Abstract
(MIF) is a broad-spectrum proinflammatory cytokine implicated in human rheumatoid arthritis. The synthesis of MIF by synovial cells is stimulated by glucocorticoids, and previous studies suggest that MIF antagonizes the anti-inflammatory effects of glucocorticoids. This has not been established in a model of arthritis. We wished to test the hypothesis that MIF can act to reverse the anti-inflammatory effects of glucocorticoids in murine antigen-induced arthritis (AIA). Cutaneous DTH reactions and AIA were induced by intradermal injection and intra-articular injection, respectively, of methylated bovine serum albumin in presensitized mice. Animals were treated with anti-MIF MoAbs, recombinant MIF, and/or dexamethasone (DEX). Skin thickness of DTH reactions was measured with callipers and arthritis severity was measured by blinded quantitative histological assessment of synovial cellularity. Cutaneous DTH to the disease-initiating antigen was significantly inhibited by anti-MIF MoAb treatment (P < 0·001). AIA was also significantly inhibited by anti-MIF MoAb (P < 0·02). DEX treatment induced a dose-dependent inhibition of AIA, which was significant at 0·2 mg/kg (P < 0·05). MIF treatment reversed the effect of therapeutic DEX on AIA (P < 0·001). DEX also significantly inhibited DTH reactions (P < 0·05) but rMIF had no effect on this effect of DEX. DTH and AIA are MIF-dependent models of inflammation and arthritis. The reversal of glucocorticoid suppression of AIA by MIF supports the concept that MIF is a counter-regulator of glucocorticoid control of synovial inflammation. Although DTH was observed to be MIF-dependent and glucocorticoid-sensitive, rMIF had no reversing effect on the suppression of DTH by glucocorticoids. This suggests that inflammatory processes in specific tissues may respond differently to MIF in the presence of glucocorticoids.
Keywords: arthritis, glucocorticoids, macrophage migration inhibitory factor
INTRODUCTION
First described simply as an activity, macrophage migration inhibitory factor (MIF) was the first cytokine to be discovered. Since its cloning led to ‘rediscovery’ as a proinflammatory cytokine in the early 1990s [1,2], the range of actions implied by the original nomenclature has been shown to massively underestimate the profile of this cytokine. Within the immune system, MIF is produced by monocyte-macrophages and T cells, is a stimulus to macrophage tumour necrosis factor-alpha (TNF-α) synthesis, and provides an essential contribution to cognate T cell activation [3,4]. In vivo, MIF is a critical factor in phenomena as diverse as cutaneous DTH reactions and endotoxic shock [5,6]. This profile of activities suggested the hypothesis that MIF could be an important cytokine in chronic inflammatory diseases such as rheumatoid arthritis (RA). This has been confirmed in animal models including adjuvant and collagen arthritis, and in recent studies in human RA tissue and cells [7–9]. Results to date indicate an upstream role for MIF in the activation of RA synovial inflammation.
A unique characteristic of MIF is its relationship with glucocorticoids. Unlike other proinflammatory cytokines, the synthesis and release of MIF are induced by low/physiological concentrations of glucocorticoids [9–11]. Moreover, MIF reverses the inhibitory effects of glucocorticoids on monocyte and T cell activation [10–12], suggesting that it may function physiologically as a counter-regulator of glucocorticoid inhibition of inflammation. The glucocorticoid-inducible expression of MIF in RA synovial cells [9], together with the recent description of the induction of matrix metalloproteinases by MIF [13], suggest that MIF may be an important contributor to the failure of low-dose glucocorticoids to completely suppress cartilage degradation and joint erosion in RA.
We sought to investigate whether the glucocorticoid-reversing effects of MIF operated in an in vivo model of arthritis. We initially established that the model of interest was dependent upon MIF and was inhibited by glucocorticoids. The chief finding of this study is that MIF does indeed reverse the effects of therapeutic glucocorticoids on a model of arthritis. This finding is consistent with the hypothesis that MIF is a key counter-regulator of glucocorticoid actions, and implicates it in steroid resistance.
