Abstract
The protective efficacy of BCG vaccination against pulmonary tuberculosis (TB) is highly variable in different populations. The reason remains to be elucidated. This study aims to investigate the possible effect of intestinal helminths on the immune response to PPD in naturally immunized or BCG-vaccinated humans. The study population was assessed for helminthic infection and those found to be positive were randomly assigned to either an albendazole treatment group or a control group who received a placebo. The immune response to PPD was compared between the two groups. In addition, subjects who were tuberculin skin test-negative in both groups were BCG vaccinated and later on tested for PPD-specific responses. Albendazole induced elimination/or reduction in intestinal worms resulting in a significant improvement in T cell proliferation and in interferon-gamma production by peripheral blood mononuclear cells (PBMC) stimulated with PPD. Moreover, BCG vaccination significantly improved PPD-specific immune responses in the treated group but not in the placebo group. The differences in the in vivo skin test responses were not significant. The data show that cellular immune responses to PPD are reduced in persons with concurrent helminthic infections, perhaps reflecting a lowered resistance to mycobacterial infections. This could explain, at least in part, the reduced efficacy of BCG against TB in helminth-endemic areas of the world.
Keywords: tuberculosis, helminth, BCG, lymphoproliferation, interferon-gamma
INTRODUCTION
Tuberculosis (TB) is a chronic infectious disease caused in most cases by Mycobacterium tuberculosis, an intracellular parasite that infects preferentially mononuclear phagocytes [1,2]. For many years the incidence of TB has declined, at least in affluent societies, and the disease had been predicted to be eradicated by the year 2010 [3]. However, between 1989 and 1992, for example, a 30% increase in the disease incidence occurred in the USA [4]. Today TB kills more people than it ever did in the history of mankind. As is the case with most other infectious diseases, the bulk of the TB problem remains in resource-poor countries.
There are several methods to control the disease: improvement in socio-economic conditions, case finding and treatment, chemoprophylaxis and vaccination [5]. BCG is the most widely used vaccine and is the only vaccine available against TB and leprosy [1]. Nevertheless, it is also the most controversial vaccine with highly variable efficacy (from 0% to 80%) against pulmonary TB [6]. This variability, as most authors suggest, could be due to varying exposure of different populations to environmental mycobacteria [7–9]. However, an overview of published literature shows that environmental mycobacterial exposure could explain only about 40% of the observed variability [10]. There are other possible explanations to account for BCG's inconsistent behaviour which include (i) genetic or physiological differences among the study populations, (ii) strain variation in BCG preparations, and (iii) nutritional differences among the vaccinees. However, none of these explanations is supported by strong evidence. Another possible factor may be immune perturbation by chronic infectious diseases. Most areas where the vaccine confers the least protection are characterized by a high endemic prevalence of chronic infectious diseases, particularly helminths [11]. The host exposed to these infections would be expected to respond to the challenge, altering the immune balance and the host immune response to subsequent infection [12]. To this end, it was shown that intestinal helminthic infection reduces the efficacy of vaccines in cattle and anti-helminthic treatment before vaccination can reverse the situation [13]. This may be expected to occur in humans as well. This study aimed to use in vivo and in vitro analysis to test the hypothesis that the lowered response to BCG in tropical countries is in part due to a strong Th2 bias of the immune response as a result of intestinal helminthic infections.
