Abstract
We studied the relationship between the HLA specificities associated with multiple sclerosis (MS) susceptibility in southern Italy and the reactivity of the human myelin basic protein (hMBP) immunogenic peptides 84–98 and 143–168, using short-term T-cell lines established from 9 MS patients and from 8 healthy individuals. In our population, DR15 was significantly associated with MS (34·9% in MS versus 13·7% in healthy controls, P < 0·05). This result is in agreement with the association found in northern Europe, but not with data obtained in a population from the island of Sardinia (Italy). In MS patients the frequency of reactive T-cell lines (TCL), tested for fine specificity against the immunodominant hMBP peptides 84–98 and 143–168, was increased for the hMBP 143–168 peptide (P < 0·05) but not for the 84–98 peptide. Although this reactivity was higher in DR15+ MS patients than in DR 15− MS patients, it seemed not to be associated with DR15 specificity in the MS population. Furthermore, there were no significant differences in frequency of reactive TCL to hMBP peptide 84–98 in DR15-positive or DR15-negative MS patients. Consequently, it appears that peptide 84–98, considered as a relevant autoantigen, is not implicated in the pathogenesis of MS in our population from southern Italy.
Keywords: HLA typing, T lymphocytes, myelin basic protein, multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by focal T-cell, plasma cell and macrophage infiltrates, leading to demyelination and progressive loss of neurological functions. The prevalence of the disease is about 1 : 1000 in the northern hemisphere (Europe and North America) and decreases notably in southern regions [1,2]. As in other autoimmune diseases, genetic factors appear to play a role in MS susceptibility: indeed, the degree of concordance for MS in monozygotic twins compared with dizygotic twins (30% and 3%, respectively) indicates a genetically determined predisposition for the disease [3–5]. In particular, the association of MS with the HLA class II-DRB1‐1501 allele and the associated allele DQB1‐0602 are significantly increased in northern European patients [6]. Notably, other MHC class II antigens were found to be more frequent in MS patients from the Italian island of Sardinia [7], Arabia (DR4) [8] and Japan (DR6) [9]. The differences in allele frequencies show that genetic factors contribute to MS pathogenesis, but also indicate that factors different from HLA, such as the environment, infections and pathogen exposure play a role in susceptibility to the disease.
T-cell responses to myelin basic protein (MBP) and proteolipid protein are thought to have pathological relevance in MS [10]. T-cell reactivity to MBP is preferentially directed against two immunodominant regions, corresponding to human MBP residues 84–98 and 143–168. Peptide 84–98 induces a higher frequency of reactivity than does peptide 143–168. The analysis of TCL reactivity in MS patients showed that MHC class II DR15 specificity is the main element of restriction for these two peptides [11–13]. Taken together, these data suggest that activation of T lymphocytes recognizing human MBP-derived peptides can trigger the inflammatory process.
Here we examine the genetic factors associated with the risk of developing MS and report the results of the in vitro analysis of T-cell reactivity against the two human MBP immunogenic peptides, i.e. 84–98 and 143–168. In Italy, conclusive data are available only for the population of the island of Sardinia, where the DR4 allele is associated with MS. Consequently we examined the MS population living in the District of Naples (Campania Region, southern Italy), given the presumably different genetic background of the two populations. As found in other Caucasian populations [6], in our group the DR15 allele was associated with MS. Furthermore, the DR4 allele, which is reported to be more frequent in the MS population of Mediterranean countries [14], was not associated with MS in our population. Lastly, we established TCL specific for human MBP and its immunodominant epitopes 84–98 and 143–168 from MS patients and healthy controls to analyse and identify the immunogenic peptide, if any, associated with the susceptibility gene in the Campanian population.
MATERIALS AND METHODS
Patients and healthy donors
Forty-three patients with MS, diagnosed according to the criteria of Poser et al. [15], and 80 healthy individuals from the Campania Region for at least two generations participated in this study. Thirty-one patients had relapsing/remitting, 10 had secondary progressive and 2 had primary progressive MS. At the onset of the study, none of the patients had received steroid, immuno-suppressive or interferon-β therapy for at least 6 months prior to enrolment in the study. Approval of the local ethical committee and informed consent of the patients were obtained before the enrolment in the study.
