Abstract
Rheumatoid factors (RFs) are autoantibodies directed against the Fc part of IgG. Considerable evidence exists that there are two classes of RFs, pathological and physiological. Whereas pathological RFs are associated with disease, physiological RFs are considered to be a normal component of the immune response. RF+ precursor B cells present as part of the B cell repertoire of healthy individuals are held responsible for the production of physiological RFs, which is a transient phenomenon with a clear correlation with an initiating stimulus such as immunization or exposure to an infection. Here we demonstrate a difference in the regulatory control of total Ig and RF production by peripheral blood (PB) B cells of both healthy controls (HC) and patients with rheumatoid arthritis (RA). Highly purified B cells from HC and patients with RA were cocultured with T cells stimulated with immobilized anti-CD3 mAb. Similar to IgM production, IgM-RF production was shown to be dependent on CD40 cross-linking. However, activation of PB B cells in the CD40 system in the presence of IL-2, IL-4, IL-10, combinations of these cytokines or supernatant of anti-CD3-stimulated T cells failed to induce detectable IgM-RF, whereas total IgM production was considerable. From these results we conclude that conditions to activate physiological RF+ B cells require additional contact besides CD40–CD40L interactions between T and B cells. Since the requirements for RF production were similar using PB B cells from HC and patients with RA it is suggested that the regulatory properties of RF+ precursors in the PB B cell compartment is equal among these groups. Together, these results indicate that conditions for the induction of total Ig and physiological RFs are different.
Keywords: peripheral blood B cells, rheumatoid factor rheumatoid arthritis, T–B cell interaction, CD40
INTRODUCTION
Rheumatoid factors (RFs), which were first discovered in the serum of patients with rheumatoid arthritis (RA), are autoantibodies directed against the Fc part of IgG. Their persistent presence in the circulation is a hallmark of RA and high titres were shown to correlate with more severe disease [1,2]. RFs in RA are believed to contribute to local inflammation by immune complex formation and complement activation [3]. However, production of RFs is not unique for patients with RA. RFs can also be detected in sera of apparently healthy individuals after secondary, T cell-dependent, antibody responses [4,5]. In contrast to RFs in RA, these RFs are of low affinity and titre and may represent a normal component of the immune response. Based on the appearance of RFs with pathological significance on the one hand and RFs as part of the normal immune physiology on the other hand, it is believed that there are two classes of RFs, pathological and physiological. Evidence exists that pathological RFs are structurally different from physiological RFs. Physiological RFs are usually polyreactive, have restricted V gene usage and are predominantly of the IgM isotype, whereas pathological RFs have a broad V gene usage and are isotype-switched and contain somatic mutations, indicative that these RFs result from an antigen-driven immune response [3,6,7]. Accordingly, recent data indicate that the persistent presence of circulating RFs is derived from terminally differentiated plasma cells [8,9]. In contrast, precursor B cells present in the B cell repertoire of healthy individuals are held responsible for the production of physiological RFs, which is a transient phenomenon with a clear correlation with an initiating stimulus. Such initiating events can be active immunization or exposure to bacterial, viral or parasitic infections [10–13]. It is believed that the activation of precursor B cells to produce RFs is T cell-dependent. RF+ B cells are thought to interact with T helper (Th) cells during a secondary immune response to foreign Ag to which the T cells react, in the context of IgG as part of an immune complex [14,15]. However, in contrast to antibodies directed against foreign Ag, physiological RFs show no increase in affinity [16]. An in vitro model for T cell-dependent precursor B cell activation has been established by utilizing immobilized anti-CD3 to activate Th cells to stimulate RF precursors.
The current studies were undertaken to compare the requirements of physiological RF production by peripheral blood (PB) B cells to those of total Ig production. For this purpose we cocultured PB B cells with T cells stimulated by anti-CD3 mAb. Here we demonstrate that both total Ig and physiological RF synthesis by PB B cells are dependent on CD40 activation. However, activation of PB B cells in the CD40 system in the presence of IL-2 and IL-10, leading to efficient total Ig production, failed to induce detectable RF. Moreover, in contrast to total Ig production RF production increased upon increasing amounts of anti-CD3 used to stimulate T cells. These results indicate differential requirements in the induction of total Ig and physiological RFs.
MATERIALS AND METHODS
Study subjects
Heparinized PB was obtained from RA patients with increased serum RF titres who were seen at the Department of Rheumatology of the Leiden University Medical Center and fulfilled the 1987 criteria of the American Rheumatism Association for RA [17]. Patients with other forms of arthritis (ankylosing spondylitis and gout) and HC without increased serum RF titres were also included.
Isolation of B cells
Peripheral blood mononuclear cells (PBMC) were purified by Ficoll-Hypaque (Pharmacia Biotech AB, Uppsala, Sweden; ρ = 1.077 g/ml) density gradient centrifugation and frozen in liquid nitrogen. PBMC were thawed and stained with the following phycoerythrin-conjugated monoclonal antibodies (Becton Dickinson, Mountain View, CA): anti-CD3, anti-CD14, anti-CD16, and anti-CD56. B cells were isolated by negative selection using a cell sorter (FACStar, Becton Dickinson). Obtained cells contained ≥ 95% CD19+ lymphocytes as determined by fluorescence activated cell sorter analysis (data not shown).
