ml) were preincubated for 10 min at 37°C in HBSS containing 80 nm (final) di-O-C5(3), after which they were transferred to disposable reaction cuvettes which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 460 nm and 510 nm, respectively. The neutrophils were then activated with FMLP (1 μm) and the subsequent alterations in fluorescence intensity monitored over a 5–10-min period. The final volume in each cuvette was 3 ml containing a total of 3 × 106 neutrophils.
Spectrofluorimetric measurement of Ca2+ fluxes
Fura-2/AM (Calbiochem Corp., La Jolla, CA) was used as the fluorescent, Ca2+-sensitive indicator for these experiments [12]. Neutrophils (1 × 107/ml) were preloaded with fura-2 (2 μm) for 30 min at 37°C in PBS (0·15 m, pH 7·4), washed twice and resuspended in indicator-free HBSS pH 7·4 containing 1·25 mm CaCl2, referred to hereafter as Ca2+-replete HBSS. The fura-2-loaded cells (2 × 106/ml) were then preincubated for 10 min at 37°C, after which they were transferred to disposable reaction cuvettes, which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 340 nm and 500 nm, respectively. After a stable base-line was obtained (1 min), the neutrophils were activated by addition of FMLP (1 μm) and the subsequent increase in fura-2 fluorescence intensity was monitored over a 5-min period. The final volume in each cuvette was 3 ml containing a total of 6 × 106 neutrophils.
The data from these experiments were used to compare the following in control and CGD neutrophils: (i) basal concentrations of Ca2+ in unstimulated cells; (ii) peak increments in cytosolic Ca2+ concentrations and the duration of these in stimulated cells; (iii) the rate of clearance of Ca2+ from the cytosol of FMLP-activated cells.
In an additional series of experiments, the effects of the cyclic AMP-elevating agent rolipram (1 μm final), an inhibitor of type 4 phosphodiesterase (PDE4), the predominant type found in human neutrophils [13,14], on FMLP-activated Ca2+ fluxes in CGD neutrophils were investigated. Rolipram was present with the neutrophils throughout the 10-min preincubation period prior to addition of FMLP.
Mn2+ quenching of fura-2 fluorescence
Cells loaded with fura-2/AM as described above were activated with FMLP 1 μm in the presence of 300 μm MnCl2 (added 5 min prior to FMLP) and fluorescence quenching as a measure of Ca2+ influx was determined at an excitation wavelength of 360 nm, which is an isosbestic wavelength, and at an emission wavelength of 500 nm [10]. These experiments were performed using cells from two control subjects and two CGD patients (DT and RS).
Radiometric assessment of Ca2+ fluxes
45Ca2+ (Calcium-45 chloride, specific activity 18·53 mCi/mg; Du Pont NEN Research Products, Boston, MA) was used as tracer to label the intracellular Ca2+ pool and to monitor Ca2+ fluxes in resting and activated neutrophils. In the assays of net efflux and influx of Ca2+ described below, the radiolabelled cation was always used at a fixed, final concentration of 2 μ Ci/ml, containing 50 nmol cold carrier Ca2+. The final assay volumes were always 5 ml containing a total of 1 × 107 neutrophils. The standardization of the procedures used to load the cells with 45Ca2+, as well as a comparison with silicone oil-based methods for the separation of labelled neutrophils from unbound isotope, have been described elsewhere [15].
Efflux of 45Ca2+ from FMLP-activated neutrophils
To measure net efflux of 45Ca2+ from neutrophils uncomplicated by concomitant influx of the radiolabelled cation, the cells (1 × 107/ml) were loaded with 45Ca2+ (2 μ Ci/ml) for 30 min at 37°C in HBSS. The neutrophils were then pelleted by centrifugation, washed once with, and resuspended in ice-cold Ca2+-replete HBSS and held on ice until use, which was always within 10 min of completion of loading with 45Ca2+. Using this procedure, the FMLP-activated fura-2 responses of neutrophils, similarly processed in HBSS containing 1 μm cold CaCl2 followed by washing with, and suspension in Ca2+-replete HBSS, did not differ from those of cells which had been maintained in Ca2+-replete HBSS throughout, indicating that at the time of measurement of efflux in the 45Ca2+ system there was no meaningful depletion of intracellular Ca2+[15]. The 45Ca2+-loaded neutrophils (2 × 106/ml) were then preincubated for 10 min at 37°C in Ca2+-replete HBSS, followed by activation with FMLP 1 μm and measurement of the kinetics (10, 30 and 60 s) of net efflux of 45Ca2+. FMLP was omitted from the corresponding control systems.
Reactions were stopped by the addition of 10 ml Ca2+-replete HBSS to the tubes which were transferred immediately to an ice bath [15]. The cells were then pelleted by centrifugation at 400 g for 5 min followed by washing with 15 ml ice-cold Ca2+-replete HBSS and the cell pellets finally dissolved in 0·5 ml of Triton X-100/0·1 m NaOH and the radioactivity assessed in a liquid scintillation spectrometer. Control, cell-free systems (HBSS and 45Ca2+ only) were included for each experiment and these values were subtracted from the relevant neutrophil-containing systems. These results are presented as the amount of Ca2+ released from the cells during efflux (pmol/107 cells).