SUBJECTS AND METHODS
Study subjects and study design
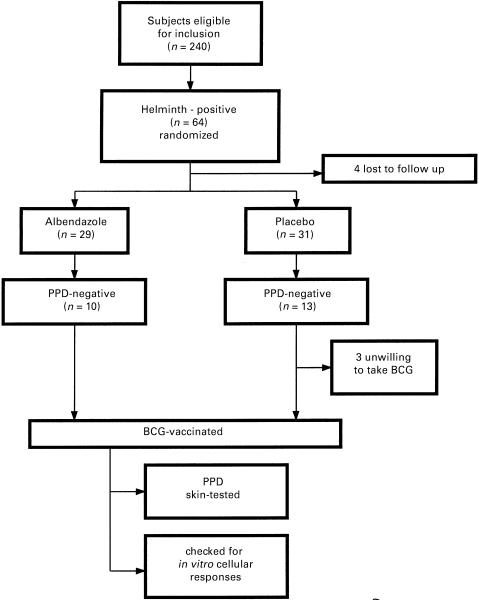
The study population consisted of college students from Addis Ababa, Ethiopia. A total of 240 students were enrolled (Fig. 1). The mean age was 21·6 years (range 18–24 years). Ten persistently helminth-free, PPD-reactive subjects were included in the study as controls. All were examined for the presence of intestinal helminths and 64/240 (26·7%) harboured one or more helminths. Four individuals did not show up for the follow-up study. Sixty individuals were then assigned randomly to either the albendazole treatment (n = 29) or placebo (n = 31) groups. History of prior BCG vaccination in either group was not assessed at entry as BCG coverage in Ethiopia at the time of birth of the study participants was very low [14]. Two doses of albendazole or placebo (Smith-Kline Beecham, London, UK), 400 mg each, were given 1 month apart. Ten of the albendazole and 13 of the placebo group were found to be PPD-negative when skin tested 6 weeks after the first dose of treatment. All PPD-negative individuals were then vaccinated with BCG, except for three from the placebo group who refused to take BCG. The BCG-vaccinated subjects of the albendazole group were given 400 mg albendazole every month until the end of the study.
Fig. 1.
Trial profile.
Informed consent was obtained from all participating individuals, and the study protocol was reviewed and approved by the local and national ethical committees. Subjects were excluded if pregnant, or if they had a chronic infectious disease.
Tuberculin skin testing and BCG vaccination
Tuberculin PPD (0·1 ml; 2 Tuberculin units (TU); Statens Serum Institute, Copenhagen, Denmark) was injected intradermally on the ventral aspect of the right forearm. After 48–72 h, the diameter of the skin induration was measured [15]. These procedures were carried out following the guidelines specified in the WHO standard Tuberculin Test Technical Guides [16].
BCG vaccine (0·1 ml; Copenhagen strain 1331; Statens Serum Institute) was injected intradermally in the left deltoid region to all PPD-negative subjects 10 weeks after the first dose of albendazole or placebo.
Parasitological examination
Before treatment, single stool samples were collected in a cap and transported to the laboratory at ambient temperature and examined the same day by direct microscopy and formol–ether concentration techniques [17]. The effect of albendazole was also checked by repeated stool examinations during the subsequent follow up.
Peripheral blood mononuclear cell separation
Heparinized venous blood (10 ml) was collected and peripheral blood mononuclear cells (PBMC) were isolated using Ficoll–Hypaque density gradient centrifugation. The cells were then resuspended in 10% DMSO in fetal calf serum (FCS; Sigma, St Louis, MO), aliquoted into Nunc tubes and frozen stepwise to −70°C overnight and preserved under liquid nitrogen until assayed. Cryopreserved PBMC were thawed, and the cell suspension adjusted to a concentration of 1 × 106 cells/ml in complete RPMI 1640 containing 100 U penicillin, 100 μg/ml streptomycin, 100 μg/ml glutamine (Life Technologies, Paisley, UK) and 10% heat-inactivated normal human AB serum (Blood Bank, Rigshospitalet, Copenhagen, Denmark).
Cellular proliferation
PBMC (2 × 105) were cultured in a round-bottomed 96-well microtitre plate. The cultures were set up in triplicate and stimulated with phytohaemagglutinin (PHA; Life Technologies) at a concentration of 3 μg/ml for 3 days and PPD (Statens Serum Institute) at a concentration of 5 μg/ml for 5 days at 37°C in a CO2 incubator. Supernatants were collected on day 3 from PHA-stimulated cultures and on day 5 from PPD-stimulated cultures and stored at −70°C until assayed. The cultures were pulsed with 1 μ Ci 3H-thymidine per well 20 h before harvesting. Proliferation was assessed by radioactive thymidine incorporation as ct/min measured in a liquid scintillation counter (LKB β-counter; LKB Wallak, Turku, Finland). Data are presented as stimulation index (SI, test ct/min/background ct/min).