PCR-specific sequence primers (PCR-SSP) HLA typing
Medium-low resolution PCR-SSP HLA molecular typing was performed on the studied populations according to the Olerup and Zetterquist method [16]. Briefly, blood was collected from donors and patients in the morning after an overnight fast, in EDTA-sterile vacutainers. After a Ficoll-Hypaque-gradient, peripheral blood mononuclear cells (PBMC) were obtained and washed extensively with phosphate buffer solution. Then, 5–10 × 106 cells were collected for DNA extraction with the DNAzol Extraction Kit (Gibco Life Technologies, Milan, Italy). After DNA purification, PCR-SSP HLA molecular typing was performed in a 10-µ l final reaction volume using primers for all the HLA-DR specificities with the human growth hormone (hGH) as internal control, synthesized and purified by Primm srl. (Milan-Italy). PCR conditions were: 5 min 94°C; 10 cycles 1 min 94°C, 30 s 60°C; 20 cycles 1 min 94°C, 30 s 58°C, 1 min 72°C; 7 min 72°C; 12·5 pmoles of each specific primer were used; hGH was 5-fold less concentrated; 0·5 units of DNA-Taq polymerase (Roche, Milan, Italy) were added to each reaction tube. dNTP mixes were at a 200 µm final concentration. A 9700-Perkin Elmer thermal cycler was used in this procedure (Norwalk, CT).
Establishment of hMBP-specific short-term T-cell lines
MBP-specific T-cell lines were generated from PBMC as previously described [11]. Briefly, PBMC from nine MS patients and eight controls were separated by Ficoll-Hypaque density gradient and plated in 96-well round-bottom plates. For each patient and control, 250 wells were plated with 2 × 105 PBMC and cultured for 13 days with hMBP (10 µg/ml) in media consisting of RPMI 1640, 10% heat-inactivated pooled human serum, 1% penicillin/streptomycin and 2% glutamine. On day 4 and then every 3–4 days thereafter, 10 IU/well of human recombinant interleukin-2 (rIL-2) (Roche) were added to the culture. On day 10 the human rIL-2 was added for the last time and on day 13 an aliquot of each T-cell line was extensively washed, plated in fresh medium for an overnight period and then analysed for reactivity to hMBP by replacing 10 000 cells from the T-cell line with 20 000 irradiated autologous PBMC as antigen-presenting cells in duplicate for 72 h in a round-bottom 96-well microtitre plate, and pulsing with (3H)-thymidine during the last 18 h of culture. Lines positive for proliferation to hMBP defined as showing both a stimulation index >3 and a Δ CPM [(CPM T cell line ±APC pulsed with antigen) – (CPM T cell line ±APC alone)] > 500 were retested 72 h later for reactivity to the immunodominant hMBP-peptides 84–98 and 143–168 (concentration: 10 µ g/ml antigen). All the background values were subtracted from those of antigen-specific proliferation and the range of the stimulation indexes was between 3 and 10. To verify the peptide specificity we resynthesized and tested the peptides twice for each individual. There were no differences between the two batches.
Preparation and purification of antigens
Human MBP was purified in the water-soluble, lipid-free form, according to Deibler et al. [17,18]. MBP peptides were synthesized by Primm srl using the solid phase method and were purified by HPLC.
T-cell proliferation assays
1 ×104 cells were incubated with 2 × 104 irradiated autologous peripheral blood lymphocytes (PBL) per well. Triplicate cultures were set up without antigen as control, with 10 µ g/well of hMBP or with 10 µ g/well of hMBP synthetic peptides. After 3 days of culture, 1 µ Ci of 3H thymidine (40 Ci/mmol; Amersham, Bucks., UK) was added per well, then the cells were harvested 18 h later and thymidine incorporation measured by scintillation counting.
Statistical analysis
Results were compared by using the chi-square test and the Fischer's exact test when appropriate. Relative risks were calculated as odds ratios according to Woolf's formula [19]. A P-value of <0·05 was considered as significant. The percentage of MBP-reactive lines was calculated separately per each subject. Furthermore, hMBP-reactive TCL were subsequently analysed for the specificity of each peptide (84–98 and 143–168) and the total percentage of reactivity was calculated.