Influx of 45Ca2+ into FMLP-activated neutrophils
To measure the net influx of 45Ca2+ into FMLP-activated neutrophils, uncomplicated by concomitant efflux of the radiolabelled cation, the cells were loaded with cold, Ca2+-replete HBSS for 30 min at 37°C, after which they were pelleted by centrifugation, then washed once with, and resuspended in ice-cold Ca2+-free HBSS and held on ice until use. Pre-loading with cold Ca2+ was undertaken to minimize spontaneous uptake of 45Ca2+ (unrelated to FMLP activation) in the influx assay. The efficiency of this loading procedure was demonstrated by measurement of the FMLP-activated fura-2 responses of the Ca2+-loaded neutrophils, which were similar to those of neutrophils maintained in Ca2+-replete HBSS [15]. The Ca2+-loaded neutrophils (2 × 106/ml) were then incubated for 10 min at 37°C in Ca2+-free HBSS followed by simultaneous addition of FMLP and 45Ca2+ (2 μ Ci/ml), or 45Ca2+ only to control, unstimulated systems. The kinetics of influx of 45Ca2+ into FMLP-activated neutrophils was then monitored over a 5-min period (at 10, 20, 30 and 60 s and 2, 3 and 5 min after the addition of FMLP) and compared with that of influx of the radiolabelled cation into the identically processed, unstimulated cells.
Elastase release
Neutrophil degranulation was measured according to the extent of release of the primary granule-derived enzyme, elastase. Neutrophils were preincubated at a concentration of 1 × 107/ml in HBSS for 10 min at 37°C. FMLP (0·1 μm) in combination with a submaximal concentration of cytochalasin B (CB) (1 μm) was then added to the cells, which were incubated for 15 min at 37°C. The tubes were then transferred to an ice bath, followed by centrifugation at 400 g for 5 min to pellet the cells. The neutrophil-free supernatants were then decanted and assayed for elastase activity using a micromodification of a standard colourimetric procedure [16]. Briefly, 125 μl of supernatant were added to the elastase substrate N-succinyl-l-alanyl-l-analyl-l-alanine-p-nitroanilide (3 mm in DMSO) in 0·05 m Tris–HCl pH 8·0 and elastase activity monitored at a wavelength of 405 nm.
In an additional series of experiments the following were also investigated: (i) the effects of rolipram (1 μm final) on the release of elastase from control and CGD neutrophils, and (ii) a comparison of elastase release from control and CGD neutrophils using submaximal and maximal combinations of FMLP with CB (0·1 μm/1 μm and 1 μm/10 μm).
Because elastase has been reported to be functionally inactivated by very high concentrations of reactive oxidants [17,18], additional experiments were performed to control for possible overestimation of elastase activity in supernatant fluids from stimulated CGD neutrophils. Hydrogen peroxide at final concentrations of 50, 100, 500 and 1000 μm or an equal volume of HBSS (50 μl) were added to 950 μl of supernatant fluid from FMLP-activated control and CGD (DT and RS) neutrophils which was then assayed for elastase activity following 15 min of incubation at 37°C. Maximally stimulated neutrophils have been reported to generate 100 nmol H2O2/106 cells over a 30-min incubation period [19].
Phospholipase A2 activity
This was measured using a radiometric thin layer chromatography procedure [20]. Neutrophils (1 × 107/ml) were co-incubated with 5 μ Ci/ml radiolabelled arachidonate (5,6,8,9,11,12,14,15–3H(N), 185 Ci/mmol; Du Pont NEN) for 15 min at 37°C in Ca2+-free HBSS containing 5 μm indomethacin, to allow incorporation of radiolabelled arachidonate into membrane phospholipids. The cells were then washed twice and resuspended to 1 × 107/ml in Ca2+-replete HBSS. The cells (2·5 × 106/ml) were then preincubated for 10 min at 37°C prior to the addition of FMLP (1 μm final) in a final volume of 2 ml. This was followed by a 3-min incubation at 37°C (predetermined in preliminary kinetics experiments), after which the reactions were terminated and 3H-arachidonate extracted by the addition of 5 ml n-hexane/isopropanol/HCl (300:200:4 v/v/v) and thorough mixing. The upper organic phase was removed and evaporated to dryness under a nitrogen stream. The lipids were reconstituted in 40 μl hexane/isopropanol and spotted onto silica gel precoated TLC plates (Merck, Darmstadt, Germany) together with 2 μm unlabelled arachidonate standard to facilitate detection. The plates were developed in chloroform/acetone (96:4 v/v) and then exposed to iodine vapours. The arachidonate spots were localized, excised and assayed for radioactivity.
Expression and statistical analysis of results
The results of each series of experiments are expressed as the mean values ± s.e.m., with the exception of the membrane potential experiments for which the traces are shown. Statistical analyses were performed using the paired Student's t-test when comparing two groups or by analysis of variance with subsequent Tukey–Kramer multiple comparisons test for multiple comparisons.
RESULTS
Superoxide generation
These results are shown in Table 1. The LECL responses of FMLP- and PMA-activated CGD neutrophils were virtually undetectable in comparison with those of cells from control subjects.
Table 1.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP)- and phorbol 12-myristate 13-acetate (PMA)-activated lucigenin-enhanced chemiluminescence (LECL) responses of neutrophils from control and chronic granulomatous disease (CGD) subjects
| LECL responses of neutrophils | ||||
|---|---|---|---|---|
| Without FMLP | With FMLP | Without PMA | With PMA | |
| Control (n = 4) | 578 ± 55 | 2129 ± 409 | 623 ± 94 | 4505 ± 488 |
| CGD (n = 4) | 15 ± 2 | 38 ± 14 | 19 ± 4 | 38 ± 14 |
Results are expressed as the mean peak LECL values in mV/s ± s.e.m.