T cell clone K15
The synovial fluid (SF), autoreactive, Vβ19+, CD4+ T cell clone K15 was derived from a T cell line of a patient with RA which responded to autologous SF in vitro as described in detail [18]. To maintain the T cell clone 3×105 T cells/ml were stimulated weekly with phytohaemagglutinin (Murex Diagnostics Ltd, Dartford, England; 1 µg/ml) and cultured in the presence of irradiated allogeneic PBMC (106/well; 30 Gy) and a B lymphoblastoid cell line (BLCL; 105/well; 50 Gy) in 24-well tissue culture plates (Greiner, Alphen a/d Rijn, The Netherlands) at 37°C, 5% CO2. Culture medium consisted of Iscove's modified Dulbecco's medium (IMDM; Gibco BRL, Breda, The Netherlands) with glutamax supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS; Gibco BRL), 100 IU/ml penicillin, 100 µg/ml streptomycin (Boehringer Mannheim GmbH, Mannheim, Germany) and 10 U/ml recombinant human IL-2 (rhIL-2; Cetus, Gouda, The Netherlands). Rested T cells were frozen in liquid nitrogen until further use.
L-CD40L cells
Murine fibroblast L cells transfected with CD40 ligand (L-CD40L cells) [19] and untransfected mouse fibroblasts (L-cells) were maintained in IMDM with glutamax supplemented with 10% (v/v) heat-inactivated FCS, penicillin and streptomycin in T75 flasks (Greiner, Alphen a/d Rijn, The Netherlands).
Proliferation assay
T cells (clone K15; 105/well) were cocultured with irradiated purified B cells (104/well; 30 Gy) in a total volume of 200 µl, in 96-well flat-bottom tissue culture plates, in IMDM with glutamax supplemented with 10% (v/v) heat-inactivated FCS, penicillin and streptomycin at 37°C, 5% CO2. T cells were stimulated with different amounts of immobilized anti-CD3 mAb OKT-3 (Cilag AG Int., Zug, Switzerland; wells coated overnight at room temperature) as indicated. A negative control was cultured in medium alone. After 72 h of incubation cells were pulsed with 1 µCi/well of[3H]-thymidine (New England Nuclear, Boston, MA) over an additional 20 h incubation period. The cells were then harvested on filtermats (Skatron Instruments, Lier, Norway) and the incorporated [3H]-thymidine was measured using a liquid scintillation counter (Skatron Instruments).
In vitro antibody production
T-B cell co-culture system
Purified B cells (104/well) were cocultured with T cells (clone K15; 105/well) in a total volume of 200 µl, in 96-well flat-bottom tissue culture plates, in IMDM with glutamax supplemented with 10% (v/v) heat-inactivated FCS, penicillin and streptomycin at 37°C, 5% CO2. T cells were stimulated with immobilized anti-CD3 mAb OKT-3 (Cilag AG Int., Zug, Switzerland; wells coated overnight at room temperature; 50 µg/ml). In some experiments T cells were stimulated with different amounts of OKT-3 as indicated. Control cultures included T cells only, B cells only, T cells with anti-CD3, B cells with anti-CD3, T cells and B cells. To study the importance of CD40 signalling in the induction of IgM-RF production, the mouse IgG1 antibodies mAb89 [20], LL48 [21] and an irrelevant isotype-matched control Ab were added at the initiation of culture in final concentrations as indicated.
CD40 system
Purified B cells (104/well) were cultured on a layer of irradiated L-CD40L or L-cells (5 × 103/well; 70 Gy). Cells were cultured in 96-well flat-bottom tissue culture plates, in the presence of rhIL-2 (40 U/ml), rhIL-4 (200 U/ml), rhIL-10 (50 ng/ml) (PreproTech Inc., Rocky Hill, NJ) or combinations of these cytokines, in a total volume of 200 µl, in IMDM with glutamax supplemented with 10% (v/v) heat-inactivated FCS, penicillin and streptomycin at 37°C, 5% CO2.
All cultures were set up in duplicate or triplicate. Cell free culture supernatants were harvested after 13 (anti-CD3-driven system) or 14 (CD40 system) days and analysed for Ig production and RF activity.
T cell supernatant
T cells of clone K15 (105/well) were cultured, in the presence of immobilized anti-CD3 mAb OKT-3 (50 µg/ml), for 5 days, in a total volume of 200 µl, in 96-well flat-bottom tissue culture plates, in IMDM with glutamax supplemented with 10% (v/v) heat-inactivated FCS, penicillin and streptomycin at 37°C, 5% CO2. T cell supernatant was collected and stored at − 70°C.
Determination of immunoglobulins
Total IgM concentration in culture supernatants was determined by immunoassay. ELISA plates (Titertek, Flow Laboratories, Zwanenburg, The Netherlands) were coated (overnight at room temperature) with mouse monoclonal antibodies directed against human IgM (clone HB57, ATCC, Rockville, MD) in a carbonate buffer, pH 9.6, followed by incubation with proper dilutions of culture supernatants (1 h at 37°C). Bound immunoglobulin was detected using horseradish peroxidase (HRP)-conjugated rabbit antihuman IgM (Dako, Denmark; 1 h at 37°C) and ortho-phenylenediamine (OPD; Sigma, St. Louis, MO; 0.2 mg/ml) in phosphate buffer, pH 5.6, containing 0.03% (v/v) H2O2 (Merck, Darmstadt, Germany). The reaction was stopped after 15 min by adding 10% (v/v) H2SO4 (Merck) and read in an ELISA reader (EL 312e Bio-kinetics Reader, Bio-Tek Instruments, Inc., Winooski, VT) at 490 nm. Dilutions of a reference serum with known quantities of total IgM (Central Laboratory Blood Transfusion (CLB), Amsterdam, The Netherlands) were used to construct a standard curve. The detection limit of the total IgM assay was 1 ng/ml.