RESULTS
HLA-DR15 is associated with MS in the southern Italian population (Campania Region)
The frequencies of HLA-DR specificities in the MS patients and the healthy controls are shown in Table 1. We identified the DR specificity in 43 MS-affected patients and in 80 healthy donors, all from the Campania Region of southern Italy, by PCR-SSP low-medium resolution molecular typing. HLA-DR15 specificity was significantly associated with MS: 15/43 patients (34·9%) versus 11/80 controls (13·7%). The relative risk was 3·3 and the P < 0·05, in agreement with northern European and northern American population frequencies [1]. Furthermore, we confirm [20,21] a decreased trend in the frequency of HLA DR7 specificity in MS patients: 7/43 patients (16·3%) versus 23/80 controls (28·7%), but the difference was not significant (P < 0·05). Conversely, the HLA DR4 allele, alleged to be more frequent in the MS population of Mediterranean countries, was not associated with MS in our Campania population.
Table 1.
Distibution of DRB1 specificities in MS patients and controls
| Controls (n = 80) | Patients (n = 43) | |||||
|---|---|---|---|---|---|---|
| DRB1 | No· | % | No· | % | Rel· risk | P |
| 11 | 33 | 41·2 | 18 | 41·9 | – | – |
| 15 | 11 | 13·7 | 15 | 34·9 | 3·3 | <0·05 |
| 7 | 23 | 28·7 | 7 | 16·3 | – | NS |
| 4 | 10 | 12·5 | 7 | 16·3 | – | NS |
| 16 | 7 | 8·7 | 6 | 13·9 | – | – |
| 17 | 8 | 10 | 9 | 20·9 | – | – |
| 13 | 10 | 12·5 | 2 | 4·6 | – | – |
| 14 | 10 | 12·5 | 3 | 7 | – | – |
| 1 | 17 | 21·2 | 4 | 9·3 | – | – |
| 8 | 3 | 3·75 | 3 | 7 | – | – |
| 10 | 2 | 2·5 | 3 | 7 | – | – |
| 12 | 4 | 5 | 2 | 4·6 | – | – |
HMBP-specific short-term T-cell lines: frequency, fine specificity for immunogenic peptides and HLA–DR15 association in MS patients and controls
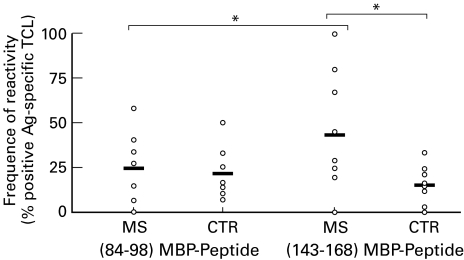
Out of a total of 1946 wells seeded from PBMC obtained from 9 patients, 91 (4·7%) responded to hMBP in the proliferation assay with a stimulation index >3-fold the basal response with medium alone (Table 2). The percentage of hMBP-reactive lines was calculated separately for each subject by dividing the number of hMBP-reactive lines by the total number of lines. The percentage of hMBP-positive wells was slightly higher in healthy donors (7%) than in MS patients (4·7%). A proliferative response to hMBP was confirmed in 58 out of 91 lines, that were positive in the initial proliferation screening. These 58 lines of hMBP-specific TCL were tested for fine specificity against the immunogenic epitopes 84–98 and 143–168. The proportion of 143–168-specific TCL was higher in MS patients than in healthy controls: 20/58 (34·5%) versus 11/89 (12·3%),P < 0·05 (Table 2 and Fig. 1). The percentage of reactivity to the other immunogenic peptide (84–98) was slightly increased, albeit to a lesser degree, in patients versus controls: 14/58 (24·1%) versus 16/89 (18·0%) (Table 2 and Fig. 1).
Table 2.