Membrane potential
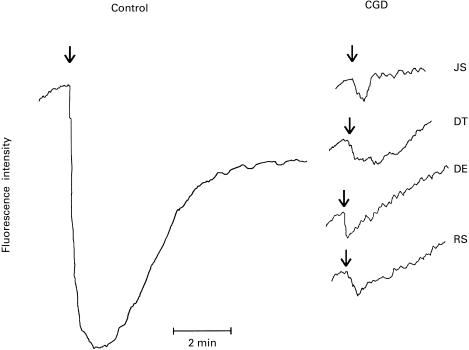
FMLP-activated alterations in membrane potential in control and CGD neutrophils are shown in Fig. 1. Exposure of control neutrophils to FMLP resulted in rapid membrane depolarization which terminated at around 30 s and was followed 60–90 s later by membrane repolarization which was complete at 5 min, but which did not recover to preactivation values. In contrast, the FMLP-activated decrease in membrane potential in CGD neutrophils was trivial (average of 9% of the control response), with variable time taken for complete repolarization.
Fig. 1.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm)-activated alterations in the membrane potential of neutrophils from one typical control subject and from four different patients with chronic granulomatous disease (CGD) (JS, DT, DE, RS). FMLP was added as indicated (↓) approximately 1 min after transfer of neutrophils to the reaction cuvettes. The assays were repeated on three and two visits several months apart on patients JS and RS, respectively, and similar results were obtained on each occasion. All traces are on the same scale.
Fura-2 responses of neutrophils
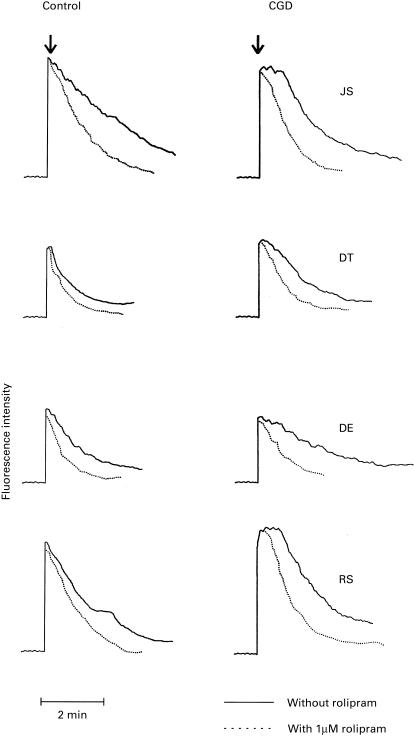
The results shown in Fig. 2 are typical traces of the FMLP-activated fura-2 responses of control and CGD neutrophils in the presence and absence of rolipram. Addition of FMLP to neutrophils was accompanied by the characteristic, abrupt increase in fura-2 fluorescence due to an increase in the cytosolic concentrations of Ca2+, the magnitude of which was similar in control and CGD neutrophils. In the case of control neutrophils, attainment of peak fluorescence was accompanied by a rapid decline in fluorescence intensity which was delayed in CGD neutrophils, indicative of impaired clearance of Ca2+ from the cytosol.
Fig. 2.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm)-activated fura-2 fluorescence responses of neutrophils from four different control and chronic granulomatous disease (CGD) subjects (JS, DT, DE, RS). Neutrophils from each control subject were paired with those of a CGD patient as shown. FMLP was added when a stable base-line was obtained (± 1 min). The assays were repeated on three, three and four visits several months apart on patients JS, DT and RS, respectively, and similar results were obtained on each occasion.
Peak increments in cytosolic Ca2+ concentrations following addition of FMLP to control and CGD neutrophils, time taken to initiation of the abrupt linear decline in fluorescence intensity and the rate of Ca2+ clearance during this phase are shown in Table 2 for a larger series of experiments, including repeated measurements on patients JS, DT and RS. There were no significant differences between control and CGD neutrophils with respect to basal and peak increments in cytosolic Ca2+, as well as the rate of clearance of cytosolic cation. However, the time taken to initiation of the abrupt decline in peak fluorescence intensity was significantly (P < 0·0001) longer in CGD neutrophils.
Table 2.
Peak increments in cytosolic Ca2+ concentrations, time taken to onset of clearance and rates of clearance of the cation in N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP)-activated control and chronic granulomatous disease (CGD) neutrophils
| Peak increments in cytosolic Ca2+ (pmol)† | Time taken (s) to onset of clearance of cytosolic Ca2+ | Clearance rate of cytosolic Ca2+ (pmol/min) | |
|---|---|---|---|
| Control neutrophils (n = 9) | 282 ± 28 | 7 ± 1 | 93 ± 9 |
| CGD neutrophils (n = 14) | 276 ± 21 | 43 ± 2* | 86 ± 6 |
Results are expressed as the mean values ± s.e.m. The basal cytosolic concentrations for unstimulated control and CGD neutrophils were 105 ± 62 and 89 ± 31 pmol/6 × 106 cells, respectively.
Peak increments in cytosolic Ca2+ were adjusted for basal values.
P < 0·001 for comparison between control and CGD neutrophils.
The effects of rolipram on the fura-2 responses of control and CGD neutrophils are also shown in Fig. 2. Addition of this agent to both normal and CDG cells did not affect either the basal levels of fluorescence in resting neutrophils or the peak fluorescence intensity following addition of FMLP. However, the rates of clearance of cytosolic Ca2+ were significantly faster in rolipram-treated cells. In normal cells the rates of Ca2+ clearance in the absence and presence of rolipram were 93·4 ± 8·8 pmol/min and 156·6 ± 12·1 pmol/min (P < 0·001), respectively, while the corresponding values for CGD cells were 86·3 ± 5·6 pmol/min and 160·6 ± 5·3 pmol/min (P < 0·001). Rolipram also significantly decreased the time taken to initiation of the abrupt linear decline in peak fluorescence intensity in FMLP-activated CGD cells (43 ± 2 s versus 6·6 ± 1 s, P < 0·001 in the absence and presence of rolipram, respectively), the value for rolipram-treated CGD cells being similar to that for stimulated control cells in the absence of rolipram (7 ± 1 s).