Interferon-gamma assay
Interferon-gamma (IFN-γ) levels in culture supernatants of PBMC were measured with a standard ELISA technique using a commercially available pair of MoAbs (1-D1K and 7-B6-1; Mabtech, Stockholm, Sweden) according to the manufacturer's instructions. Recombinant IFN-γ (Life Technologies) was used as a standard. The results were given as means of duplicate wells and IFN-γ levels are shown as those in culture supernatants of PHA- or PPD-stimulated PBMC minus those in supernatants of cells cultured with medium alone.
Statistical analysis
The data were expressed as means and s.e.m. Student's t-test was used for testing the significance of the differences. P <0·05 was considered significant. Statistical analysis was performed using the SPSS statistical package (SPSS Inc., Chicago, IL).
RESULTS
Prevalence of intestinal parasites in the study population
The prevalence of parasites was unexpectedly low, 26·7% for worms and 18% for protozoan parasites, compared with other reports performed in Ethiopia, whereby the prevalence of intestinal parasites in the community is between 50% and 70% (Wolday et al., personal communication). The most prevalent helminths were Ascaris lumbricoides (13%), hookworms (9·2%), Trichuris trichiura (4·1%), Strongyloides stercoralis (2·3%), Hymenolepis nana (2·3%), and Taenia spp. (1%). Entamoeba histolytica (12·3%) and Giardia lamblia (7%) were the two common protozoan parasites observed. Overall, intestinal parasites were identified in 40·2% of the subjects. Of these, 26·7% harboured one or more helminths, whereas 18% were positive for protozoan parasites (E. histolytica or G. lamblia, or both). Double infection with protozoa and helminths occurred in 4·7% of the population. A similar proportion of individuals was infected with helminths in both groups, and after albendazole treatment stool samples were 94% negative for helminths. The drug had no significant effect on protozoan infection.
Tuberculin reactivity and skin test conversion after BCG vaccination
A tuberculin skin test was performed 6 weeks after albendazole or placebo treatment of both groups. Induration of ≥7 mm to 2 TU PPD was considered positive [15]. The tuberculin reactivity rate tended to be higher in the albendazole-treated group compared with the placebo group and the sizes of the DTH responses tended to be higher in the treated group. However, the differences were not statistically significant (Table 1).
Table 1.
Skin-test reactivity to PPD in subjects infected with helminths
| Category of study subjects | |||
|---|---|---|---|
| Albendazole group | Placebo group | P* | |
| Tuberculin-reactive | 19/29 | 18/31 | NS |
| (65·5)† | (58·1) | ||
| DTH size in mm (mean ±s.e.m.) | 14·6 ± 0·5 | 13±5 ± 0·4 | NS |
Student's t-test, χ 2 test or Fisher's exact test.
Numbers in parentheses are percentages.
NS, Not significant.
