Abstract
Pregnancy is an immunological balancing act. Trophoblasts do not express MHC class I or II, except HLA-C and G, but express Fas ligand (FasL), which confers immune privilege. RCAS1 (receptor-binding cancer antigen expressed on SiSo cells) has recently been recognized to play a role in immune evasion of the tumour cells. We therefore studied the involvement of RCAS1 and FasL in the infiltration of NK cells by examining the curettaged uterine contents of 20 cases of early stage of pregnancy. The cases were clinically divided into two groups; curettage was performed (A) due to the absence of foetal heart beats, and (B) due to spontaneous uterine bleeding and abortion. In group A, RCAS1 was expressed in the uterine glands and extravillous cytotrophoblasts, as was FasL. Infiltration of NK cells around the uterine glands was scarcely detected. In contrast, in group B, expression of both RCAS1 and FasL was strikingly decreased in both the level of expression and the numbers of RCAS1/FasL-positive cells and massive infiltration of NK cells was frequently detected around the uterine glands. These findings suggest that a reduction in RCAS1 and FasL expression seems to be closely associated with activation and infiltration of maternal NK cells and destruction of uterine glands, resulting in rejection of the foetus. Thus, expression of RCAS1 and FasL in the uterine glands and cytotrophoblasts may play a role in the downregulation of the maternal immune response, thereby maintaining pregnancy at early stage.
Keywords: RCAS1, FasL, NK cells, pregnancy
INTRODUCTION
The placenta separates the foetal and maternal blood and lymphoid systems. Fetal trophoblasts are considered to play a major role in evading recognition by the maternal immune system. The potential immunological mechanisms involved in the maintenance of pregnancy were originally described by Medawar [1], and are that (1) the foetus does not engender an immune response (2) the maternal immune response is suppressed (3) the uterus is an immunologically privileged site, and thus the placenta is a barrier between the mother and foetus [2]. The most important factors for the maintenance of pregnancy appear to lie at the uterus–placenta interface. In particular, expression of FasL and complement regulatory proteins, and failure to express MHC class I and II molecules in the placenta are thought to be crucial factors for maintence of pregnancy [2–6].
Trophoblast cells fail to express MHC class I or class II molecules, except HLA-C and HLA-G [7–9]. In addition, the trophoblast also protects itself by expression of Fas Ligand (FasL) [4,5], thereby conferring immune privilege in a similar way as the cornea and Sertoli cells of the testis [10]. Fas is expressed on many cells, whereas FasL expression is restricted to sites of immune privilege and activated CTL and CD4 positive Th1 cells. In mice, FasL is also expressed on uterine glandular epithelial cells and decidual cells in placental trophoblasts. Predominant expression of FasL in mice is found in the uterus at 6–10 gestation days, and this then shifts to the placenta at 12–14 gestation days during pregnancy. Recently, FasL expression was also reported in first trimester and term human placental villi [11]. Thus, expression sites of FasL are obviously positioned to induce apoptosis in maternal Fas positive immune cells, such as NK and T cells [4].
NK cell activity is depressed in the decidua at the early stages of normal pregnancy. The maternal–foetal interaction partly depends on an NK allorecognition system [12]. However, it has been reported that the relative proportion of decidual NK cells and their activity are significantly higher in anembryonic pregnancy and recurrent spontaneous abortion, in comparison to normal pregnancy. Thus, NK cells are considered to play a role in the maternal immune response against foetus [13,14].
Tumour-associated antigens that can be recognized by the immune system include the MAGE-family, p53, MUC-1, HER2/neu, p21ras and so on [15,16]. Despite expression of these distinct antigens, tumour elimination by the immune system is often inefficient. Tumour cells may also evade immune attack by expressing FasL or other molecules that induce apoptosis in activated T cells [17]. Recently Nakashima et al. [18] described RCAS1 (receptor-binding cancer antigen expressed on SiSo cells), a membrane molecule expressed on human cancer cells, which are detected by 22–1-1 monoclonal antibodies for RCAS1 [19]. RCAS1 appears to act as a ligand for a putative receptor present on various human cells and normal peripheral lymphocytes such as T, B and NK cells. RCAS1 inhibits in vitro growth of the receptor-expressing cells and induces apoptotic cell death. Thus, RCAS1 is considered to play a role in the escape of tumour cells from immune surveillance [18].