Influx of Ca2+ using Mn2+ quenching of fura-2 fluorescence
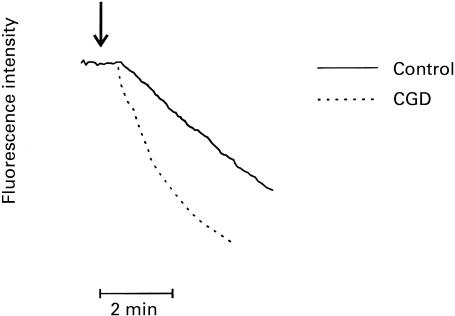
The results of the indirect measurement of Ca2 influx into FMLP-activated control and CGD neutrophils are shown in Fig. 3. In control cells the decrease in fluorescence intensity, which indicates influx of the cation, was delayed for about 30 s after addition of FMLP, followed by an almost linear decrease over 3–4 min. In the case of CGD cells the decrease in fluorescence intensity also occurred at around 30 s after the addition of FMLP, but proceeded at almost double the rate to that observed in control cells (P < 0·001) over the initial 2-min period of the time course (data from six repeat measurements on control cells and cells from patient DT). Similar differences (not shown) were observed using cells from patient RS.
Fig. 3.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm)-activated Mn2+ quenching of the fura-2 responses of control and chronic granulomatous disease (CGD) (DT) neutrophils. FMLP was added as indicated (↓) and the results shown are typical traces of six replicates.
Efflux of 45Ca2+
In these experiments, control and CGD neutrophils which had been preloaded with 45Ca2+ and then washed and transferred to Ca2+-replete HBSS (to minimize re-uptake of radiolabelled cation) were activated with FMLP followed by measurement of the amount of cell-associated 45Ca2+. Exposure of both control and CGD neutrophils to FMLP resulted in an abrupt efflux of the radiolabelled cation which terminated approximately 30 s after addition of the stimulant, resulting in the loss of about 40% of cell-associated 45Ca2+. The rates and extent of efflux of 45Ca2+ from FMLP-activated control and CGD neutrophils were not significantly different. The amounts of cation discharged from FMLP-activated control and CGD neutrophils 30 s after activation were 157 ± 17 and 160 ± 14 pmol Ca2+/107 cells, respectively. There was no loss of cell-associated 45Ca2+ from unstimulated neutrophils over the brief 60-s time course during which efflux was measured (not shown).
Influx of 45Ca2+
For these experiments control and CGD neutrophils were preloaded with cold Ca2+, then transferred to Ca2+-free HBSS prior to activation with FMLP, which was added simultaneously with 45Ca2+. This step (loading with cold Ca2+) was undertaken to minimize spontaneous uptake of 45Ca2+ by neutrophils [15]. The results of these experiments, which were designed to measure net influx of 45Ca2+ into FMLP-activated control and CGD neutrophils, are shown in Table 3.
Table 3.
Kinetics of influx of 45Ca2+ into N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP)-activated control and chronic granulomatous disease (CGD) neutrophils
| Influx of Ca2+ into FMLP-activated neutrophils (pmoles cell-associated Ca2+/107 neutrophils) | ||
|---|---|---|
| Time after addition | Contro lneutrophils (n = 6) | CGD neutrophils (n = 6) |
| 10 s | 0 | 1 ± 1 |
| 20 s | 0 | 18 ± 10 |
| 30 s | 4 ± 4 | 34 ± 16 |
| 60 s | 16 ± 8 | 54 ± 9* |
| 2 min | 56 ± 9 | 107 ± 18* |
| 3 min | 109 ± 13 | 166 ± 13* |
| 5 min | 156 ± 16 | 167 ± 15 |
The results are expressed as the mean values for six different control subjects and the four different CGD patients with repeat evaluations performed on DT and RS.
P < 0·05 – P < 0·001 for comparison between the uptake of 45Ca2+ by control and CGD cells at the corresponding times.
The amount of influx of Ca2+ into unstimulated control and CGD neutrophils at 5 min was 17 ± 6 and 27 ± 4 pmol/107 cells, respectively.
Activation of control neutrophils with FMLP under these experimental conditions resulted in a delayed uptake of 45Ca2+ which was detectable after a lag phase of about 30–60 s and continued beyond 5 min after addition of the stimulus. In the case of FMLP-activated CGD cells the mean time taken for detection of influx of 45Ca2+ was shorter, although not significantly so (20 s and 30 s for CGD and control cells, respectively). However, influx of the cation into FMLP-mediated CGD neutrophils proceeded at a significantly faster rate over the first 3 min of the time course of the experiment and was complete at 3 min after addition of the stimulus in comparison with 5 min in the case of control cells. Influx of 45Ca2+ was a true consequence of activation of neutrophils with FMLP, since there was only trivial influx of the radiolabelled cation over the same time course into control identically processed neutrophils which had not been exposed to FMLP (Table 3).