IgM-RF activity was measured in a capture ELISA. To determine IgM-RF activity, ELISA test plates were coated (overnight at room temperature) with human IgG Fc fragments (Cappel, Durham, NC) in a carbonate buffer, pH 9.6. After an incubation with appropriately diluted culture supernatants (1 h at 37°C) the plates were incubated with biotinylated mouse antihuman IgM (biotin-HB57; 1 h at 37°C). Subsequently, the plates were successively incubated with streptavidin-HRP (Zymed Laboratories, Inc., San Francisco, CA; 50 min at 37°C), biotin-tyramine (Sigma; 1 h at 37°C), and streptavidin-HRP (50 min at 37°C). The colour reaction (OPD) was stopped after 15 min by adding 10% (v/v) H2SO4 and read at 490 nm in an ELISA reader. Relares serum (200 IU/ml IgM-RF; CLB) was used for standardization of the IgM-RF assay. The detection limit of the IgM-RF assay was 6 mIU/ml.
Statistical analysis
Significant differences between experimental groups were calculated using the Mann–Whitney U-test. A P < 0.05 was considered significant.
RESULTS
RF production by PB B cells in the presence of anti-CD3-stimulated T cells
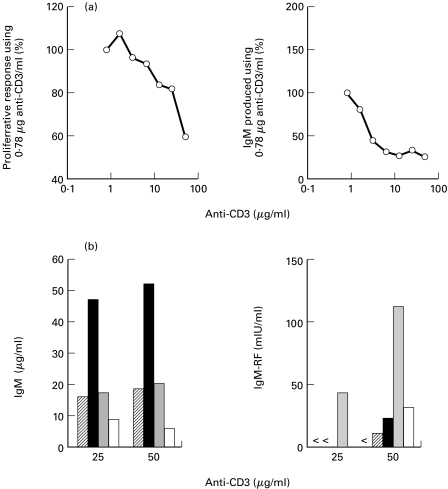
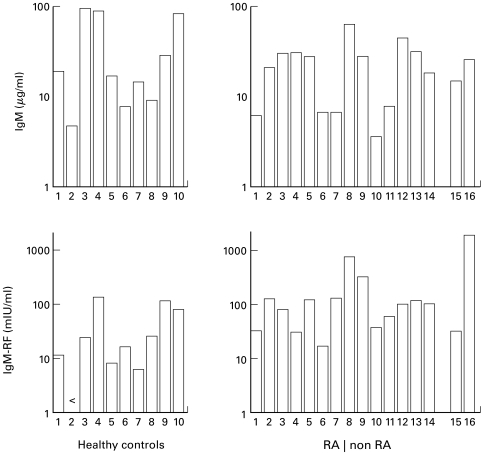
Activated T cells are potent inducers of RF production by PB B cells [22–24]. To examine the ability of T cells to induce physiological RF production in more detail, T cells of a Th1 clone (K15) were cocultured with highly purified PB B cells from HC in wells which were coated with increasing concentrations of anti-CD3 mAb. Optimal IgM production was observed using an anti-CD3 concentration of approximately 1 µg/ml, whereas high amounts of anti-CD3 favoured IgM-RF production (Fig. 1a,b). The preponderance of IgM-RF production at high concentration of anti-CD3 used to coat the wells was inversely correlated with the capacity of T cells to proliferate (Fig. 1a,b). Analysis of physiological RF production, using 50 µg/ml of anti-CD3, revealed IgM-RF production in nine out of 10 HC tested (Fig. 2; mean (s.d.): 42.2 (49.5) mIU/ml (n = 10)). In order to determine RF regulation in the PB B cell compartment of patients with arthritis, B cells were isolated from the PB of patients and cultured in the anti-CD3-driven system. Peripheral B cells of all patients with RA synthesized IgM-RF (Fig. 2; mean (s. d.): 143.1 (183.6) mIU/ml (n = 14)). B cells of patients with RA produced significantly (P = 0.014; Mann–Whitney U-test) more IgM-RF compared with B cells from HC. Peripheral B cells derived from patients with ankylosing spondylitis or gout synthesized comparable amounts of IgM-RF in the anti-CD3-stimulated cultures (Fig. 2).
Fig. 1.
(a) PB B cells (104/well) from healthy controls were cocultured with T cells (clone K15; 105/well) in the presence of different amounts of immobilized anti-CD3. Proliferative responses (triplicate experiments) were determined after 4 days. The percentages of the proliferative response using 0.78 μg anti-CD3/ml are shown. After 13 days triplicate culture supernatants were harvested and analyzed for IgM concentrations. Percentages of IgM produced using 0.78 μg anti-CD3/ml are shown. Results shown are representative of two independent experiments. A high concentration of anti-CD3 favours induction of IgM-RF production by T cell clone K15. (b) PB B cells of healthy controls or patients with RA (104/well) were cocultured simultaneously with T cells (clone K15; 105/well) in the presence of two different amounts of immobilized anti-CD3 mAb (25 or 50 μg/ml). Duplicate culture supernatants were harvested after 13 days and analyzed. Data shown are the results of two independent experiments ( healthy control 1; ▪ healthy control 2; patient with RA 1; □ patient with RA 2; < below detection limit of 6 mIU/ml).