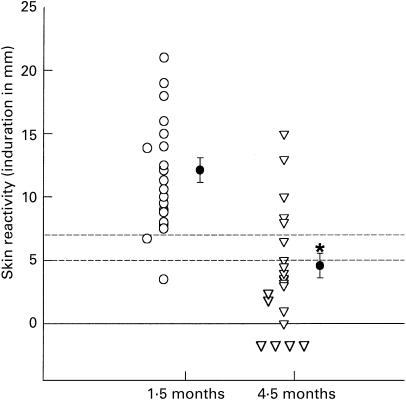
Those who were skin-tested and found negative were BCG-vaccinated and 1·5 months and 4·5 months later the tuberculin skin test was performed again. As shown in Fig. 2, of the 20 initially PPD-negative vaccinees, and tuberculin skin-tested 1·5 months after BCG vaccination, all but one became positive, irrespective of the treatment (placebo or albendazole). The results concur with a previous report that BCG vaccination does cause skin test conversion [18]. However, only 25% of the vaccinees from both groups when tested 4·5 months after vaccination were found to have sustained PPD skin test reactivity. When the cut-off for a positive PPD skin reactivity was reduced from 7 mm to 5 mm, the rate of PPD reactivity increased to only 35% (Fig. 2). When compared with the rate of reactivity at 1·5 months using the same cut-off value, there was an overall reduction in reactivity of 60% (P = 0·02). Further reduction of the cut-off value to the presence of any induration (> 0 mm) accounted for 50% increase in the rate of skin reactivity. Overall, there was a significant reduction in the mean skin induration from 12·1 mm at 1·5 months to 4·6 mm at 4·5 months (P < 0·001). There was no difference in the waning of skin reactivity to PPD between the placebo and albendazole treatment group, however.
Fig. 2.
Dot plot showing tuberculin skin test reactivity 1·5 and 4·5 months post-BCG vaccination (n = 20). Cut-off values (dashed horizontal lines) for positivity were based on indurations of 5 or 7 mm. *P < 0·001. ○, Individual values; •, mean values.
PPD-specific in vitro cellular responses
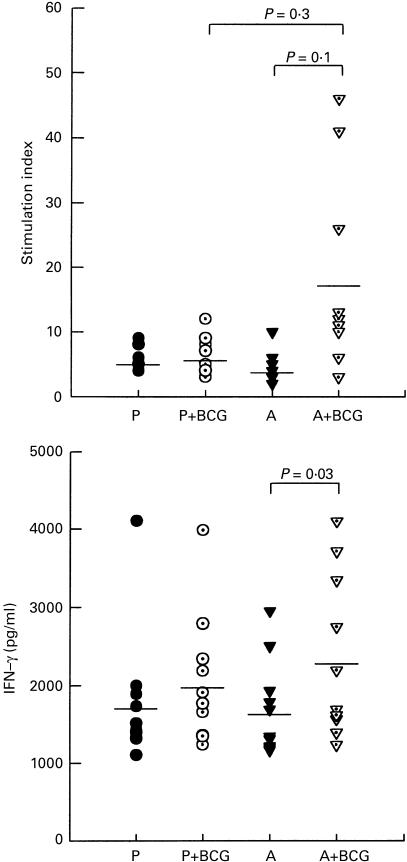
Cellular responses to PPD were investigated using PBMC obtained 8 weeks after the first dose of albendazole or placebo. Twenty-nine donors from both the albendazole and placebo groups were investigated for proliferative responses. As shown in Fig. 3, proliferative responses to PPD were significantly higher in the albendazole-treated group compared with the placebo controls (P = 0·02). Also, the proliferative response to the control mitogen PHA was significantly higher in the albendazole group when compared with the controls (P < 0·001).
Fig. 3.
Effect of treatment of helminths with albendazole on proliferative responses (a) and IFN-γ secretion (b) to mycobacterial antigen. Peripheral blood mononuclear cells (PBMC) obtained 8 weeks after the first dose of albendazole (□) or placebo (▪) were stimulated without or with phytohaemagglutinin (PHA) or PPD. Proliferative responses are presented as stimulation index (SI (test ct/min/background ct/min)). IFN-γ levels are shown as those in culture supernatants of PHA- or PPD-stimulated PBMC minus those in supernatants of cells cultured with medium alone. The proliferative responses and IFN-γ production in cells cultured with medium alone were <750 ct/min and 148 pg/ml, respectively. Data are means +s.e.m. from 29 (a) or 15 (b) donors from each group.
Levels of IFN-γ produced in the culture supernatants of PBMC in 15 donors from each group were also measured. Significantly higher amounts of IFN-γ were measured in the albendazole group in response to PHA and PPD compared with their placebo counterparts (Fig. 3, P = 0·03 and 0·04, respectively).