MATERIALS AND METHODS
Antigen-induced arthritis (AIA) was induced in male C57Bl6 mice (9–12 weeks). Groups of at least six animals were used for all studies. Methylated bovine serum albumin (mBSA; 100 μg) (Sigma, Sydney, Australia) in 100 μl Freund's complete adjuvant (Sigma) was injected subcutaneously on each flank and Bordetella pertussis (0·4 × 109 organisms/400 μl saline per mouse) (Bioscientific, Sydney, Australia) was injected intraperitoneally. Antigen challenge was performed 8 days later with 60 μg mBSA/10 μl saline administered by intra-articular injection into the left knee joint. As a control, an equal volume of saline was injected into the right knee joint. All reagents were screened for endotoxin using the Limulus assay (Sigma).
Cutaneous DTH was induced by intradermal injection of 50 μg mBSA/20 μl saline (right footpad) or saline alone in the control left footpad on day 4 post intra-articular antigen challenge. Skin thickness was measured on day 5 using calibrated skin fold callipers, and results expressed as the difference in skin thickness (mm) between mBSA- and saline-injected sites.
Treatment of mice with dexamethasone (DEX; Sigma) 0·02–0·2 mg/kg and rMIF (1 mg/kg) was performed by daily i.p. injection on days 3 and 4 post-antigen challenge. Treatment with anti-MIF MoAb, which recognizes murine MIF [8,14], was performed on days 0, 2 and 4 after antigen challenge. Control mice were treated with isotype-matched IgG1.
Mice were killed on day 5 post-antigen challenge. Serum was obtained from tail vein puncture prior to killing. Synovium from knee joints was collected for frozen section and routine haematoxylin and eosin (H–E) staining as described [7]. Briefly, specimens of whole mouse knee joint were fixed in perodate-lysine-paraformaldehyde (PLP) and decalcified in a solution of 7·5% polyvinylpyrrolidone (PVP; Sigma) and 10% ethylenediamine tetra acetic acid (EDTA; BDH Chemicals, Sydney, Australia). Decalcified joints were embedded in OCT (Tissue Tek, Westhaven, CT) and frozen. Frozen tissue was cut into 7-μ m sections using a cryostat (Reichardt-Jung Cryocut 1800; Nussloch, Germany). Histological counts were obtained by counting cells in two to four 0·2-mm2 areas of the synovium using a graticule. Counts were averaged and results expressed as mean ±s.e.m. cells/0·2 mm2.
RESULTS
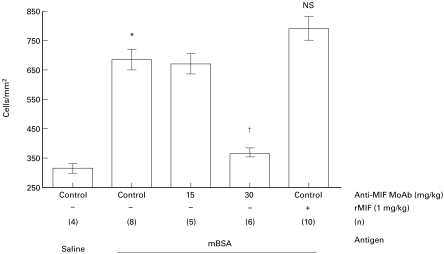
Compared with non-pre-immunized animals (313 ± 34 cells/mm2) or to contralateral saline-injected joints in pre-immunized animals (323 ± 13 cells/mm2), intra-articular injection of mBSA in pre-immunized animals was associated with a significant increase in synovial cellularity (694 ± 55 cells/mm2, P < 0·005) (Figs 1 and 2). Anti-MIF MoAb 30 mg/kg treatment completely prevented AIA (368 ± 24 cells/mm2, P < 0·02) (Figs 1 and 2), while a weaker inhibitory effect of anti-MIF MoAb 15 mg/kg was non-significant (653 ± 70 cells/mm2, NS). All control animals received isotype-matched control mouse IgG in matched concentrations. Administration of rMIF 1 mg/kg induced a trend towards increased severity of AIA (791 ± 69 cells/mm2) which did not reach statistical significance (n = 10) (Fig. 2).
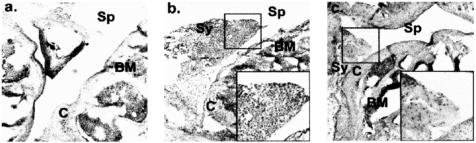
Fig. 1.