Since RCAS1 expression appears to inactivate the host immune response, it is postulated that RCAS1 may also play a role in placental immune privilege. In the present study, the relationship between expression of RCAS1 and FasL, and NK cell infiltration in the human placenta was investigated at an early stage of pregnancy.
MATERIALS AND METHODS
Tissue samples
20 samples of the products of conception at early pregnancy stages (6–10 weeks) were selected from the tissue specimen files maintained in the Department of Pathology, Fukuoka University, from 1998 to 1999. The cases were divided into two groups according to clinical diagnosis: curettage performed due to either the absence of foetal heart beats (group A), or spontaneous uterine bleeding (group B). The samples were fixed in buffered formalin, embedded in paraffin, and then stained with haematoxylin-eosin. As controls, we used 3 samples of normal term placental tissue and 3 samples of endometrial tissue,which were obtained during operations for leiomyoma.
Immunohistochemistry
Serial tissue samples of 3 µm thick dewaxed paraffin sections were prepared and incubated at 121°C for 10 min. Then tissue sections were incubated with the following monoclonal antibodies; L26 (CD20) monoclonal antibody (MAb) specific for B cells, anti-CD3 MAb for T cells (Dakopatts, Copenhagen, Denmark), anti-CD56 MAb for natural killer cells (T Cell Diagnostic, Cambridge, MA) and monoclonal antibody to Granzyme B (Pharmacell, Paris, France). An anti-Fas ligand (FasL) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was also used for immunohistochemical staining, as described previously [20]. Biotinylated horse anti mouse IgG antibody (Vector, Burlingame, CA) was used as the second antibody. Immunohistochemical staining was visualized by alkaline phosphatase conjugated streptavidin (Dakopatts).
For detection of RCAS1, the 22–1-1 hybridoma culture supernatants, which were diluted 1 : 20 in PBS, were utilized, as described in a previous report [19]. For the second antibody, biotinated goat F(ab)′2 antimouse IgM antibody, absorbed with human and mouse tissues (Vector), was used.
In the case of RCAS1 and FasL staining, samples with less than 5% reactive cells per field were considered negative (–), 5–10% reactive cells were weakly positive (+ –), and over 10% reactive cells were defined as positive (+).
For NK cells (CD56-positive cells), mononuclear cells with less than 1% reactive cells were defined as negative (–), 1–5% were weakly positive (+ –), 5–10% were positive (+), 10–20% were strongly positive (+ +), and > 20% were very strongly positive (+ + +).
RESULTS
Decreased expression of RCAS1 and FasL in trophoblasts and uerine glands in cases with spontaneous foetus rejection
Samples of the products of conception were collected from cases of early pregnancy with the absence of foetal heart beats (group A in Table 1). In these cases, there were no clinical and histological signs of graft rejection by maternal immune cells. As shown in Fig. 1A), RCAS1 was expressed in extravillous cytotrophoblasts but not in villous cytotrophoblasts or syncytiotrophoblasts. RCAS1 expression was also detected in the uterus glands (Fig. 1E). FasL was expressed in the extravillous cytotrophoblasts but also in villous cytotrophoblasts and syncytiotrophoblasts (Fig. 1B), in contrast to RCAS1. FasL was also positive in the uterine glands of some cases (Fig. 1F). Besides the expression of RCAS1 and FasL in trophoblasts, RCAS1-positive histiocytes were intermingled in chorionic villi (Fig. 1C). FasL-positive histiocytes were also detected in chorionic villi and decidual tissues (Fig. 1D).
Table 1.