Fig. 2.
Induction of IgM-RF synthesis by PB B cells in the anti-CD3-driven system. Peripheral B lymphocytes (104/well) of healthy controls and patients with RA or other forms of arthritis (15: ankylosing spondylitis; 16: gout) were cocultured with T cells (clone K15; 105/well) in the presence of immobilized anti-CD3 mAb (50 μg/ml). Duplicate culture supernatants were harvested after 13 days and analyzed for IgM and IgM-RF concentrations. Data shown are the result of eight independent experiments.
IgM-RF production by PB B cells is dependent on CD40 activation
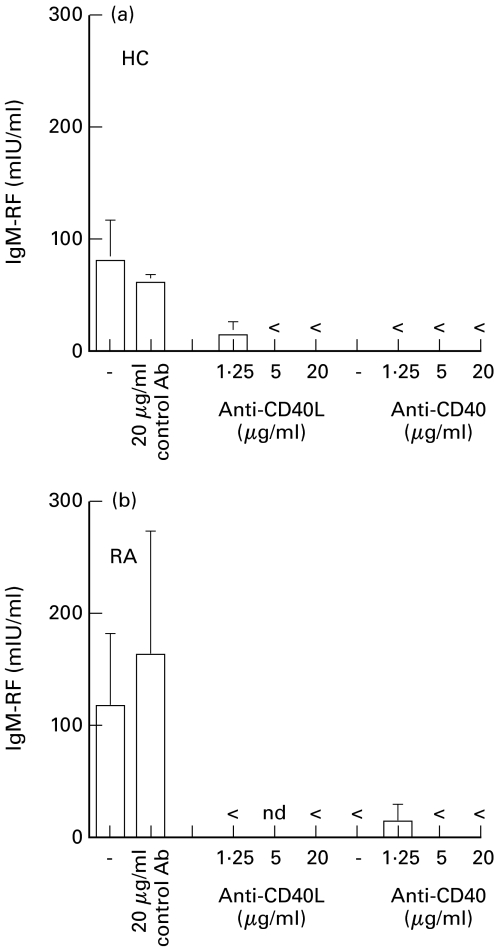
To further discern the factors necessary for differentiation of RF+ B cells into Ab-secreting cells we investigated to what extent the CD40–CD40L interaction was involved in the induction of RF production in the anti-CD3-driven cultures. Purified B cells of HC or patients with RA were cocultured with T cells which were stimulated with 50 µg/ml anti-CD3 mAb. At the initiation of culture, antibodies directed against CD40 or CD40L were added in final concentrations as indicated. After 14 days IgM and IgM-RF production were determined and compared with those measured in anti-CD3-stimulated cultures which were performed in the absence or presence of an isotype-matched control Ab. Similar to IgM production (data not shown), IgM-RF production by PB B cells of HC and patients with RA was abrogated in the presence of anti-CD40 or anti-CD40L (Fig. 3).
Fig. 3.
Inhibition of CD40 signalling abrogates IgM-RF production in T-B cell cultures stimulated with anti-CD3. PB B cells of (a) healthy controls or (b) patients with RA (104/well) were cocultured with T cells (clone K15; 105/well) and immobilized anti-CD3 mAb (50 μg/ml) in the absence or presence of mAb89 (anti-CD40), LL48 (anti-CD40L) or control Ab added at the initiation of culture in final concentrations as indicated. Culture supernatants were harvested after 13 days and analyzed for IgM-RF activity. Results are expressed as mean+s.d. of culture triplicates. 1 representative experiment out of three independent experiments (< below detection limit of 6 mIU/ml; nd not done).