Percentage of hMBP-, (84–98)MBP-peptide and (143–168)MBP-peptide reactive TCLs in MS paptients and controls
| MBP | (84–98)MBP-peptide | (143–168)MBP-peptide | |||||
|---|---|---|---|---|---|---|---|
| HLA-DR | No· positive | % positive | No· positive | % positive | No· positive | % positive | |
| Patients | |||||||
| MS1 | 17 | 17/200 | 8·5 | 3/11 | 27·3 | 5/11 | 45·4 |
| MS2 | 7/16 | 11/250 | 4·4 | 1/7 | 14·2 | 0/7 | 0·0 |
| MS3 | 14/17 | 27/250 | 10·8 | 1/16 | 6·2 | 4/16 | 35·0 |
| MS4 | 103/13 | 6/250 | 2·4 | 2/5 | 40·0 | 1/5 | 20·0 |
| MS5 | 1/7 | 1/250 | 0·4 | 0/1 | 0·0 | 1/1 | 100·0 |
| MS6 | 7/16 | 10/218 | 44·6 | 4/7 | 57·1 | 2/7 | 28·6 |
| MS7 | 15/16 | 6/200 | 3·0 | 1/3 | 33·3 | 2/3 | 66·6 |
| MS8 | 15/17 | 8/80 | 6·2 | 0/4 | 0·0 | 1/4 | 25·0 |
| MS9 | 103/15 | 5/250 | 2·0 | 2/5 | 40·0 | 4/5 | 80·0 |
| Total | – | 91/1946 | 4·7* | 14/58 | 24·1† | 20/58 | 34·5‡ |
| Controls | |||||||
| C1 | 7/16 | 10/250 | 4·0 | 2//8 | 33·0 | 1/8 | 12·5 |
| C2 | 4/9 | 31//250 | 12·4 | 3/28 | 10·7 | 1/28 | 3·5 |
| C3 | 11 | 15/250 | 6·0 | 3/12 | 25·5 | 0/12 | 0·0 |
| C4 | 10/17 | 7/250 | 2·8 | 1/6 | 16·6 | 2/6 | 33·3 |
| C5 | 7/17 | 18/250 | 7·0 | 3/6 | 50·0 | 1/6 | 16·6 |
| C6 | 15/16 | 19/63 | 30·0 | 1/7 | 14·2 | 1/7 | 14·2 |
| C7 | 9/15 | 11/280 | 3·9 | 2/8 | 25·0 | 2/8 | 25·0 |
| C8 | 15/18 | 17/218 | 7·8 | 1/14 | 7·1 | 3/14 | 21·4 |
| Total | – | 128/1811 | 7·0 | 16/89 | 18·0 | 11/89 | 12·3 |
NS versus MBP control
NS versus (84–98) control
P < 0·05 versus (143–168) control; P < 0·05 versus (84–98) MS
Fig. 1.
MS patients display increased percentage of reactivity against MBP-peptide (143–168) when compared with MBP-peptide (84–98) and healthy controls. Each dot represents the percentage of positive antigen-reactive TCL measured each patient (see Table 2). *P < 0·05.
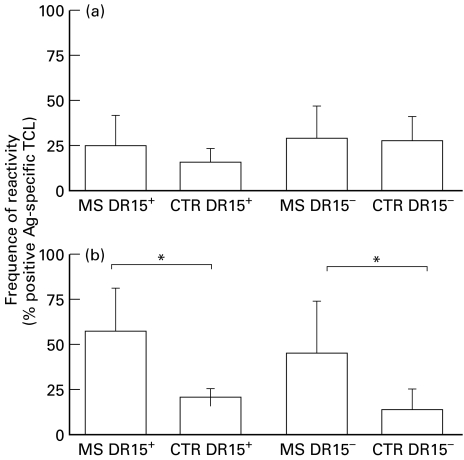
In the Campania population, as well as in northern Europe, the HLA-DR15 specificity was associated with MS. We tested our TCL for reactivity against the immunogenic peptides in patients and controls, dividing the groups according to the presence or absence of the DR15 allele. Our aim was to verify whether predominant responses to different MBP regions were associated with the presence of the DR15 allele (Fig. 2). The percentage of TCL reactivity toward residues 84–98 did not differ significantly between DR15-negative patients and controls: MS DR15-negative, 23·4% versus CTR DR15-negative, 20·0% (Fig. 2). The DR15-positive patients had a little increase in reactivity with respect to DR15-positive controls: MS DR15-positive, 25·0% versus CTR DR15-positive, 13·8% (Fig. 2). Conversely, the percentage of TCL reactivity against the immunogenic peptide 143–168 was significantly increased in DR15-positive patients as compared with controls: MS DR15-positive, 58·3% versus CTR DR15-positive, 20·7%, P < 0·05 (Fig. 2). Similarly, when peptide 143–168 was tested in DR15-negative subjects, the frequency of TCL reactivity was significantly higher in patients than in controls (MS DR15-negative, 27·6% versus CTR DR15-negative, 8·3%, P < 0·05) (Fig. 2). The analysis of reactivity among the group of DR15-negative/DR15-positive patients showed no significant difference in the percentage of TCL raised against peptide 84–98 (23·4% versus 25·0%), whereas the reactivity against peptide 143–168 was significantly increased in DR15-positive patients versus DR15-negative patients (58·3% versus 27·6%, P < 0·05) (Fig. 2). Similarly, we observed the same trend of TCL reactivity against peptide 143–168 in DR15-positive and DR15-negative controls (20·7% versus 8·3%).