The findings of RCAS1 and FasL expression and NK cell infiltration
| RCAS | FasL | NK cell infiltration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trophoblast | Trophoblast | Trophoblast | |||||||||
| Patient | Age/sex | Group | villous | extra | Uterine glands | villous | extra | Uterine glands | villous | extra | Uterine glands |
| 1 | 38F | A | − | + | + | + ‡ | + | + − | − | − | + − |
| 2 | 25F | A | − * | + | + | + ‡ | + | + − | − | − | + − |
| 3 | 32F | A | − | + | + − | + ‡ | + | + | − | − | + − |
| 4 | 28F | A | − | + | + − | + ‡ | + | + − | − | − | − |
| 5 | 35F | A | − * | + | + | + ‡ | + | + − | − | − | + − |
| 6 | 24F | A | − * | + | + | + ‡ | + | + − | − | − | − |
| 7 | 29F | A | − * | + | + | + ‡ | + | + | − | − | − |
| 8 | 29F | A | − * | + | + | + ‡ | + | + | − | − | − |
| 9 | 27F | A | + − * | + | + | + ‡ | + | + | − | − | − |
| 10 | 30F | B | − | + − | − | + − ‡ | + − | − | − | + − | + + † |
| 11 | 25F | B | − | + | + − | − ‡ | − | − | − | + | + + † |
| 12 | 30F | B | − | − | + − | − ‡ | − | − | − | − | + + + † |
| 13 | 27F | B | − | − | + − | − ‡ | + − | − | − | − | + + † |
| 14 | 23F | B | − | + − | + − | + − ‡ | + − | − | − | − | + + + † |
| 15 | 23F | B | − | + − | + − | + − ‡ | − | + − | − | − | + + † |
| 16 | 37F | B | − | + − | + − | − ‡ | − | + − | − | − | + + † |
| 17 | 39F | B | − | + − | + − | + − ‡ | + − | + − | − | − | + + † |
| 18 | 24F | B | − | − | − | − ‡ | + − | − | − | − | + † |
| 19 | 24F | B | − | − | + − | − ‡ | + − | + − | − | − | + + † |
| 20 | 24F | B | − | + − | − | + − ‡ | + − | + − | − | − | + |
villous, villous cytotrophoblasts and syncytiotrophoblasts; extra, extravillous cytotrophoblast
RCAS1-positive histiocytes are present
FasL-positive histiocytes are present.
Most of CD56 positive NK cells express granzyme B and FasL.
Uterine glands were destroyed by infiltrated NK cells.For RCAS1 and FasL, the regions with < 5% reactive cells were considered negative (–), those with 5–10% reactive cells were weakly positive (+ –), and those with > 10% reactive cells were defined as positive (+).For NK cells (CD56-positive cells), the mononuclear cells with < 1% reactive cells were defined as negative (–), with 1–5% were weakly positive (+ –), with 5–10% were positive (+), 10–20% were strongly positive (+ +),and > 20% were (+ + +).
Fig. 1.
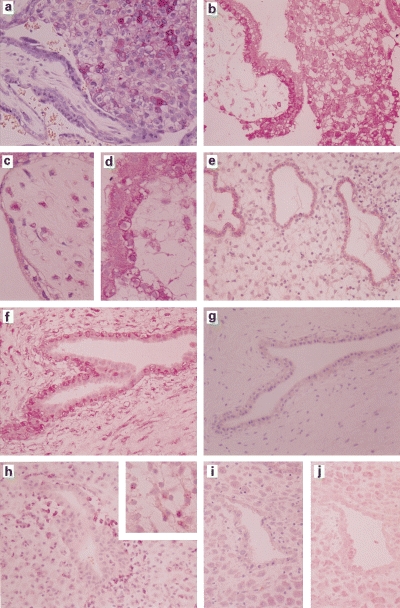
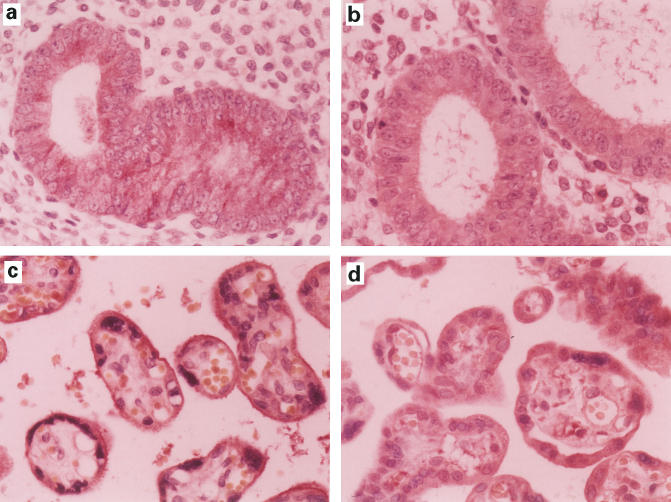
Immunohistochemical staining. (a-d) Trophoblasts and uterine glands from group A cases. RCAS1 is expressed in extravillous cytotrophoblasts, but not in villous cytotrophoblasts and syncytiotrophoblasts (a). FasL is expressed in extravillous cytotrophoblasts, villous cytotrophoblasts, and syncytiotrophoblasts (b). RCAS1-positive histiocytes were intermingled in chorionic villi (c), and FasL-positive histiocytes were also detected in chorionic villi (d). The uterine glands expressed RCAS1 (e) as well as FasL (f). No CD56-positive NK cells infiltrate the uterine glands (g). (h-j) Trophoblasts and uterine glands from the group B cases. CD56-positive NK cells infiltrate the uterine glands (h). The NK cells contain granzyme B in the cytoplasm (window in h); However, expression of RCAS1 (i) and FasL (j) are not detected.