CD40 cross-linking plus cytokines is insufficient for induction of RF production
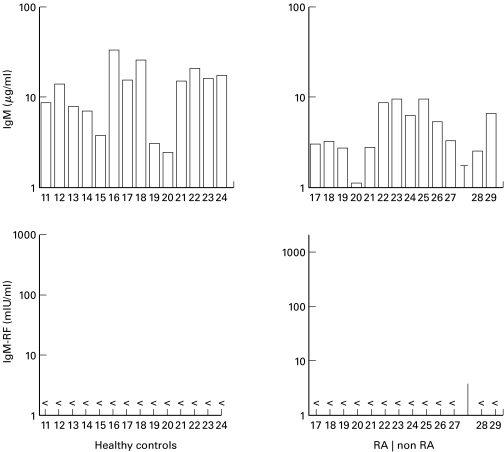
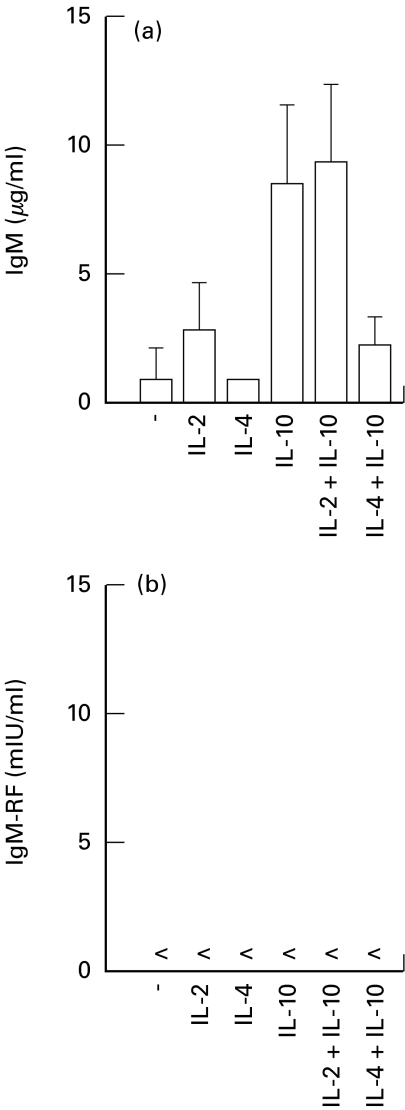
To determine whether the CD40-CD40L B cell activation system was sufficient for the induction of RF production, PB B cells from HC, patients with RA, ankylosing spondylitis or gout were tested in this system using IL-2 plus IL-10. Despite a considerable production of total IgM by these B cells, no IgM-RF activity could be determined in these cultures (Fig. 4). In order to investigate whether other conditions in the CD40 system were necessary for induction of RF production by PB B cells of HC or patients with RA, IL-2, IL-4, IL-10 and combinations of these cytokines were tested. However, non of the conditions applied resulted in production of detectable RFs despite considerable IgM production (Fig. 5). The level of IgM production by PB B cells stimulated by CD40 cross-linking in the presence of IL-10 and the combination of IL-2 and IL-10 were the highest and equalled the amounts produced in the presence of activated T cells (Figs 2,5,6). Thus the defect in RF production could not be explained by an overall decrease in the responsiveness to CD40 activation compared with usage of activated T cells. Similar observations were made using PB B cells from patients with RA (data not shown). Hence, PB RF+ B cells from neither HC nor patients with arthritis, able to synthesize RFs in the T-B cell coculture systems, were triggered to RF production in the CD40 system under the conditions applied.
Fig. 4.
T cell help essential for induction of IgM-RF production by PB B cells of healthy controls and patients with arthritis. PB B cells (104/well) of healthy controls and patients with RA or other forms of arthritis (28: ankylosing spondylitis; 29: gout) were cultured on a layer of irradiated L-CD40L cells (5×105/well; 70 Gy) in the presence of rhIL-2 (40 U/ml) and rhIL-10 (50 ng/ml). Duplicate culture supernatants were harvested after 14 days and analyzed. Data shown are the result of four independent experiments (< below detection limit of 6 mIU/ml).
Fig. 5.
IgM and IgM-RF production as determined in culture supernatants of the CD40 system. PB B cells of healthy controls (104/well) were cultured on a layer of irradiated L-CD40L cells (5×105/well; 70 Gy) in the absence or presence of rhIL-2 (40 U/ml), rhIL-4 (200 U/ml), rhIL-10 (50 ng/ml) or combinations of these cytokines. Duplicate culture supernatants were harvested after 14 days and analyzed. Data shown are the result of two independent experiments. Results are expressed as mean ± s.d. (healthy controls n =4; < below detection limit of 6 mIU/ml).
Fig. 6.
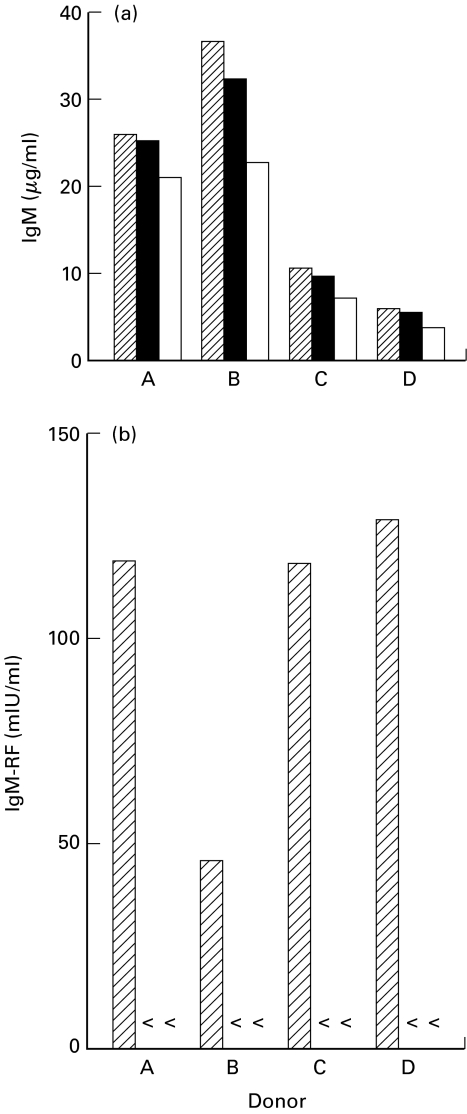
CD40 triggering, in the presence of cytokines, is insufficient to promote IgM-RF secretion in the complete absence of T cells. B lymphocytes of healthy controls or patients with RA (104/well) were cocultured with T cells (clone K15; 105/well) in the presence of immobilized anti-CD3 mAb (50 μg/ml) or cultured on a layer of irradiated L-CD40L cells (5 × 105/well; 70 Gy) in the presence of rhIL-2 (40 U/ml) and rhIL-10 (50 ng/ml) or 50% supernatant (SN) of anti-CD3-stimulated T cell clone K15. Duplicate culture supernatants were harvested after 14 days and analyzed for total IgM and IgM-RF activity. Data shown are the result of two independent experiments. anti-CD3 + T cells; ▪ L-CD40L cells + rhIL-2 + rhIL-10; □ L-CD40L + SN. (A & B, healthy controls; C & D, patients with RA; < below detection limit of 6 mIU/ml).