Persistently helminth-free but tuberculin-reactive subjects were included as controls to see if treatment with albendazole by itself had any effect on the in vitro immune response and to rule out the possibility of a direct immune potentiating effect of the drug. The results indicate that the drug by itself had no direct effect on the cellular immune response (data not shown), and that the differences in immunological parameters observed between albendazole and placebo-treated populations were likely to be due to its effect on the worms.
PPD-specific cellular responses after BCG vaccination
PPD-negative subjects from both groups were BCG-vaccinated 10 weeks after the first dose of albendazole or placebo and their in vitro cellular immune responses to PPD measured. Ten donors from each group were included in this analysis and the cellular responses obtained after BCG vaccination were compared with the prevaccination levels.
The albendazole-treated group had significantly higher PPD-induced T cell proliferative responses compared with prevaccination levels or the responses of placebo group post-vaccination (Fig. 4, P = 0·01 and 0·02, respectively). Interestingly, there were no significant differences in the pre- and post-vaccination proliferative responses of PBMC from the placebo group.
Fig. 4.
Cellular immune responses to PPD in the placebo (P) and albendazole (A) groups before and after vaccination with BCG. Ten donors from each group, who were initially tuberculin-negative, were vaccinated with BCG 10 weeks after the first dose of placebo or albendazole. Blood samples were then analysed before and 8 weeks after vaccination. The proliferative responses and IFN-γ production in unstimulated cultures were below 970 ct/min and 366 pg/ml, respectively. Data are presented as dot plots and horizontal lines are mean values.
Vaccinees in the albendazole and placebo groups were compared with regard to the change in the levels of IFN-γ secretion in response to PPD after BCG vaccination with reference to prevaccination levels (Fig. 4). The albendazole-treated group produced a significantly greater amount of IFN-γ after vaccination compared with prevaccination levels (P = 0·03), whereas in the placebo group the differences were not significant.
DISCUSSION
An established immune response profile to a given infection may influence the immune responses mounted to an unrelated antigen or the outcome of a concurrent heterologous infection. This was demonstrated by Curry and colleagues [19] when the cytokine and antibody production accompanying the immune response to Schistosoma mansoni egg antigens in vivo were found to affect the outcome of an infection with Tr. muris. When mice susceptible to infection with Tr. muris are co-infected with Sch. mansoni, they acquire the capacity to resolve Tr. muris infection. The situation may be similar in humans. Although the number of studies addressing this problem is small, analysis of data from some laboratories indicates that infectious diseases, especially helminthic infections endemic in Africa and other developing countries, profoundly affect the host immune system. Such an altered immune background makes the host more susceptible to, for example, HIV infection and less capable of controlling that infection once it is acquired [12,20,21].
In this study, we hypothesized that the lowered efficacy of BCG vaccine in a country such as Ethiopia is, in part, due to a strong Th2 bias of the immune response caused by chronic helminthic infection. Our results are in general agreement with this hypothesis. A reduction or elimination of intestinal worms using broad-spectrum anti-helminthic treatment resulted in enhancement of T cell proliferation and IFN-γ production by PBMC stimulated with PPD. A similar improvement in T cell responses to PPD was observed in filarial worm-infected subjects after treatment with diethylcarbamazine [22–25]. We have not analysed the correlation of parasite burden in relation to responsiveness to mycobacterial antigens in this study.
The functional impairment of T cells (reduced proliferation and IFN-γ production) in helminth-exposed Ethiopians favours the notion that chronic infections result in a functional alteration of T cells similar to, but of a lesser extent than, that occurring in peripheral T cells from HIV-infected patients [26]. In the absence of HIV infection or other causes of immunosuppression, only about 10% of adult people infected with M. tuberculosis develop overt TB, while the remaining 90% mount effective immune responses and as a result either clear the infection or keep it at bay [27]. An effective immune response against mycobacterial infection is thought to be mediated by co-operative interaction between T lymphocytes and mononuclear phagocytes, with key roles played by CD4 lymphocytes [28]. This interaction is dependent upon the interplay of cytokines produced mainly by CD4+ T cells, with IFN-γ being a central cytokine in inducing adequate macrophage activation culminating in the killing of intracellular mycobacteria [29].