On the other hand, in group B, in which the samples of the conception products were collected from cases with spontaneous uterine bleeding and abortion, expression of RCAS1 was rarely detected in the villous cytotrophoblasts, extravillous cytotrophoblasts or syncytiotrophoblasts. In the uterine glands, a few RCAS1-positive cells were detected. Not only RCAS1, but also FasL, expression was markedly decreased in the trophoblasts and uterine glands (Fig. 1I,J). Interestingly, RCAS1-positive histiocytes were rarely intermingled in the chorionic villi, whileFasL-positive histiocytes were still detected and intermingled in the chorionic villi.
In the controls, RCAS1 was expressed in the extravillous cytotrophoblasts of term placental tissue, and in uterine glands, as for group A. FasL was also expressed in trophoblasts and uterine glands (Fig. 2).
Fig. 2.
Immunohistochemical staining of controls. (Samples obtained during operation for leiomyoma). RCAS1 (a) and FasL (b) were expressed in uterine glands. RCAS1 was expressed in villous cytotrophoblasts of term placental tissue (c). FasL was expressed in trophoblasts (d).
Infiltration of NK cells around uterine glands in cases with spontaneous foetus rejection
In order to investigate infiltration of NK cells in uterine glands and decidual tissues in both group A and B cases, tissue samples were stained with either anti-CD56 antibodies or antibodies to granzyme B. As shown in Fig. 1H, CD56 positive cells are frequently detected around the uterine glands in group B, but not group A samples (Fig. 1G). NK cells infiltrated into the uterine glands were strongly positive for granzyme B (Fig. 1H, window), indicating that they were activated and appeared to attack uterine glands. In both group A and B, CD3-positive T cells were rarely observed in decidual and uterine tissues.
In the controls, NK cells were rarely encountered in term placental tissue and endometrium.
DISCUSSION
In the present study, the corelation between RCAS1 or FasL expression and the infiltration of NK cells was studied in the human uterus at an early stage of pregnancy. In cases where a maternal immune response against foetal cells appeared not to be evident, RCAS1 was expressed in the uterine glands and extravillous cytotrophoblasts. In addition, FasL was also expressed in the villous trophoblasts, uterine glands and extravillous cytotrophoblasts. There was no infiltration of NK cells into deciduas. On the other hand, in cases of deciduas and uterine glands from curettaged samples collected because of spontaneous bleeding and rejection, expression of RCAS1 and FasL was strikingly decreased in both level of expression and the numbers of RCAS1/FasL-positive cells. In contrast, NK cells were frequently detected around the uterine glands. These findings suggest that a reduction in RCAS1 and FasL expression closely related to infiltration and activation of NK cells at the maternal–foetal interface.
The placenta separates the foetus from the maternal blood and lymphoid systems. The chorionic villi are composed of foetal stem vessels and mesenchyme surrounded by the villous cytotrophoblast and syncytiotrophoblast. Extravillous cytotrophoblasts proliferate from chorionic villi, forming a shell, and invade into the maternal deciduas. Anatomically, the trophoblasts must play a major role in evading recognition by maternal immune system [6]. Functionally, trophoblasts fail to express MHC class I or class II molecules, except HLA-C and HLA-G [7–9] and the extravillous cytotrophoblast cells express FasL, which might induce apoptosis in Fas-positive immune cells [4,5]. In addition, trophoblasts also express complement regulatory proteins, such as CD46, CD55, and CD59 [21], and produce various kinds of cytokines, which shift regional helper T cells from a Th1 to Th2 cell type [22,23]. NK cells become a prominent cell population in the rodent uterus during pregnancy, but their activity is depressed in the decidua at early stages of normal pregnancy, but is high in abortion [13,14]. Therefore, NK cells are considered to play an important role in rejection of the foetus by the maternal immune response during pregnancy. These findings indicate that there must be a generalized immunosuppression in the uterus during pregnancy in order to protect the foetus from the maternal immune reaction. However, allografts placed in an intrauterine site are still rejected [6], suggesting the existence of a specific immunosuppression mechanism in the placenta.