Fig. 2.
Frequency of hMBP-peptide-reactive TCL in DR15− and DR15+ MS patients and controls. The reactivity against (a) (84–98) and (b) (143–168) MBP-peptides was evaluated in patients and controls according to the HLA-DR15 allele presence. A significant increase in reactivity toward the (143–168) MBP-peptide was observed in MS patients, when compared with controls, independently by the presence of the DR15 allele. * P < 0·05.
DISCUSSION
Epidemiological studies indicate that MS is a multifactorial disease caused by environmental factors acting on genetically susceptible individuals [22]. Genomic screenings highlight the complexity of MS genetics and suggest that more than one locus contributes significantly to the familial risk. Association of MS with the established Caucasian HLA haplotype (DR15-DQ6) [23] is the most consistent finding, except in the Mediterranean area where two haplotypes (DR4-DQ3 and DR3-DQ2) were found to be associated with the disease [14,24]. Our results contribute a new point to the immunogenetic map of MS in Europe, confirming in the Campania region the association between DR15 and MS in Caucasians. In contrast, DR4 specificity, believed to be more frequent in the MS population of the Mediterranean, does not appear to be associated with MS in our district. These results suggest that the association of the DR4 allele with MS as observed in the Sardinian population reflects a genetic isolate not shared by other Italian populations [24], and that genetic, historical and enviromental factors determine the notable difference in MS risk between Sardinia and the rest of Italy [25]. Differently, the Campania Region is a heterogenous community with a more variable genetic and historical background as compared with Sardinia. In our region the DR association with MS, similar to that observed in Northern Europe, could be explained partially considering the characteristic historical events. In the past, the Campania area has been a very complex racial ‘crucible’ due to the different foreign dominations by the French and the Spanish, which have been alternated for several centuries. These observations point out that racial susceptibility, together with the enviromental factors, determines the distribution of the disease and the specific DR association in the various geographical regions [25].
In an attempt to understand the mechanisms of MHC class II-mediated susceptibility in our region, we analysed the immunodominant epitopes of target autoantigens and the responsive T-cell populations. We examined the T-cell response to hMBP in 9 patients and in 8 healthy individuals, and found that MS patients and controls had a comparable reactivity to hMBP, with a slightly higher frequency of responding TCL in controls (Table 2). The presence of MBP-specific T cell reactivity in healthy donors is a common observation as demonstrated by different authors [11,12,26,27].What induces this autoreactivity and what is its function in the induction or regulation of autoimmunity has been debated. The recent observation that immunological relevant myelin proteins were detected in lymphoid tissues of healthy subjects [28–30] has led to the reevaluation of immune tolerance to myelin antigens. In this context both the absence of costimulation molecules [31], as well as a T cell dependent regulatory activity, could contribute to modulate and mantain a MBP-specific tolerance status.
Earlier reports on MS and control populations suggested that some portions of the hMBP molecule are more immunogenic than others: aa 87–106 and 154–172 [32], 84–102 and 143–168 [33], 84–102 and 149–171 [34]. Based on these data, the middle portion of the molecule and the C-terminal end emerged as distinct predominant regions. These epitopes may be relevant to the pathogenesis of MS since both peptides can be presented by DR15 molecules of the disease-associated haplotype [35]. To correlate the reactivity of immunogenic peptides 84–98 and 143–168 with the observed increased frequency of DR15 specificity in the Campania population, we studied autoreactive T-cell responses in MS patients and healthy controls expressing the MS-associated HLA DR15 allele. We found that the immunodominant epitope 143–168 had a significantly higher reactivity in MS patients than in controls. Although this increased reactivity in DR15-positive patients could have suggested that this epitope was a relevant autoantigen in MS in the Campania region, the frequency of 143–168 peptide-specific TCL was also increased in DR15-positive controls (Fig. 2). In addition, analysing the behaviour of hMBP (143–168) peptide-specific TCL reactivity within each group of MS patients and controls, we observed that the presence of DR15 molecules in both patients and controls seems to favour the response of TCL to peptide 143–168 in the Campania population. These findings seem to suggest that the hMBP peptide 143–168 is not associated with a specific DR15 allele in MS patients and probably not directly involved in the pathogenesis of MS, but is rather related to an epitope-spreading mechanism in our population. Furthermore, the percentage of reactivity of TCL to hMBP-residues 84–98 was higher in DR15-positive MS patients than in DR15-positive controls. In addition, peptide 84–98 was recognized equally well by TCL from DR15-positive and DR15-negative MS patients. These findings seem to suggest that peptide 84–89 does not play a direct role as an autoantigen at least in our examined patient cohort.