T cell contact is essential for induction of RF production by PB B cells
To investigate whether other soluble factors released by the T cell clone upon stimulation are essential for induction of physiological RF production, T cell supernatant of T cells, cultured in the presence of 50 µg/ml anti-CD3, was tested in the CD40 system. Substitution of cytokines by culture supernatant of anti-CD3-stimulated T cell clone K15 did not result in RF production in the CD40 system (Fig. 6).
DISCUSSION
It has long been known that B cell precursors with the potential to secrete RFs are frequently present in the normal PB B cell repertoire [22–25]. Utilization of distinct mechanisms for B cell activation allowed us to identify a clear difference in the regulatory control of total Ig and RF production by PB B cells. The difference in activation requirements were similar among HC and patients with RA. Usage of anti-CD3-stimulated T cells induced a significant CD40-dependent RF response in PB B cells, whereas application of the CD40 activation system was insufficient to induce detectable RF whilst comparable amounts of IgM were produced. These results suggest that precursors of RF-secreting cells in the PB compartment have specific activation requirements.
In vivo, RF+ B cells are exposed to IgG. Apparently, B cells in RF-seronegative healthy individuals, capable of synthesizing physiological RFs in vitro, are anergic. In vitro, anti-CD3-stimulated T cells may break the state of tolerance and induce the production of physiological RFs. It is possible that the in vitro conditions are a reflection of physiological processes that occur during, e.g. immunization, since under this circumstance physiological RFs appear in the circulation of healthy individuals [4,5]. The data indicate that RF production by PB B cells in patients with RA has the same induction requirements as physiological RF production by PB B cells in HC. Using the anti-CD3-driven system, only quantitative differences in RF production by PB B cells between HC and patients with RA were observed. B cells from patients with RA produced more RF. This is in line with findings of others [23] who, using a similar approach, reported that patients with RA had a clear expansion of the anti-CD3-driven RF-producing B cell population when compared with HC.
Help provided by anti-CD3-stimulated T cells was an absolute requirement for induction of physiological RF production by PB B cells. Although the T cell help was completely CD40-dependent we were not able to mimic this help by using the CD40 B cell activation system. We have attempted to substitute for T cell involvement by adding T cell-derived cytokines to the CD40 activation system. Neither IL-2, IL-4, IL-10, combinations of these cytokines nor supernatant from activated T cells was sufficient to provide the required T helper signals. The failure to detect RF could not be ascribed to a lower stimulatory capacity of this mode of stimulation since considerable amounts of total IgM were produced which sometimes even equalled production levels found in supernatants of T cell-stimulated B cells. From this result we conclude that conditions to activate physiological RF+ B cells require additional contact between T and B cells besides CD40–CD40L interaction.
Efficient T cell help for the induction of significant RF production was accomplished by using high density anti-CD3-activated T cells. Triggering of T cells by high density immobilized anti-CD3 is known to result in proliferative unresponsiveness [26–30]. This state is characterized as a failure of the cell to produce and secrete IL-2 and proliferate [31,32]. In accordance, in our cultures we observed a inverse relationship between the amounts of immobilized anti-CD3 and T cell proliferation. In this respect it is interesting to mention that we and others have demonstrated that usage of the bacterial superantigen (SAg) staphylococcal enterotoxin D (SE D) in a T-B cell coculture system gives considerable RF production [24–33]. A SAg like SE D functions by cross-linking HLA class II molecules on the surface of B cells with αβ TCR molecules in a Vβ-restricted fashion [34,35]. As such, stimulation by SE D can be regarded as high affinity/density triggering of the TCR resulting in T cell unresponsiveness [35,36]. He and colleagues [33] provided evidence that SE D preferentially stimulated RF+ B cells in a T-B cell coculture system, although the cells did not proliferate. This finding made them suggest that SE D probably acts primarily on the B cell. Together, the combined data lend evidence to hypothesize a role for proliferative unresponsive T cells in the regulation of physiological RF production. Consequently, the production of physiological RF may be linked to the functional status of T cells. Studies with human and murine T cells revealed that anergic T cells have an activated phenotype [36,37] and express CD40L [38,39], which may explain the CD40-dependent mechanism of RF production.
Terminally differentiated CD20−, CD38+ plasma cells present in the synovium of seropositive RA patients are held responsible for the production of pathological RF [8]. In contrast to physiological RF+ B cells these cells actively secrete RF in the absence of a stimulus [8]. These plasma cells are thought to originate from germinal centre (GC)-like structures present in the inflamed synovium [40–44]. T cells which are abundantly present in the inflamed joints, play an important role to allow GC cells to differentiate into plasma blasts. Functional analysis of synovial T cells revealed that these cells are predominantly of the TH1 type [45] and that despite an activated phenotype, these cells show impaired cytokine production and decreased responsiveness toward mitogenic stimulation [46–48]. The functional and biochemical features of these cells, which appear to be specific for RA, are reminiscent to those described for anergic T cells [49]. If functional dichotomy in T cells is related to a specific type of help to B cells, it is tempting to speculate that the persistent presence of hyporesponsive synovial T cells allows RF+ precursors to expand and affinity maturate in high affinity RF-secreting plasma cells.