FasL, a member of the TNF gene family [24], restricts migration of activated lymphocytes into the testis and anterior chamber of the eye by delivering a death signal to the lymphocytes through their Fas receptors [25]. In mice, at gestation days (g.d.) 6–10, immunoreactive FasL was prominent in the glandular epithelial cells and decidual cells [4]. Between g.d. 12 and 14, FasL expression shifted to the placental trophoblast cells bordering the maternal blood spaces and foetal placental endothelial cells. Thus, FasL is appropriately positioned during pregnancy, first in the uterus and then in the placenta, to deter trafficking of activated Fas-positive immune cells. In gld mice, a mutant strain lacking functional FasL, extensive leucocytic infiltrates and necrosis at the decidual–placental interface were observed [4]. In addition, NK cells were lost from the uteri of pregnant Fas antigen-deficient lpr mice, whose placenta was bigger than normal [14]. These observations are consistent with the idea that FasL protects the placenta against a maternal leukocytic influx at the maternal–foetal interface which would otherwise reduce fertility [4]. Recently, FasL expression was detected in first trimester and human placental villi [11]. and functionally FasL expressed human trophoblasts inhibited the growth of Fas-bearing Jurkat T cells in a coculture study [26]. In the present study, the reduction of FasL expression in human unterine glands was closely associated with NK cell infiltration and destruction of the glands. The findings also support the notion that FasL inhibits NK cells and plays an immune privileged role to maintain pregnancy. Recently, it has been reported that the extravillous cytotrophoblast cells express HLA-G, which may downregulate NK cell function [3,7].
Maternal T cells acquire a transient state of tolerance specific for paternal alloantigens at least in part through Fas/FasL interactions [5]. However, approximately 60% of lymphocytes, which are present in the vicinity of the feto-placental allograft, are NK cells and 15% are CTLs [27]. In the present study, the majority of the cells found in the human placenta at early stage of pregnancy with spontaneous bleeding rejection were also NK cells and expressed granzyme B, which mediates perforin cytotoxicity, in the cytoplasm [28,29]. These findings support a role of actived NK cells in the maternal rejection of the foetus.
Tumour cells may also evade immune attack by expressing FasL or other molecules that induce apoptosis in activated T and NK cells. Nakashima et al. [18] recently described RCAS1 as a candidate molecule for an immune evasion system of human cancer cells. Previously, RCAS1 was found to be expressed at a high frequency on uterine and ovarian tumour cells as well as on various kinds of nongynecological cancers, such as colon, skin and lung cancers [19]. In addition, RCAS1 was highly expressed on invasive carcinomas, and expression of RCAS1 was correlated with poor prognosis in uterine cancers [30]. RCAS1 appears to act as a ligand for a putative receptor present on various human cells including normal lymphocytes such as T, B and NK cells. RCAS1 receptor expression on T cells and NK cells is strongly enhanced once the cells are activated. Addition of recombinant or soluble RCAS1 to receptor positive cells inhibits cell proliferation and induces apoptotic cell death [18]. Given these results, tumour cells may evade immune surveillance by expression of RCAS1, which would suppress clonal expansion and induce apoptosis in RCAS1 receptor-positive T and NK cells [18]. In the present study, RCAS1 was expressed in uterine glands and extravillous cytotrophoblasts in cases without maternal rejection. In contrast, expression of RCAS1 strikingly decreased in cases with maternal rejection, which were accompanied by the marked infiltration and activation of NK cells. In addition to FasL, the reduction of RCAS1 expression seems to be closely associated with NK cell activation and destruction of uterine glands. There is also the possibility, however, that the down regulation of FasL and RCAS1, and activation of NK cells might be a consequence of the abortion process rather than its cause. Although it was not clear in the present study whether RCAS1 or FasL plays a more important role in maintence of the pregnancy, RCAS1 may play a role as one of the failsafe mechanisms to inhibit maternal immune attack and ensure that pregnancy is maintained.