Elastase release
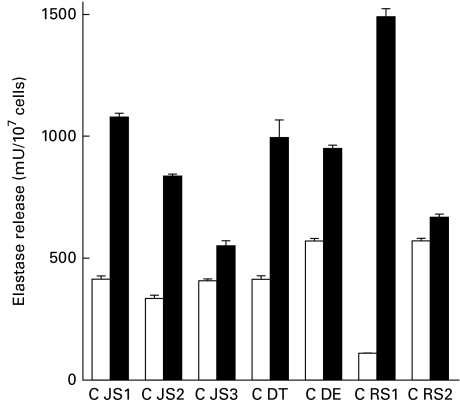
Elastase release from FMLP/CB-activated control and CGD neutrophils is shown in Fig. 4. Activation of neutrophils from all four CGD patients on each occasion tested resulted in a significantly higher release of elastase than that observed with control neutrophils. In a single experiment designed to investigate the effects of increasing the potency of the activator of degranulation, the differential release of elastase from CGD neutrophils from a single subject (JS), relative to that of control cells, appeared to be independent of the concentration of the stimulus. The release of elastase from control and CGD neutrophils activated with 0·1 μm FMLP/1 μm CB was 407 ± 8 and 552 ± 21 mU elastase/107 cells, respectively (P < 0·001), while the corresponding values for cells activated with 1 μm FMLP/10 μm CB were 1282 ± 38 and 1988 ± 58 mU elastase (P < 0·001). The background values for unstimulated control and CGD neutrophils were 15 ± 1 and 34 ± 3 mU elastase, respectively.
Fig. 4.
N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP)/cytocholasin B (CB) (0·1 μm/1 μm)-activated release of elastase from control (□) and chronic granulomatous disease (CGD; ▪) (JS, DT, DE, RS) neutrophils. The paired responses of control and CGD cells are expressed as the mean amount of elastase released in milliunits enzyme/107 cells with three to six replicates for each value. CGD patient JS was evaluated on three separate occasions several months apart (JS1, JS2, JS3), patient RS on two occasions (RS1, RS2) and the others (DT, DE) on one occasion. The range of values for the spontaneous release of elastase from unstimulated neutrophils from control and CGD subjects (all subjects on all occasions tested) was 15–36 (x¯ = 29) and 31–41 (x¯ = 35) mU elastase/107 cells, respectively. The level of statistical significance for comparison of elastase release from FMLP/CB-activated control and CGD neutrophils was P < 0·0001.
The effects of rolipram on the release of elastase from FMLP/CB-activated control and CGD neutrophils are shown in Table 4. The PDE4 inhibitor significantly reduced the release of elastase from both control and CGD neutrophils.
Table 4.
Effects of rolipram on the release of elastase from N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP)/CB-activated control and chronic granulomatous disease (CGD) neutrophils
| Release of elastase from FMLP/CB activated neutrophils | ||
|---|---|---|
| Without rolipram | With rolipram (1 μm) | |
| Control neutrophils (n = 3) | 269 ± 57 | 134 ± 26* |
| CGD neutrophils (n = 4) | 707 ± 49 | 88 ± 1** |
The results are expressed as the mean values ± s.e.m. in milliunits enzyme/107 cells and the assay was performed in triplicate for each subject. The values for unstimulated neutrophils from control and CGD subjects were 39 ± 4 and 41 ± 5 mU enzyme/107 cells, respectively.
P < 0·002
P < 0·0002.
Co-incubation of the cell-free supernatants from FMLP/CB-activated control and CGD neutrophils with added H2O2 (50–1000 μm) for 15 min at 37°C did not inhibit, but rather modestly increased, the functional reactivity of elastase. In systems exposed to 1000 μm H2O2 the activities of elastase (mU/107 cells) in the supernatants of control neutrophils without and with H2O2 (1000 μm) were 173 ± 10 and 216 ± 2, respectively, while the corresponding values for CGD cells (DT) were 388 ± 10 and 469 ± 10. In a second experiment the corresponding values for control neutrophils were 174 ± 10 and 227 ± 3, while those for CGD cells (RS) were 296 ± 2 and 322 ± 3. These data have not been included in Fig. 4. In a series of control experiments it was found that H2O2 (1000 μm) did not affect the assay system for detection of elastase activity.
Phospholipase A2 activity
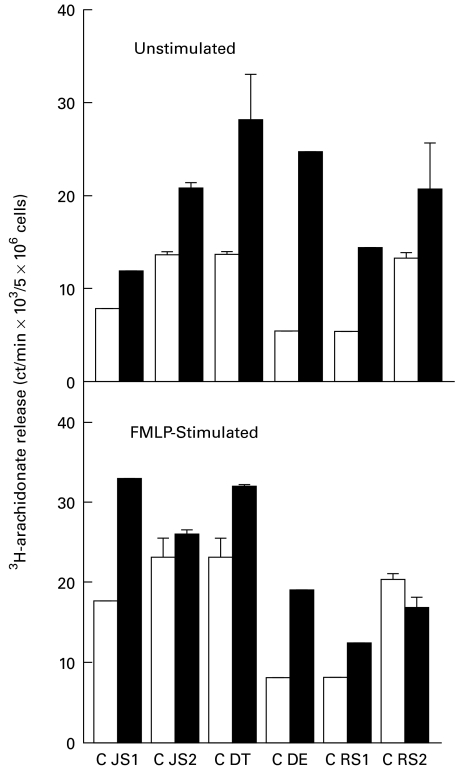
These results are shown in Fig. 5. Both spontaneous and FMLP-activated phospholipase A2 (PLA2) activity was consistently higher in CGD neutrophils in comparison with the corresponding responses of control neutrophils, but the results did not achieve statistical significance.
Fig. 5.