Mice received were pre-immunized with methylated bovine serum albumin (mBSA) 8 days prior to intra-articular injection into the knee of (a) saline or (b,c) mBSA. Arthritis on day 5 after intra-articular injection was analysed by histological analysis of H–E-stained decalcified whole joint sections. Main photomicrographs × 50, insets × 200. Compartments of the joint are labelled Sp (space), Sy (synovium), C (cartilage) and BM (bone marrow). Saline and mBSA-injected mice received isotype control IgG. Compared with saline injection (a), injection of mBSA (b) was associated with synovial hyperplasia characterized by a marked infiltration of leucocytes. In contrast, anti-MIF MoAb administration (c) was associated with a marked reduction in synovial hypercellularity.
Fig. 2.
Mice received were preimmunized with methylated bovine serum albumin (mBSA) 8 days prior to injection of mBSA into the knee. Arthritis on day 5 after intra-articular injection was analysed by histological analysis of H–E stained decalcified whole joint sections. Compared with saline-injected joints in pre-immunized mice (saline), mBSA injection in pre-immunized mice was associated with a significant increase in synovial cellularity (cells/mm2, *P < 0·005). Compared with control mice which received isotype control IgG, administration of anti-MIF MoAb (15 mg/kg or 30 mg/kg) on days 0, 2 and 4 was associated with a significant, dose-dependent inhibition of AIA (†P < 0·02). Administration of recombinant MIF 1 mg/kg was associated with a moderate but non-significant increase in synovial cellularity. In each case, n refers to the numbers of animals studied.
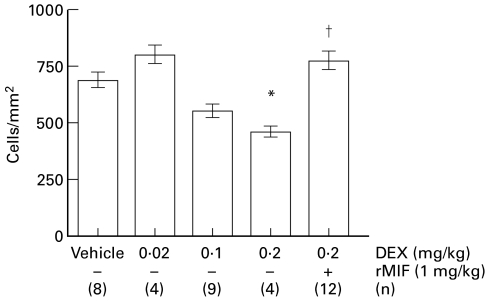
Dexamethasone treatment of AIA was associated with dose-dependent inhibition of arthritis (Fig. 3). All control animals received vehicle (ethanol) in matched concentrations. At a dose of 0·2 mg/kg, the effect of DEX on AIA reached statistical significance (467 ± 23 cells/mm2, P < 0·05) (Figs 3 and 4). Treatment with rMIF 1 mg/kg completely reversed inhibition of AIA by DEX 0·2 mg/kg (780 ± 67 cells/mm2, P < 0·001) (Figs 3 and 4). Indeed, mice treated with rMIF and DEX had AIA severity indistinguishable from mice treated with rMIF alone (P = 0·92).
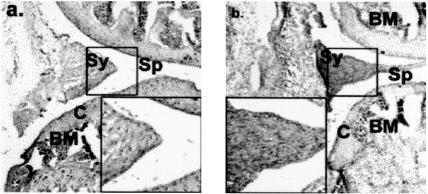
Fig. 3.
Mice with evolving antigen-induced arthritis were treated with dexamethasone (DEX) (a) or DEX plus recombinant MIF (b). Main photomicrographs × 50, insets × 200. Compartments of the joint are labelled Sp (space), Sy (synovium), C (cartilage) and BM (bone marrow). Recombinant MIF treatment was associated with marked reversal of the effects of DEX on synovial hypercellularity.
Fig. 4.
Compared with vehicle treatment, antigen-induced arthritis (AIA) was dose-dependently inhibited by dexamethasone (DEX) 0·02–0·2 mg/kg, and this inhibition reached statistical significance at 0·2 mg/kg (*P < 0·05). AIA inhibition by DEX 0·2 mg/kg was significantly reversed by rMIF 1 mg/kg (†P < 0·001). In each case, n refers to the numbers of animals studied.