Acknowledgments
Purified hMBP was obtained from Paolo Riccio, University of Basilicata, Potenza, Italy. This work was supported by grants from the Associazione Italiana Sclerosi Multipla (AISM). We thank Drs G. Ruggiero and G. Terrazzano for critical reading of the manuscript.
REFERENCES
- 1.Robertson NP, Fraser M, Deans J, Clayton D, Walker N, Compston DAS. Age adjusted recurrence risks for relatives of patients with multiple sclerosis. Brain. 1996;119:449–55. doi: 10.1093/brain/119.2.449. [DOI] [PubMed] [Google Scholar]
- 2.Dean G, Bhigjee AIG, Bill PLA, et al. Multiple sclerosis in black South Africans and Zimbabweans. J Neurol Neurosurg Psych. 1994;57:1064–9. doi: 10.1136/jnnp.57.9.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebers GC, Bulman DE, Sadovnick AD, et al. A population-based study of multiple sclerosis in twins. N Engl J Med. 1986;315:1638–41. doi: 10.1056/NEJM198612253152603. [DOI] [PubMed] [Google Scholar]
- 4.Sadovnick AD, Armstrong H, Rice GPA, et al. A population based study of multiple sclerosis in twins: updated. Ann Neurol. 1993;33:281–5. doi: 10.1002/ana.410330309. [DOI] [PubMed] [Google Scholar]
- 5.Ebers GC, Sadovnick AD, Rische NJ the Canadian Collaborative Study Group. A genetic basis for familial aggregation in MS. Nature. 1995;377:150–1. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 6.Francis DA, Thompson AJ, Brookes P, et al. Multiple sclerosis and HLA. is the susceptibility gene really HLA-DR or DQ? Hum Immunol. 1991;32:119–24. doi: 10.1016/0198-8859(91)90108-l. [DOI] [PubMed] [Google Scholar]
- 7.Marrosu MG, Murru MR, Costa G, et al. Multiple sclerosis in Sardinia is associated and in linkage dysequilibrium with HLA-DR3 and -DR4 alleles. Am J Hum Genet. 1997;61:454–7. doi: 10.1016/S0002-9297(07)64074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaqub BA, Daif AK. Multiple sclerosis in Saudi Arabia. Neurology. 1988;328:621–5. doi: 10.1212/wnl.38.4.621. [DOI] [PubMed] [Google Scholar]
- 9.Naito S, Kuroiwa Y, Itoyama T, et al. HLA and Japanese MS. Tissue Antigens. 1978;12:19–24. [PubMed] [Google Scholar]
- 10.Hafler DA, Benjamin DS, Burks J, Weiner HL. Myelin basic protein and proteolipid protein reactivity of brain and cerebrospinal fluid derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1998;139:68–72. [PubMed] [Google Scholar]
- 11.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 12.Martin RD, Jarequemada D, Flerlage M, et al. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990;145:540–8. [PubMed] [Google Scholar]
- 13.Zhang JW, Chou GA, Hashim GA, Medaer R, Raus JCM. Preferential peptide and HLA restriction of MBP specific cell clones derived from MS patients. Cell Immunol. 1990;129:189–98. doi: 10.1016/0008-8749(90)90197-y. [DOI] [PubMed] [Google Scholar]
- 14.Coraddu F, Reyes-Yanez MP, Parra A, et al. HLA associations with multiple sclerosis in the Canary Islands. J Neuroimmunol. 1998;87:130–5. doi: 10.1016/s0165-5728(98)00074-5. [DOI] [PubMed] [Google Scholar]
- 15.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 16.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 17.Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–65. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 18.Deibler GE, Boyd LF, Kies MW. Proteolytic activity associated with purified myelin basic protein. In: Alvord EC Jr, Kies MW, Suckling AJ, editors. Experimental Allergic Encephalomyelitis: a Useful Model for Multiple Sclerosis. New York: Alan R. Liss; 1984. pp. 249–56. [Google Scholar]