Spontaneous and N-formyl-l-methionyl-l-leucyl-l-phenylalanine (FMLP; 1 μm)-activated release of 3H-arachidonate from control (□) and chronic granulomatous disease (CGD; ▪) (JS, DT, DE, RS) neutrophils as a measure of phospholipase A2 activity. The paired responses of control and CGD cells are expressed as the mean amount of 3H-arachidonate released in absolute ct/min with three replicates for each value. CGD subjects JS and RS were each evaluated on two occasions several months apart (JS1, JS2, RS1, RS2) and the others (DT, DE) on one occasion.
DISCUSSION
Although the relationship between activation of granulocyte membrane-associated oxidative metabolism and alterations in membrane potential is well recognized, the functional consequences of attenuated depolarization secondary to the absence of NADPH-oxidase in phagocytes from CGD subjects remain unknown [4–6]. Interestingly, the abruptly occurring depolarization which accompanies activation of various types of inflammatory cell, including basophils, mast cells and neutrophils, has been shown to limit the influx of extracellular Ca2+[7–9]. It has been proposed that when the cells are depolarized the driving force for entry of Ca2+ is markedly reduced because the electrical component of the electrochemical gradient for Ca2+ is abolished [7,8]. Carefully regulated influx of Ca2+ during recovery of membrane potential may facilitate diversion of incoming cation into stores, thereby preventing flooding of the cytosol with Ca2+ and possible hyperactivation of the cells.
In the current study, the relationship between NADPH-oxidase-dependent alterations in membrane potential and maintenance of Ca2+ homeostasis has been investigated using FMLP-activated neutrophils from control and CGD subjects. Neutrophils from all four CGD subjects (two with X-linked and two with autosomal recessive CGD) demonstrated markedly blunted membrane depolarization responses following stimulation with FMLP. This observation is consistent with several previous reports and supports the contention that NADPH-oxidase is the major contributor to the alterations in membrane potential which accompany activation of neutrophils with stimuli of membrane-associated oxidative metabolism [4–6]. The initial decrease in membrane potential has been attributed to the electrogenic activity of the oxidase [21,22], as well as to the action of a rapidly activated H+ conductance with resultant influx of H+ and depolarization [23]. Recovery of membrane potential is achieved, at least in part, through the action of a slowly activatable H+ conductance which allows only H+ extrusion and repolarization [21,22].
Ca2+ fluxes in FMLP-activated control and CGD neutrophils were measured and compared using fura-2 spectrofluorimetry together with radiometric procedures which enable distinction between efflux and influx of Ca2+. When used in combination these procedures facilitate the identification of the origins (extracellular or intracellular) of cytosolic Ca2+[15].
Exposure of fura-2-loaded CGD neutrophils to FMLP was accompanied by an immediate increase in fluorescence intensity which was of similar magnitude to that observed in control cells, confirming that PLC/ITP3-mediated mobilization of intracellular Ca2+ is normal in CGD neutrophils [10,24]. However, the decline in peak fluorescence intensity was delayed by up to 40–50 s in CGD cells, indicative of impairment of the clearance of Ca2+ from the cytosol. This could not be attributed to defective efflux of Ca2+, because the rate and extent of extrusion of the cation, as has been reported previously [25], were similar in control and CGD cells, demonstrating that, although coincident, Ca2+ efflux and membrane depolarization are not interdependent events in FMLP-activated neutrophils.
The observation that the early occurring efflux of Ca2+ is unimpaired in FMLP-activated CGD neutrophils suggested that uncontrolled influx may be responsible for the sustained elevation of cytosolic Ca2+. This contention was supported by data from the radiometric procedure which demonstrated accelerated influx of Ca2+ into FMLP-activated CGD neutrophils. Influx of the cation was detected earlier, proceeded at a faster rate, and terminated earlier in CGD cells in comparison with control cells, which demonstrated the typical delayed, store-operated influx of the cation [15,26,27].
The altered fura-2 responses observed in neutrophils from all four CGD subjects in the present study differ from those reported by other authors who used either fura-2 [10] or quin-2 [24] as the Ca2+-sensitive intracellular fluorescent dyes. Although Geiszt and colleagues [10] were unable to detect alterations in the fura-2 responses of FMLP-activated CGD neutrophils, relative to those of control cells, they did, however, observe an immediate influx of Ca2+ into CGD cells, while uptake of the cation by control cells was detectable only after a lag period of 2 min. This differential influx of Ca2+ into control and CGD neutrophils was detected using an indirect Mn2+/fura-2 fluorescence quenching procedure [10]. The applicability of this procedure to the measurement of Ca2+ influx through store-operated Ca2+ channels has been questioned, largely because these channels are Ca2+-selective with a very small single channel conductance [26]. Nevertheless, the observation made by Geiszt et al. of accelerated influx of Ca2+ in the setting of trivial membrane depolarization in FMLP-activated CGD cells [10] is clearly supported by the data from the present study in which both radiometric and Mn2+/fura-2 fluorescence quenching procedures were used to detect influx of Ca2+ into neutrophils. Geiszt and colleagues [10] also excluded the alterations in intracellular pH which accompany activation of normal neutrophils, but which are absent in CGD cells [1], as being involved in the regulation of Ca2+ movements. Moreover, the fluorescence intensity of fura-2 is stable to pH 6·75 [12].