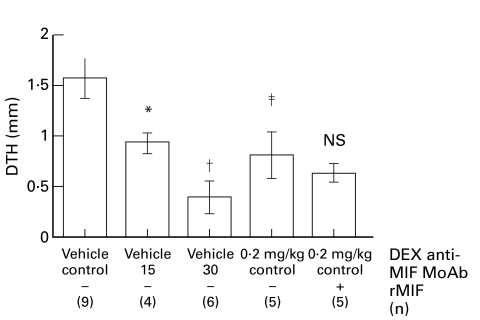
Confirmation of a T cell-mediated immune response to mBSA was sought by eliciting cutaneous DTH reactions. A significant difference in skin thickness between mBSA-injected and saline-injected sites was observed in pre-immunized mice (δ skin thickness 1·6 ± 0·2 mm, P < 0·001) (Fig. 5). Dependence of the DTH response on MIF was investigated by anti-MIF MoAb treatment. Cutaneous DTH reactions were inhibited by anti-MIF MoAb 30 mg/kg (0·4 ± 0·2 mm, P < 0·001, Fig. 5), and this effect was dose-dependent (15 mg/kg anti-MIF MoAb 0·9 ± 0·1 mm, P = 0·054). All control animals received isotype-matched control mouse IgG in matched concentrations. Cutaneous DTH reactions to mBSA were significantly inhibited by DEX 0·2 mg/kg (P < 0·05, Fig. 5). All control animals received vehicle (ethanol) in matched concentrations. Daily treatment with rMIF 1 mg/kg had no significant effect on the inhibition of DTH by DEX (Fig. 5).
Fig. 5.
Cutaneous DTH reactions were induced by intradermal injection in mice pre-immunized with methylated bovine serum albumin (mBSA). Control ice received isotype control IgG and/or vehicle (ethanol). Reactions were measured by callipers and expressed as the mean (± s.e.m.) difference between saline- and mBSA-injected sites in the same animals, in mm. Compared with control IgG, anti-MIF MoAb (15–30 mg/kg) (*P = 0·054, †P < 0·005) dose-dependently inhibited cutaneous DTH. Compared with vehicle, dexamethasone 0·2 mg/kg (DEX) also significantly inhibited DTH (‡P < 0·05). rMIF had no significant effect on the inhibition of DTH by DEX. In each case, n refers to the numbers of animals studied.
DISCUSSION
The relative importance of the various proinflammatory cytokines present in RA synovium is the subject of much study. The aetiology of RA is unknown, but contributions from local/resident fibroblast-like synoviocytes, cells of monocyte-macrophage lineage, and T cells, are known to be of importance. No cytokine previously identified in RA lesions has been shown to be a functionally important product of all these cell types. Recently however, MIF has been shown to be produced by monocyte-macrophages [3,9], T cells [4], and fibroblast-like synoviocytes [9], and the range of biological effects of MIF is both broad and highly relevant to the pathology of RA. For example, MIF is essential for the activation of both innate and cognate immune responses in vivo, exemplified by endotoxic and septic shock, and by cutaneous DTH responses and T cell activation by recall antigens, respectively [5,6,15]. In support of these observations, endotoxin-induced lethality and monocyte TNF-α production are reduced markedly in mice with absent MIF expression due to targeted gene disruption [16]. We have also shown reduced mortality in adrenalectomized rats with adjuvant-induced arthritis treated with anti-MIF MoAb [11]. Anti-MIF antibodies are protective in models of Gram-positive bacterial toxic shock [17], and are protective against lethality from bacterial sepsis even in TNF-α−/− mice, again supporting the idea that MIF occupies an ‘upstream’ position in the cytokine hierarchy of innate immunity [15].
The hypothesis that MIF is a central cytokine in the development and/or persistence of RA is supported more directly by recent data. Antagonism of MIF in vivo significantly suppresses the collagen-induced and adjuvant-induced arthritis models of RA [7,8]. We recently reported the expression of MIF in human RA synovial tissues and in both freshly isolated and cultured RA synovial cells [9]. MIF has been implicated in neovascularization and endothelial cell proliferation in tumours [18,19], suggesting it may have such a role in synovial pathology in RA. MIF released by synoviocytes is capable of inducing monocyte TNF-α production, suggesting that MIF may have a significant ‘upstream’ role in the RA cytokine hierarchy [9]. In terms of direct effects on synovial cells, few data are available, but recently the induction of synoviocyte phospholipase A2 (PLA2) and cyclooxygenase-2 expression has been demonstrated [20]. Lastly, the recent description of the induction of synoviocyte matrix metalloproteinases by MIF [13] suggests MIF may be a participant in cartilage and bone erosion in RA.