- 19.Woolf B. On estimating the relation between blood group and disease. Am Hum Genet. 1955;9:251–6. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 20.Madigand M, Oger JJ, Fauchet R, Sabouraud O, Genetet B. HLA profiles in multiple sclerosis suggest twoforms of disease and the existence of protective haplotypes. J Neurol Sci. 1982;53:519–29. doi: 10.1016/0022-510x(82)90248-9. [DOI] [PubMed] [Google Scholar]
- 21.Spurkland A, Ronningen KS, Vandvik B, Thorsby E, Vartdal F. HLA-DQA1 and HLA-DQB1 genes may jointly determine susceptibility to develop multiple sclerosis. Hum Immunol. 1991;30:69–75. doi: 10.1016/0198-8859(91)90073-i. [DOI] [PubMed] [Google Scholar]
- 22.Hogancamp WE, Rodriguez M, Weinshenker BG. The epidemiology of multiple sclerosis. Mayo Clin Proc. 1997;72:871–8. doi: 10.4065/72.9.871. [DOI] [PubMed] [Google Scholar]
- 23.Haines JL, Terwedow HA, Burgess K, et al. Linkage of the MHC to familial multiple sclerosis suggests genetic heterogeneity. Hum Mol Genet. 1998;7:1229–34. doi: 10.1093/hmg/7.8.1229. [DOI] [PubMed] [Google Scholar]
- 24.Marrosu MG, Murru MR, Costa G, Murru R, Muntoni F, Cucca F. DRB1-DQA1-DQB1 loci and multiple sclerosis predisposition in the Sardinian population. Hum Mol Genet. 1998;7:1235–7. doi: 10.1093/hmg/7.8.1235. [DOI] [PubMed] [Google Scholar]
- 25.Rosati G, Pirastru MI, Mannu L, Sanna G, Sau GF, Sotgiu S. Epidemiology of multiple sclerosis in Northwestern Sardinia: further evidence for higher frequency in Sardinians compared to other Italians. Neuroepidemiology. 1996;15:10–9. doi: 10.1159/000109884. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–84. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzanti B, Vergelli M, Riccio P, et al. T-cell response to myelin basic protein and lipid-bound myelin basic protein in patients with multiple sclerosis and healthy donors. J Neuroimunol. 1998;82:96–100. doi: 10.1016/S0165-5728(97)00194-X. [DOI] [PubMed] [Google Scholar]
- 28.Grima B, Zelenika D, Javoy-Agid F, Pessac B. Identification of new human myelin basic protein transcripts in the immune and central nervous sistems. Neurobiol Dis. 1994;1:61–6. doi: 10.1006/nbdi.1994.0008. [DOI] [PubMed] [Google Scholar]
- 29.Zelenika D, Grima B, Pessac B. A new family of transcripts of myelin basic protein gene: expression in the brain and immune sistem. J Neurochem. 1993;60:1574–7. doi: 10.1111/j.1471-4159.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 30.Prybil TM, Campagnoni CW, Kampf T, et al. The human myelin basic protein gene is included within a 179-kilobase transcription unit. Proc Natl Acad Sci USA. 1993;90:10695–9. doi: 10.1073/pnas.90.22.10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskhul RR. Myelin protein expression in lymphoid tissues: implications for peripheral tolerance. Immunol Rev. 1998;164:81–92. doi: 10.1111/j.1600-065x.1998.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin R, Howell MD, Jaraquemada D, et al. A myelin basic protein peptide is recognized by cytotoxic T cell in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991;173:19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valli A, Sette A, Kappos L, et al. Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T-cells from multiple sclerosis patients. J Clin Invest. 1993;91:616–28. doi: 10.1172/JCI116242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Medaer R, Hashim GA, Ying C, van den Berg-Loonen E, Raus ICM. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursors frequency, fine specificity, and cytotoxicity. Ann Neurol. 1992;32:330–8. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- 35.Pette M, Fujita K, Wilkinson D, et al. Myelin autoreactivity in multiple sclerosis: Recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci USA. 1990;87:7968–72. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]