REFERENCES
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–8. [Google Scholar]
- 2.Wood GW. Is restricted antigen presentation the explanation of fetal allograft survival? Immunol Today. 1994;17:407–9. doi: 10.1016/0167-5699(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 3.Le Bouteiller P, Mallet VHLA-G. and pregnancy. Rev Reprod. 1997;2:7–13. doi: 10.1530/ror.0.0020007. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JS, Vassmer D, Ferguson TA, Miller L. FasLigand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–8. [PubMed] [Google Scholar]
- 5.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 6.Weetman AP. The immunology of pregnancy. Thyroid. 1999;9:643–6. doi: 10.1089/thy.1999.9.643. [DOI] [PubMed] [Google Scholar]
- 7.Ellis SA, Palmer MS, McMichael AJ. Human trophoblasts and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J Immunol. 1990;144:731–5. [PubMed] [Google Scholar]
- 8.Hammer A, Hutter H, Dohr G. HLA class I expression on the materno–fetal interface. Am J Reprod Immunol. 1997;38:150–7. doi: 10.1111/j.1600-0897.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 9.Proll J, Blaschitz A, Hutter H, Dohr G. First trimester human endovascular trophoblast cells express both HLA-C and HLA-G. Am J Reprod Immunol. 1999;42:30–6. doi: 10.1111/j.1600-0897.1999.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 10.Streilein JW. Unravelling immune privilege. Science. 1995;270:1158–9. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 11.Hammer A, Dohr G. Expression of Fas-ligand in first trimester and term human placental villi. J Reprod Immunol. 2000;46:83–90. doi: 10.1016/s0165-0378(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 12.King A, Burrows T, Loke YW. Human uterine natural killer cells. Nat Immun. 1996;15:41–52. [PubMed] [Google Scholar]
- 13.Chao KH, Yang YS, Ho HN, et al. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J Reprod Immunol. 1995;34:274–80. doi: 10.1111/j.1600-0897.1995.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 14.Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol. 1996;59:262–269. [PubMed] [Google Scholar]
- 15.Van den Eynde BJ, Van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:1115–23. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 16.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 17.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (Apo-1/Fas) ligand-expressing tumor cells – a mechanism of immune evasion? Nature Med. 1996;12:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med. 1999;5:938–42. doi: 10.1038/11383. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda K, Nakashima M, Kaku T, Kamura T, Nakano H, Watanabe T. A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer. 1996;77:1501–9. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1501::AID-CNCR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Kume T, Ohshima K, Yamashita Y, Shirakusa T, Kikuchi M. Relationship between FasLigand expression on carcinoma cell and cytotoxic T-lymphocyte response in lympoepithelioma-like cancer of the stomach. Int J Cancer. 1999;84:339–43. doi: 10.1002/(sici)1097-0215(19990820)84:4<339::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Holmes CH, Simpson KL. Complement and pregnancy: new insight into the immunology of the fetomaternal relationship. Bailliere's Clin Obstet Gynaecol. 1992;6:439–60. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 22.Nahmias AJ, Kourtis AP. The pregnant woman, placenta, fetus, and infectious agents. Clin Perinatol. 1997;24:497–521. [PubMed] [Google Scholar]
- 23.Lim KJH, Odukoya OA, Ajjan RA, Li TC, Weetman AP, Cooke ID. Profile of cytokine mRNA expression in periplantation human endometrium. Mol Hum Reprod. 1998;4:77–81. doi: 10.1093/molehr/4.1.77. [DOI] [PubMed] [Google Scholar]
- 24.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the FasLigand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 25.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 26.Balkundi DR, Hanna N, Hileb M, Dougherty J, Sharma S. Labor-associated changes in Fas ligand expression and function in human placenta. Pediatr Res. 2000;47:301–8. doi: 10.1203/00006450-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Rukavina D, Podack ER. Abundant perforin expression at the maternal–fetal interface: guarding the semiallogeneic transplant? Immunol Today. 2000;21:160–3. doi: 10.1016/s0167-5699(00)01603-0. [DOI] [PubMed] [Google Scholar]
- 28.Kägi D, Vignaux F, Ledermann B, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 29.Shiver JW, Su L, Hankart PA. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71:315–22. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- 30.Kaku T, Sonoda K, Kamura T, et al. The prognostic significance of tumor-associated antigen 22-1-1 expression in adenocarcinoma of the uterine cervix. Clin Cancer Res. 1999;5:1449–53. [PubMed] [Google Scholar]