In the current study we show for the first time that development of the AIA model of RA is dependent on MIF. AIA was chosen as a model for these experiments because of the opportunity it provides to examine a restricted set of immune phenomena. In contrast to other models of RA, AIA is induced by T cell-cognate immunity to a known exogenous antigen. By comparison, while the contribution of T cells to rat adjuvant arthritis is clear, adjuvant arthritis is also significantly dependent upon the contribution of macrophages, neutrophils and their products [21–23]. In the current study, we demonstrate prevention of synovial hypercellularity by the administration of anti-MIF MoAb. The administration of recombinant MIF also exacerbated arthritis severity in this model, although the effect did not reach statistical significance. Anti-MIF MoAbs have now been shown to be effective in adjuvant-induced, collagen-induced, and antigen-induced arthritis models, suggesting the potential for effectiveness in RA where similarities to all three of these models exist.
A unique aspect of the biology of MIF is its relationship with glucocorticoids. Previous studies have suggested that MIF is able to counteract the inhibitory effects of glucocorticoids on phenomena including T cell activation and macrophage TNF-α production [4,10,24]. Glucocorticoids are widely used to treat RA, but despite a broad range of anti-inflammatory and immune suppressive effects, glucocorticoids do not cure RA and there is debate about their effect on cartilage and bone erosions. We have recently shown that glucocorticoids induce the synthesis of MIF by RA synoviocytes in vitro [9], and that endogenous adrenal glucocorticoids support synovial MIF production in adjuvant arthritic rats in vivo [11]. A key aim of the current study was therefore to investigate whether the reported interaction between MIF and glucocorticoids could be reproduced in arthritis. We report that MIF does indeed reverse the effects of therapeutic glucocorticoids in vivo in AIA. This is consistent with the hypothesis that MIF functions in vivo as a counter-regulator of endogenous glucocorticoid inhibition of immune function. Given the induction of MIF synthesis by glucocorticoids in human RA synoviocytes, it is conceivable that low-dose glucocorticoid administration in RA is associated with increases in tissue MIF. As MIF has been reported to induce synoviocyte matrix metalloproteinases [13], this may lead to abrogation of some of the potential benefits of glucocorticoid therapy on joint destruction.
The mechanism of the reversal of glucocorticoid effects by MIF remains conjectural. No receptor has been identified for MIF, and consequently the intracellular signal transduction pathways used by MIF are not well understood. Mitchell et al. [25] have shown the involvement of p44/ERK MAP kinase pathway in the activation of PLA2 by MIF in vitro. Onodera et al. [13] have reported increased c-jun and c-fos mRNA in MIF-treated synoviocytes, implying activation of separate MAP kinase-dependent signalling pathways. No study has reported activation of nuclear factor κ B (NF-κ B) by MIF, and preliminary studies indicate a lack of induction of NF-κ B by MIF in human synoviocytes (Morand et al., unpublished observations). Of note, NF-κ B-mediated cell activation is highly sensitive to glucocorticoid inhibition [26,27], whereas MAP kinase-mediated is far less so [28]. Exploration of the signal transduction events operative in the effects of MIF on glucocorticoid-induced immunosuppression may provide an explanation for the glucocorticoid antagonism properties of MIF.
In the current study we observed a marked inhibitory effect of MIF immunoneutralization on cutaneous DTH reactions, confirming the essential role of MIF previously shown in experiments in which tuberculin was the disease-initiating antigen [5]. In the current experiments, DEX was also effective in suppressing DTH. In contrast to the results in the joint, however, inhibition of DTH by DEX was not reversed by rMIF. We have previously shown that profound inhibition of rat adjuvant arthritis synovial inflammation by inhibition of nitric oxide synthesis or neutrophil depletion is unaccompanied by effects on cutaneous DTH [22,23], suggesting that T cell-initiated joint inflammation and DTH are regulated independently. The current study demonstrates that even in an animal responding in two different sites to the same antigen, differences in the regulatory response to glucocorticoids and MIF can be demonstrated. The difference between the effects of MIF in AIA and in DTH underlies the importance of confirming its actions in models of arthritis rather than relying on data from other models of immune responsiveness.