Acknowledgments
The authors thank Dr J. H. L. M. Groenendael and Dr P. P. Tak for clinical material and data. This work was supported by the Dutch League against Rheumatism (grant NR 842).
REFERENCES
- 1.Withrington RH, Teitsson I, Valdimarsson H, Seifert MH. Prospective study of early rheumatoid arthritis. II. Association of rheumatoid factor isotypes with fluctuations in disease activity. Ann Rheum Dis. 1984;43:679–85. doi: 10.1136/ard.43.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zeben D, Hazes JMW, Zwinderman AH, Cats A, Van der Voort EAM, Breedveld FC. Clinical significance of rheumatoid factors in early rheumatoid arthritis. Ann Rheum Dis. 1992;51:1029–35. doi: 10.1136/ard.51.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan JH. Pathogenetic concepts and origins of rheumatoid factor in rheumatoid arthritis. Arthritis Rheum. 1993;36:1–6. doi: 10.1002/art.1780360102. [DOI] [PubMed] [Google Scholar]
- 4.Tarkowski A, Czerkinsky C, Nilsson L-Å. Simultaneous induction of rheumatoid factor- and antigen-specific antibody-secreting cells during the secondary immune response in man. Clin Exp Immunol. 1985;61:379–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Welch MJ, Fong S, Vaughan J, Carson D. Increased frequency of rheumatoid factor precursor B lymphocytes after immunization of normal adults with tetanus toxoid. Clin Exp Immunol. 1983;51:299–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Carson DA, Chen PP, Fox RI, et al. Rheumatoid factor and immune networks. Ann Rev Immunol. 1987;5:109–26. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- 7.Carson DA, Chen PP, Kipps TJ. New roles for rheumatoid factor. J Clin Invest. 1991;87:379–83. doi: 10.1172/JCI115007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reparon-Schuijt CC, Van Esch WJE, Van Kooten C, Levarht EWN, Breedveld FC, Verweij CL. Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum. 1998;41:2211–20. doi: 10.1002/1529-0131(199812)41:12<2211::AID-ART17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Reparon-Schuijt CC, Van Esch WJE, Van Kooten C, et al. Regulation of synovial B cell survival in rheumatoid arthritis by VCAM-1 (CD106) expressed on fibroblast-like synoviocytes. 2000;43:1115–21. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Peltier A, Christian CL. The presence of the ‘rheumatoid factor’ in sera from patients with syphilis. Arthritis Rheum. 1959;2:1–7. doi: 10.1002/1529-0131(195902)2:1<1::aid-art1780020102>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Svec KH, Dingle JH. The occurrence of rheumatoid factor in association with antibody response to influenza A2 (Asian) virus. Arthritis Rheum. 1965;8:524–9. doi: 10.1002/art.1780080406. [DOI] [PubMed] [Google Scholar]
- 12.Carson A, Bayer AS, Eisenberg RA, Lawrance S, Theofilopoulos A. IgG rheumatoid factor in subacute bacterial endocarditis: relationship to IgM rheumatoid factor and circulating immune complexes. Clin Exp Immunol. 1978;31:100–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Harboe M. Rheumatoid factors in leprosy and parasitic diseases. Scand J Rheumatol (Suppl) 1988;75:309–13. doi: 10.3109/03009748809096783. [DOI] [PubMed] [Google Scholar]
- 14.Roosnek E, Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991;173:487–9. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tighe H, Chen PP, Tucker R, et al. Function of B cells expressing a human immunoglobulin M rheumatoid factor autoantibody in transgenic mice. J Exp Med. 1993;177:109–18. doi: 10.1084/jem.177.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terness P, Opelz G. Natural anti-immunoglobulin autoantibodies: Irrelevant by-products or immunoregulatory molecules? Int Arch Allergy Immunol. 1998;115:270–7. doi: 10.1159/000069457. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Maurice MM, Res PCM, Leow A, et al. Joint-derived T cells in rheumatoid arthritis proliferate to antigens present in autologous synovial fluid. Scand J Rheumatol Supplement. 1995;24:169–77. doi: 10.3109/03009749509100922. [DOI] [PubMed] [Google Scholar]
- 19.Garrone P, Neidhardt E-M, Garcia E, Galibert L, Van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallé A, Zuber CE, Defrance T, Djossou O, De Rie M, Banchereau J. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989;19:1463–7. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- 21.Hermann P, Van Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25:2972–7. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 22.Hirohata S, Inoue T, Miyamoto T. Frequency analysis of human peripheral blood B cells producing IgM-rheumatoid factor. Differential effects of stimulation with monoclonal antibodies to CD3 and Staphylococcus aureus. J Immunol. 1990;145:1681–6. [PubMed] [Google Scholar]
- 23.He X, Goronzy JJ, Weyand CM. The repertoire of rheumatoid factor-producing B cells in normal subjects and patients with rheumatoid arthritis. Arthritis Rheum. 1993;36:1061–9. doi: 10.1002/art.1780360806. [DOI] [PubMed] [Google Scholar]