Our findings show that antigen-specific T cell responses to M. tuberculosis, particularly Th1 responses (IFN-γ production), both before and after BCG vaccination, were down-regulated in persons with concurrent helminthic infection. The implication of this could be that chronic helminth-infected individuals may have an increased susceptibility to infections normally cleared by Th1-dependent immunity, as well as altered immune responses to potential vaccines directed against intracellular infections. Indeed, epidemiological studies show that the efficacy of BCG is the least, and the prevalence of TB is the highest, in that part of the world where helminths are also highly prevalent [30].
In addition, PPD-negative subjects were vaccinated with BCG and later tested for mycobacterial antigen-specific immune responses in vivo and in vitro. The results were that PPD-specific proliferation of T cells and the production of IFN-γ were significantly improved in the treated group compared with prevaccination levels. In contrast, the difference between pre- and post-BCG vaccination was not significant in the placebo group. Although the number of vaccinees followed up to the completion of the study was limited, the findings indicate that immunization with BCG sensitizes individuals without worms better than it does those with chronic worm infection. History of prior BCG vaccination in either group was not assessed at entry. However, we do not think that this would affect the responses, as BCG coverage in Ethiopia at the time of the birth of the study participants was very low [14]. In addition, it seems that prior BCG vaccination has no significant effect either on the in vitro or the in vivo responses to PPD [31]. That treatment of helminthic infections with albendazole during BCG vaccination was associated with significantly increased proliferative and IFN-γ responses to PPD has the implication that exposure to intestinal helminths during BCG vaccination may contribute to a decreased T cell response to mycobacterial antigens. Although it is tempting to speculate that the combination of albendazole and BCG alone might be responsible for the observed increased T cell response to mycobacterial antigens, testing this hypothesis by evaluating T cell responses in skin test-negative controls without intestinal helminths exposed to albendazole and BCG is ethically problematic. However, we have noted that albendazole alone (in helminth-negative or helminth-positive subjects) has no direct effect on the cellular immune responses (Data not shown). Chronic helminthic infections induce predominantly Th2-type immune responses [20]. However, protective immunity against intracellular pathogens, including mycobacteria, require Th1 responses [24]. As Th1/Th2 responses are mutually exclusive, and helminths by capitalizing on the Th2 response may cross-inhibit Th1 response required to clear mycobacteria [20] it has been suggested that the removal of intestinal helminths by deworming would remove the inhibitory effects of Th2 on Th1 responses [20,32].
Studies conducted to determine the protective efficacy of BCG vaccination show that the vaccine provides the least protection in developing countries such as Malawi and India [33,34] where helminths are also endemic. In contrast to this, the MRC trial in the UK showed up to 80% protection against pulmonary TB [10]. Most authors argue that this variability could be due to differences in environmental mycobacterial exposure [7–9]. However, an overview of published literatures by Colditz and colleagues concluded that environmental mycobacterial exposure could account only for about 40% of the observed variation between vaccine efficacy trials [10]. An alternative explanation that may account for part of the variation and for which we have some data is that areas where the vaccine conferred the least protection, such as the developing tropical countries, are characterized by high prevalence of endemic and chronic infectious diseases [11]. The host confronted with such an infectious burden would be expected to mount a prolonged immune response to this challenge, which would alter the normal immune balance [12]. Bentwich and colleagues suggested that changes in the host immune response are caused by endemic infections, particularly helminths [20], and such infections may abrogate effective immune responses against HIV-1 and TB [32]. The fact that albendazole treatment enhanced PPD-specific T cell proliferation and IFN-γ production provides ground for these assertions. Thus, the fact that BCG confers the least protection in areas where there is high endemic prevalence of helminths may be because the baseline immunity in individuals living in these areas is perturbed, and such effects may have a bearing on the immune response to subsequent mycobacterial exposure and vaccination.