Measurement of the release of elastase from, and activity of PLA2 in activated CGD neutrophils, both of which are Ca2+-dependent functions [28,29], suggested that dysregulation of Ca2+ influx results in altered proinflammatory functions of these cells. Increased activity of elastase, which could not be attributed to the absence of oxidative inactivation of the protease, following exposure of CGD neutrophils to FMLP/CB, as well as increased spontaneous and FMLP-stimulated PLA2 activity, suggest that hyperactivity of these oxygen-independent, proinflammatory functions is consequent to disordered Ca2+ homeostasis in CGD neutrophils. In addition to the inability of CGD phagocytes to oxidatively inactivate mediators of inflammation including leukotrienes and other chemoattractants (reviewed in [1]), the mechanisms described in the current study may also contribute to poorly controlled inflammatory responses and granuloma formation in this disease. It is noteworthy in this respect that neutrophil primary granules contain, in addition to elastase, chemoattractants for monocytes, as well as for CD4+ and CD8+ T lymphocytes [30,31].
Alternatively, increased release of primary granule enzymes and enhanced PLA2 activity may partially compensate for the absence of oxidant-mediated antimicrobial activity in these cells [32–35]. In several previous studies employing particulate stimuli and different markers of neutrophil granule release to those used in the present study, no enhancement of degranulation was reported [36–38], while Voetman et al. reported that release of granule enzymes from activated CGD neutrophils was two-to-three-fold greater than that from normal neutrophils [39].
The proposed relationship between sustained elevation of cytosolic Ca2+ and increased release of the primary granule enzyme elastase in activated CGD neutrophils was further investigated using the type 4 PDE inhibitor, rolipram. This agent has previously been demonstrated to increase cAMP in neutrophils, leading to accelerated clearance of cytosolic Ca2+[40]. This has been attributed to enhanced activity of the cAMP-dependent protein kinase-up-regulated, Ca2+-sequestering/re-sequestering endo-membrane Ca2+-ATPase, and down-regulation of the proinflammatory activities of the cells [40]. In the current study, co-incubation of CGD neutrophils, as well as control neutrophils, with rolipram resulted in accelerated clearance of Ca2+ from the cytosol following activation with FMLP, which was associated with a marked reduction in the release of elastase from these cells. Importantly, treatment with rolipram converted CGD neutrophils to a normal phenotype with respect to both Ca2+ clearance and degranulation. These observations suggest that rolipram, presumably by causing cAMP-dependent up-regulation of the activity of the endo-membrane Ca2+-ATPase, can restore, albeit indirectly, Ca2+ homeostasis in activated CGD neutrophils. Second-generation type 4 PDE inhibitors which retain efficient PDE4 inhibitory activity in the setting of attenuation of side-effects [13,14] may be useful in the treatment of disordered inflammatory responses in CGD.
Two additional lines of evidence not included in the current study support the contention that altered Ca2+ influx and hyperactivation of the oxygen-independent proinflammatory functions of stimulated neutrophils occur in CGD. First, we (unpublished) and others [41,42] have observed that treatment of normal neutrophils with the protein kinase inhibitor staurosporine (200 nm) converts normal neutrophils to a CGD-like phenotype characterized by decreased FMLP-activated superoxide production and membrane depolarization, in the setting of prolonged Ca2+ transients and increased release of elastase. Ideally, we would have preferred to use diphenylene iodonium, a direct inhibitor of NADPH oxidase [43]. However, we have found that this agent, at concentrations which inhibit the phagocyte oxidase, also inhibits PLA2, as well as release of Ca2+ from intracellular stores in activated neutrophils (unpublished observations). Second, to exclude the possibility that the inability of CGD neutrophils to oxidatively inactivate FMLP [44] may explain the prolonged peak Ca2+ transients in activated neutrophils, we have measured the fura-2 responses of normal neutrophils activated with N-formyl-norleucyl-leucyl-phenylalanine (1 μm), an oxidation-insensitive chemotactic peptide [44]. The fura-2 responses of normal neutrophils activated with this oxidation-insensitive chemotactic peptide were similar to those observed with FMLP, demonstrating that differences in the fura-2 responses of normal and CGD neutrophils are not attributable to differences in the abilities of these cells to inactivate FMLP.
In conclusion, failure of depolarization in CGD neutrophils is associated with Ca2+ overload due to accelerated influx of the cation and hyperactivity of several proinflammatory activities of these cells.