- 24.Van Esch WJE, Reparon-Schuijt CC, Van Kooten C, Breedveld FC, Verweij CL. Regulation of rheumatoid factor production by B cells from healthy individuals and patients with rheumatoid arthritis. Ann NY Acad Sci. 1997;815:361–3. doi: 10.1111/j.1749-6632.1997.tb52084.x. [DOI] [PubMed] [Google Scholar]
- 25.Koopman WJ, Schrohenloher RE. In vitro synthesis of IgM rheumatoid factor by lymphocytes from healthy adults. J Immunol. 1980;125:934–9. [PubMed] [Google Scholar]
- 26.Wolf H, Müller Y, Salmen S, Wilmanns W, Jung G. Induction of anergy in resting human T lymphocytes by immobilized anti-CD3 antibodies. Eur J Immunol. 1994;24:1410–7. doi: 10.1002/eji.1830240626. [DOI] [PubMed] [Google Scholar]
- 27.Müller Y, Wolf H, Wierenga E, Jung G. Induction of abortive and productive proliferation in resting human T lymphocytes via CD3 and CD28. Immunology. 1999;97:280–6. doi: 10.1046/j.1365-2567.1999.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins MK, Chen C, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 29.Williams ME, Lichtman AH, Abbas AK. Anti-CD3 antibody induces unresponsiveness to IL-2 in Th1 clones but not in Th2 clones. J Immunol. 1990;144:1208–14. [PubMed] [Google Scholar]
- 30.Williams ME, Shea CM, Lichtman AH, Abbas AK. Antigen receptor-mediated anergy in resting T lymphocytes and T cell clones. Correlation with lymphokine secretion patterns. J Immunol. 1992;149:1921–6. [PubMed] [Google Scholar]
- 31.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: Specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–12. [PubMed] [Google Scholar]
- 32.Gutierrez-Ramos JC, Moreno de Alboran I, Martínez AC. In vivo administration of interleukin-2 turns on anergic self-reactive T cells and leads to autoimmune disease. Eur J Immunol. 1992;22:2867–72. doi: 10.1002/eji.1830221117. [DOI] [PubMed] [Google Scholar]
- 33.He X, Goronzy J, Weyand C. Selective induction of rheumatoid factors by superantigens and human helper T cells. J Clin Invest. 1992;89:673–80. doi: 10.1172/JCI115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 35.Lavoie PM, Thibodeau J, Erard F, Sékaly R-P. Understanding the mechanism of action of bacterial superantigens from a decade of research. Immunol Rev. 1999;168:257–69. doi: 10.1111/j.1600-065x.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 36.O'Hehir RE, Lamb JR. Induction of specific clonal anergy in human T lymphocytes by Staphylococcus aureus enterotoxins. Proc Natl Acad Sci USA. 1990;87:8884–8. doi: 10.1073/pnas.87.22.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier CC, Bhandoola A, Borden W, Yui K, Hayakawa K, Greene MI. Unique molecular surface features of in vivo tolerized T cells. Proc Natl Acad Sci USA. 1998;95:4499–503. doi: 10.1073/pnas.95.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telander DG, Mueller DL. Impaired lymphokine secretion in anergic CD4+ T cells leads to defective help for B cell growth and differentiation. J Immunol. 1997;158:4704–13. [PubMed] [Google Scholar]
- 39.Fasler S, Aversa G, Terr A, Thestrup-Pedersen K, De Vries JE, Yssel H. Peptide-induced anergy in allergen-specific human Th2 cells results in lack of cytokine production and B cell help for IgE synthesis. Reversal by IL-2, not by IL-4 or IL-13. J Immunol. 1995;155:4199–206. [Google Scholar]
- 40.Olee T, Lu EW, Huang D-F, et al. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992;175:831–42. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randen I, Brown D, Thompson KM, et al. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992;148:3296–301. [PubMed] [Google Scholar]
- 42.Hakoda M, Ishimoto T, Hayashimoto S, et al. Selective infiltration of B cells committed to the production of monoreactive rheumatoid factor in synovial tissue of patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1993;69:16–22. doi: 10.1006/clin.1993.1144. [DOI] [PubMed] [Google Scholar]
- 43.Dechanet J, Merville P, Durand I, Banchereau J, Miossec P. The ability of synoviocytes to support terminal differentiation of activated B cells may explain plasma cell accumulation in rheumatoid synovium. J Clin Invest. 1995;95:456–63. doi: 10.1172/JCI117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–5. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolhain RJEM, Van der Heiden AN, Ter Haar NT, Breedveld FC, Miltenburg AMM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 46.Lotz M, Tsoukas CD, Robinson CA, Dinarello CA, Carson DA, Vaughan JH. Basis for defective responses of rheumatoid arthritis synovial fluid lymhocytes to anti-CD3 (T3) antibodies. J Clin Invest. 1986;78:713–21. doi: 10.1172/JCI112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nouri AME, Panayi GS. Cytokines and the chronic inflammation of rheumatic disease. III. Deficient interleukin-2 production in rheumatoid arthritis is not due to suppressor mechanisms. J Rheumatol. 1987;14:902–6. [PubMed] [Google Scholar]
- 48.Thomas R, McIlraith M, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992;35:1455–65. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- 49.Maurice MM, Lankester AC, Bezemer AC, et al. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J Immunol. 1997;159:2973–8. [PubMed] [Google Scholar]