Another interesting observation in this study was that BCG vaccination in our study population caused skin test conversion in 95% of the vaccinees 6 weeks after vaccination. However, only 25% retained skin test reactivity 4·5 months later. Moreover, PPD skin reactivity waned in most individuals (65%) even when the cut-off for a positive reactivity was reduced from 7 mm to 5 mm. Compared with the rate of reactivity at 1·5 months at the same cut-off value, there was an overall reduction in reactivity of 60%. Further reduction of the cut-off value to the presence of any induration (> 0 mm) accounted for only a 50% increase in the rate of skin reactivity. Taken together, the data indicate that BCG vaccination does cause skin test conversion, but that skin test positivity of BCG-vaccinated individuals is lost within a relatively short period of time. The result, if confirmed by a larger study, would have important implications, because the Mantoux test is used in many countries as a diagnostic tool for exposure to M. tuberculosis, but its use is limited in BCG-vaccinated populations. However, since the vaccine-induced tuberculin reactivity seems to be short-lived, the use of the test could be extended to BCG-vaccinated populations at least in an environment like the one in Ethiopia.
In conclusion, our findings indicate that there is a functional change in the peripheral T cells of helminth-exposed populations and that such changes can be reversed in a relatively short time following chemotherapy directed against the helminths. Moreover, the improved mycobacterial antigen-specific cellular response in BCG-vaccinated persons after treatment may indicate the importance of such infections in the efficacy of the vaccine in helminth-endemic parts of the world. Control of helminthic infections in developing countries may deserve attention, as such approaches may modulate the base line immune response and lead to enhanced ability to cope with subsequent infections. Extensive investigation of the factors responsible for the compromised efficacy of BCG vaccine is recommended, as we are at present very far away from the realization of a better and new vaccine against TB, the world's major killing infectious disease.
Acknowledgments
We are grateful to Sister Genet Amare for her help in the Mantoux test and blood collection. We thank Smith Kline Beecham Co. for providing us with albendazole and placebo. The Gondar College of Medical Sciences sponsored the living costs of D.E. throughout the study period. The study was supported by funds from the Armauer Hansen Research Institute (sponsored by the governments of Ethiopia, Norway and the Swedish International Development Agency SAREC/Sida) and the School of Graduate Studies of the Addis Ababa Univsedityity (Ethiopia).
REFERENCES
- 1.Fine PEM. Immunities in and to tuberculosis: implications for pathogenesis and vaccination. In: Porter JDH, McAdam PWJ, editors. Tuberculosis back to the future. Chichester: John Wiley & Sons; 1994. pp. 53–80. [Google Scholar]
- 2.Dannenberg AM., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–33. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 3.Bloom BR, Murray CJ. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease control and Prevention. Tuberculosis morbidity and mortality—United States. MMWR. 1992;42:696–704. [Google Scholar]
- 5.Rodrigues LC, Smith PJ. Tuberculosis in developing countries and methods for its control. Trans R Soc Trop Med Hyg. 1990;84:739–44. doi: 10.1016/0035-9203(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 6.Bloom BR, Fine PEM. The BCG experience: implications for future vaccine against tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection and control. Washington, DC: American Society of Microbiology; 1994. pp. 531–57. [Google Scholar]
- 7.Palmer CE, Long MW. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966;4:553–68. doi: 10.1164/arrd.1966.94.4.553. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SH, Walker KB, Lowrie DB, et al. Monocyte antimycobacterial activity before and after Mycobacterium bovis BCG vaccination in Chingleput, India, and London, United Kingdom. Infect Immun. 1993;61:4501–3. doi: 10.1128/iai.61.10.4501-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CA, Brown IN, Swinburne R. The effect of oral Mycobacterium vaccae on subsequent responses of mice to BCG sensitization. Tubercle. 1985;66:251–60. doi: 10.1016/0041-3879(85)90062-5. [DOI] [PubMed] [Google Scholar]