REFERENCES
- 1.Segal GH, Leto TL, Gallin JI, et al. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cale CM, Jones AM, Goldblatt D. Follow up of patients with chronic granulomatous disease diagnosed since 1990. Clin Exp Immunol. 2000;120:351–5. doi: 10.1046/j.1365-2249.2000.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark RA. Activation of the neutrophil respiratory burst oxidase. J Infect Dis. 1999;179(Suppl. 2):S309–17. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 4.Seligmann BE, Gallin JI. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980;66:493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitagawa S, Ohta M, Nojiri H, et al. Functional maturation of membrane potential changes and superoxide producing capacity during differentiation of human granulocytes. J Clin Invest. 1984;73:1062–71. doi: 10.1172/JCI111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuura R, Kobayashi M, Usui T. Membrane potential changes in polymorphonuclear leukocytes of patients with chronic granulomatous disease. Hiroshima J Med Sci. 1984;33:173–7. [PubMed] [Google Scholar]
- 7.Mohr FC, Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987;104:783–92. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Lew PD, Andersson T, et al. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987;262:4574–9. [PubMed] [Google Scholar]
- 10.Geiszt M, Kapus A, Nemet K, et al. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes: alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–8. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- 11.Minkenberg I, Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NADPH-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 1984;71:61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 13.Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157:351–70. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Wu P, Ohleth K, et al. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Molec Pharmacol. 1999;56:170–4. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- 15.Anderson R, Goolam Mahomed A. Calcium efflux and influx in f-met-leu-phe (fMLP)-activated human neutrophils are chronologically distinct events. Clin Exp Immunol. 1997;110:132–8. doi: 10.1046/j.1365-2249.1997.5051403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty K, Robertie P, Senior RM, et al. Determination of oxidized alpha-1-proteinase inhibitor in serum. J Lab Clin Med. 1982;100:186–92. [PubMed] [Google Scholar]
- 17.Vissers MCM, Winterbourn CC. Myeloperoxidase-dependent oxidative inactivation of neutrophil neutral proteinases and microbicidal enzymes. Biochem J. 1987;245:277–80. doi: 10.1042/bj2450277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean RT, Nick HP, Schnebli HP. Free radicals inactivate human neutrophil elastase and its inhibitors with comparable efficiency. Biochem Biophys Res Commun. 1989;159:821–7. doi: 10.1016/0006-291x(89)90068-5. [DOI] [PubMed] [Google Scholar]
- 19.Test ST, Weiss SJ. Quantitative and temporal characterisation of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984;259:399–405. [PubMed] [Google Scholar]
- 20.Bradova V, Smid F, Ledinova J, et al. Improved one-dimensional thin layer chromatography for the separation of phospholipids in biological material. J Chromatogr. 1990;533:297–9. doi: 10.1016/s0378-4347(00)82217-3. [DOI] [PubMed] [Google Scholar]
- 21.Schrenzel J, Serrander L, Bánfi B, et al. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–7. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 22.Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential: quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J Biol Chem. 1999;274:26098–104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 23.Bánfi B, Schrenzel J, Nüsse O, et al. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–94. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew DP, Wollheim C, Seger RA, et al. Cytosolic free calcium changes induced by chemotactic peptide in neutrophils from patients with chronic granulomatous disease. Blood. 1984;63:231–3. [PubMed] [Google Scholar]
- 25.Herlin T, Borregaard N. Early changes in cyclic AMP and calcium efflux during phagocytosis by neutrophils from normals and patients with chronic granulomatous disease. Immunology. 1983;48:17–26. [PMC free article] [PubMed] [Google Scholar]
- 26.Favre CJ, Nüsse O, Lew DP, et al. Store-operated Ca2+ influx: what is the message from the stores to the membrane? J Lab Clin Med. 1996;128:19–26. doi: 10.1016/s0022-2143(96)90110-9. [DOI] [PubMed] [Google Scholar]
- 27.Montero M, Alvarez J, Garcia-Sanchez J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991;289:761–6. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lew PD, Monod A, Waldvogel FA, et al. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986;102:2197–204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takenawa T, Homma Y, Nagai Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J Immunol. 1983;130:2849–55. [PubMed] [Google Scholar]
- 30.Taub DD, Anver M, Oppenhelm JJ, et al. T lymphocyte recruitment by interleukin-8 (IL-8): IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97:1931–41. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–40. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 32.Belaauoaj A, McCarthy R, Baumann M, et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nature Med. 1998;4:615–8. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 33.De Bruyn EE, Steel HC, Van Rensburg CEJ, et al. The riminophenazines clofazimine and B669 inhibit potassium transport in Gram-positive bacteria by a lysophospholipid-dependent mechanism. J Antimicrob Chemother. 1996;38:349–62. doi: 10.1093/jac/38.3.349. [DOI] [PubMed] [Google Scholar]
- 34.Kondo E, Kanai K. Mechanism of bactericidal activity of lysolecithin and its biological implication. Jap J Med Sci Biol. 1985;38:181–94. doi: 10.7883/yoken1952.38.181. [DOI] [PubMed] [Google Scholar]
- 35.Laine VJ, Grass DS, Nevalainen TJ. Protection by group II phospholipase A2 against Staphylococcus aureus. J Immunol. 1999;162:7402–8. [PubMed] [Google Scholar]
- 36.Kauder E, Kahle LL, Morene H, et al. Leukocyte degranulation and vacuole formation in patients with chronic granulomatous disease of childhood. J Clin Invest. 1968;47:1753–62. doi: 10.1172/JCI105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baehner RL, Karnovsky MJ, Karnovsky ML. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969;48:187–92. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandell GL, Hook EW. Leukocyte function in chronic granulomatous disease of childhood. Am J Med. 1969;47:473–86. doi: 10.1016/0002-9343(69)90231-9. [DOI] [PubMed] [Google Scholar]
- 39.Voetman AA, Weening RS, Hamers MN, et al. Phacocytosing human neutrophils inactivate their own granular enzymes. J Clin Invest. 1981;67:1541–9. doi: 10.1172/JCI110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson R, Goolam Mahomed A, Theron AJ, et al. Effect of rolipram and dibutyryl cyclic AMP on resequestration of cytosolic calcium in FMLP-activated human neutrophils. Br J Pharmacol. 1998;124:547–55. doi: 10.1038/sj.bjp.0701849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewald B, Thelen M, Wymann P, et al. Staurosporine inhibits the respiratory burst and induces exocytosis in human neutrophils. Biochem J. 1989;264:879–84. doi: 10.1042/bj2640879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong K, Kwan-Yeung L, Turkson J. Staurosporine clamps cytosolic free Ca2+ concentrations of human neutrophils. Biochem J. 1992;283:499–505. doi: 10.1042/bj2830499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross AR. Inhibitors of the leucocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med. 1990;8:71–93. doi: 10.1016/0891-5849(90)90147-b. [DOI] [PubMed] [Google Scholar]
- 44.Clark RA, Szot S, Venkatasubramanian K, et al. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980;124:2020–6. [PubMed] [Google Scholar]