In conclusion, the current study extends to AIA the observation that antagonism of MIF is associated with significant inhibition of arthritis severity. Moreover, the ability of rMIF to antagonize the effects of glucocorticoids is shown for the first time in a model of arthritis. Taken with the recent demonstration that low concentrations of glucocorticoids induce MIF in human synoviocytes, these data suggest that anti-MIF therapies may be synergistic or additive to the effects of glucocorticoids in RA. The lack of effect of rMIF on glucocorticoid suppression of cutaneous DTH, despite the demonstration of the MIF-dependence of this lesion, strongly supports the contention that different tissues respond differently to the various molecules that control the inflammatory response. Further studies are required to establish the mechanisms of the MIF–glucocorticoid interaction.
Acknowledgments
Supported by the National Health and Medical Research Council, Arthritis Foundation of Australia, and Royal Australasian College of Physicians, Australia.
REFERENCES
- 1.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144–55. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 2.Bucala R. MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996;10:1607–13. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacher M, Metz CN, Calandra T, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhagen J, Bacher M, Calandra T, et al. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–82. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 7.Leech M, Metz CN, Santos LL, et al. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 1998;41:910–7. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Mikulowska A, Metz CN, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–7. [PubMed] [Google Scholar]
- 9.Leech M, Metz CN, Smith M, et al. Macrophage migration inhibitory factor (MIF) in rheumatoid arthritis: evidence for pro-inflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999;42:1601–8. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 11.Leech M, Santos LL, Metz C, Holdsworth SR, Bucala R, Morand EF. Control of macrophage migration inhibitory factor (MIF) by endogenous glucocorticoids in rat adjuvant arthritis. Arthritis Rheum. 2000;43:827–33. doi: 10.1002/1529-0131(200004)43:4<827::AID-ANR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Calandra T, Bucala R. Macrophage migration inhibitory factor—a counter-regulator of glucocorticoid action and critical mediator of septic shock. J Inflammation. 1996;47:39–51. [PubMed] [Google Scholar]
- 13.Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000;275:444–50. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin MF, Bucala R. Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF) J Immunol. 1995;154:3863–70. [PubMed] [Google Scholar]
- 15.Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nature Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 16.Bozza M, Satoskar AB, Lin G, et al. Targeted disruption of Migration Inhibitory Factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–6. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA. 1998;95:11383–8. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–91. [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa H, Nishihira J, Sato Y, et al. An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine. 2000;12:309–14. doi: 10.1006/cyto.1999.0562. [DOI] [PubMed] [Google Scholar]
- 20.Sampey AV, Hall P, Bucala R, Morand EF. Macrophage migration inhibitory factor (MIF) activation of rheumatoid synoviocytes. Arthritis Rheum. 1999;42:S283. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 21.Kinne RW, Schmidt CB, Buchner E, Hoppe R, Nurnberg E, Emmrich F. Treatment of rat arthritides with clodronate-containing liposomes. Scand J Rheumatol. 1995;101:91–97. doi: 10.3109/03009749509100907. [DOI] [PubMed] [Google Scholar]
- 22.Santos LL, Morand EF, Hutchinson P, Boyce NW, Holdsworth SR. Anti-neutrophil monoclonal antibody therapy inhibits the development of adjuvant arthritis. Clin Exp Immunol. 1997;107:248–54. doi: 10.1111/j.1365-2249.1997.263-ce1154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos LL, Morand EF, Holdsworth SR. Suppression of adjuvant arthritis and synovial macrophage inducible nitric oxide by N-iminoethyl-l-ornithine, a nitric oxide synthase inhibitor. Inflammation. 1997;21:299–311. doi: 10.1023/a:1027397816209. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly SC, Haslett C, Reid PT, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nature Med. 1997;3:320–3. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem. 1999;274:18100–6. doi: 10.1074/jbc.274.25.18100. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 27.Wissink S, van Heerde EC, vand der Burg B, van der Saag PT. A dual mechanism mediates repression of NF-kappaB activity by glucocorticoids. Mol Endocrinol. 1998;12:355–63. doi: 10.1210/mend.12.3.0081. [DOI] [PubMed] [Google Scholar]
- 28.Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–82. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]