- 10.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 11.Hori H, Watanabe F, Sakurai M. Infectious diseases in African children. Acta Paediatr Jpn. 1993;35:553–8. doi: 10.1111/j.1442-200x.1993.tb03110.x. [DOI] [PubMed] [Google Scholar]
- 12.Bentwich Z, Weisman Z, Moroz C, Bar Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakubovski MV, Varenich SE, Sviziqava AP. Influence of intestinal parasites on postvaccination immunity against bacterial and viral infections. Proc Acad Sciences, Belarus Sci Agriculture, Minsk. 1990;4:101–8. [Google Scholar]
- 14.Azbite M. National tuberculin test survey in Ethiopia. Ethiop Med J. 1992;30:215–323. [PubMed] [Google Scholar]
- 15.World Health Organization. Standard tuberculin test technical guide. Geneva: WHO; 1963. [Google Scholar]
- 16.Sokal J. Measurement of delayed type skin test responses. N Engl J Med. 1975;263:501–2. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 17.Cheesbrough M. Medical laboratory manual for tropical countries. Vol. 1. Hertford, UK: Stephen Austin and Sons Ltd; 1981. [Google Scholar]
- 18.Das SD, Narayanan PR, Kolappan C, Colston MJ. The cytokine response to Bacille Calmette Guerin vaccination in South India. Int J Tuberc Lung Dis. 1998;10:836–43. [PubMed] [Google Scholar]
- 19.Curry AJ, Else KJ, Jones F, Bancroft A, Grencis RK, Dunne DW. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–74. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 21.Anzala OA, Nagelkerke NJ, Bwayo JJ, et al. Rapid progression to disease in African sex workers with human immunodeficiency virus type 1 infection. J Infect Dis. 1995;171:686–9. doi: 10.1093/infdis/171.3.686. [DOI] [PubMed] [Google Scholar]
- 22.Rougemont A, Bobson-Pontal ME, Pontal PG, et al. Tuberculin skin tests and BCG vaccination in hyperendemic area of onchocerciasis. Lancet. 1977;2:309. doi: 10.1016/s0140-6736(77)91857-8. [DOI] [PubMed] [Google Scholar]
- 23.Ottesen EA, Weller PF, Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33:413–21. [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart GR, Boussinesq M, Coulson T, et al. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–23. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartono E, Kruize YC, Kurniawan A, et al. Elevated cellular immune responses and interferon-gamma release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J Infect Dis. 1995;171:1683–7. doi: 10.1093/infdis/171.6.1683. [DOI] [PubMed] [Google Scholar]
- 26.Vingerhoets J, Dohlsten M, Penne G, et al. Superantigen activation of CD4+ and CD8+ T cells from HIV-infected subjects: role of costimulatory molecules and antigen-presenting cells (APC) Clin Exp Immunol. 1998;111:12–19. doi: 10.1046/j.1365-2249.1998.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zumla A, Grange J. Tuberculosis. BMJ. 1998;316:1962–4. doi: 10.1136/bmj.316.7149.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellner JJ. The immune response in human tuberculosis—implications for tuberculosis control. J Infect Dis. 1997;176:1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 29.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–9. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 31.Legesse M. Addis Ababa University; 2000. In vitro T-cell proliferation and cytokine production as a surrogate marker for HIV-1 infection progression in Ethiopians. MSc Thesis. [Google Scholar]
- 32.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of worms change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–735. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 33.Tuberculosis prevention Trial, Madras. Trial of BCG vaccines in south India for tuberculosis prevention. Indian J Med Res. 1990;72(Suppl. 1):1–74. [PubMed] [Google Scholar]
- 34.Ponnighaus JM, Fine PEM, Sterne JA, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339:636–9. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]