Abstract
The thiol antioxidant N-acetyl-l-cysteine (NAC), known as a precursor of glutathione (GSH), is used in AIDS treatment trials, as a chemoprotectant in cancer chemotherapy and in treatment of chronic bronchitis. In vitro, GSH and NAC are known to enhance T cell proliferation, production of IL-2 and up-regulation of the IL-2 receptor. The 120-kD CD30 surface antigen belongs to the tumour necrosis factor (TNF) receptor superfamily. It is expressed by activated T helper (Th) cells and its expression is sustained in Th2 cells. We have analysed the effect of GSH and NAC on the cytokine profile and CD30 expression on human allergen-specific T cell clones (TCC). TCC were stimulated with anti-CD3 antibodies in the presence of different concentrations of GSH and NAC. Both thiols caused a dose dependent down-regulation of IL-4, IL-5 and IFN-γ levels in Th0 and Th2 clones, with the most pronounced decrease of IL-4. Furthermore, they down-regulated the surface expression of CD30, and the levels of soluble CD30 (sCD30) in the culture supernatants were decreased. In contrast, the surface expression of CD28 or CD40 ligand (CD40L) was not significantly changed after treatment with 20 mm NAC. These results indicate that GSH and NAC favour a Th1 response by a preferential down-regulation of IL-4. In addition, the expression of CD30 was down regulated by GSH and NAC, suggesting that CD30 expression is dependent on IL-4, or modified by NAC. In the likely event that CD30 and its soluble counterpart prove to contribute to the pathogenesis in Th2 related diseases such as allergy, NAC may be considered as a future therapeutic agent in the treatment of these diseases.
Keywords: CD30, GSH, NAC, T cell clones, cytokines
INTRODUCTION
Glutathione (GSH) is present in millimolar concentrations in mammalian cells. It plays numerous roles, for instance in defence against oxidative stress and as an electron donor to glutaredoxin, which is involved in deoxyribonucleotide and DNA synthesis. GSH acts as a redox buffer to preserve the intracellular reduced environment [1, 2]. A precursor of GSH, the thiol antioxidant N-acetyl-l-cysteine (NAC), is known as a mucolytic agent [3]. It has been shown that NAC can restore the intracellular levels of GSH in HIV infected patients [4], and NAC has therefore been proposed as an anti-HIV agent [5]. NAC is also used in cancer treatment [6]. In vitro, GSH and NAC are known to enhance T cell proliferation, as well as the production of interleukin (IL)-2 and synthesis and turnover of the IL-2 receptor [7, 8]. These thiols decrease the IL-4 production in human T cells [9, 10] and reduce the production of IgE and IgG4 by B cells [9]. Moreover, recent studies in vivo and in vitro reveal that GSH depletion in antigen presenting cells (APC) inhibits T helper (Th) 1 associated cytokines, and/or favours Th2 associated responses [11]. In studies using B cell lines, Yanagihara et al. [12] show that NAC regulates the IgE isotype switching by inhibiting the activation of nuclear factor-κB (NF-κB). In a recently published study it has also been shown that NAC inhibits primary human T cell responses elicited by dendritic cells [13].
The 120 kD CD30 is preferentially expressed by activated lymphoid cells, particularly activated peripheral blood B and T cells. Small populations of CD30+ cells can also be found in the peripheral lymphoid organs, as well as in the thymus [14–17]. This cellular distribution suggests that CD30 is involved in the regulation of immune responses. The CD30 ligand (CD30L) has been identified [18]. Ligation of CD30 with recombinant CD30L or crosslinking with certain anti-CD30 antibodies can activate T cells to proliferate and produce cytokines [19, 20]. It has also been suggested that CD30 can work as a costimulatory molecule in a secondary T cell proliferative response [21] and that CD30 ligation promotes the development of human Th2 cells [22]. Increased CD30 expression appears to play a role in diseases characterized by increased levels of CD4 or CD8 lymphocytes which produce Th2 cytokines [23, 24]. We have previously demonstrated elevated levels of the soluble counterpart of CD30 (sCD30, 88 kD) in patients with atopic dermatitis (AD) [25]. In addition, large numbers of lymphocytes expressing CD30 and producing Th2 type cytokines are also found in the skin of patients with AD but not in patients with allergic contact dermatitis [26]. CD30 appears to be important in AIDS, where CD30 signalling enhances HIV replication via tumour necrosis factor receptor-associated factor (TRAF)-2-mediated NF-κB activation [27], and elevated levels of sCD30 in serum correlate with disease progression [28]. Increased expression of CD30 antigen and elevated levels of sCD30 have also been found in patients with systemic sclerosis [29], or systemic lupus erythematosus (SLE) [30]. CD30 expression by tissue-infiltrating activated T lymphocytes and increased levels of sCD30 in serum are also typical of Omenn's syndrome, a severe immunodeficiency characterized by a Th2 type response [31].
An appropriate Th1/Th2 balance is critical for an effective and nonpathological immune function. The fact that NAC can regulate cytokine production, HIV replication and IgE switch, makes it very interesting as a potential therapy for immunological disorders. To further investigate the effect of GSH and NAC on T helper lymphocytes, and the role of these thiols in inhibiting a Th2 response, we have conducted a study, using human allergen-specific TCC, where we have examined whether there is a change in cytokine production after GSH/NAC treatment. We have also determined the surface CD30 expression, as well as the presence of sCD30 in culture supernatants, after treating the cells with GSH and NAC. If CD30 is shown to play a role in the pathogenesis of Th2 related diseases like allergy, novel therapies that inhibit CD30 expression, or molecules that are involved in CD30 signal transduction could help treat these diseases.
MATERIALS AND METHODS
Compounds
GSH and NAC were purchased from Sigma Chemical Company (Sigma-Aldrich Sweden AB, Stockholm, Sweden). Both compounds were dissolved in AIM-V culture medium (Life Technologies, Täby, Sweden) or PBS; the pH was adjusted to 7·4 and the solutions were sterile filtered (0·22 µm) before use.
T cell clones
TCC specific for Pityrosporum orbiculare (also denoted Malassezia furfur) were generated from freshly isolated peripheral blood mononuclear cells (PBMC) and skin biopsy specimens from two patients with atopic dermatitis (AD), as previously described [32]. The T cell clones were cultured in flat-bottomed 12-or 24-well plates (Costar, Cambridge, UK), in Iscove's modified Dulbecco's medium (Life Technologies) supplemented with gentamycin (25 U/ml), penicillin (100 IU/ml), streptomycin (100 IU/ml), l-glutamine (2 mm) (Merck, Darmstadt, Germany), and 10% heat inactivated foetal calf serum (FCS, Hyclone Laboratories Inc., Lagen, UT, USA). The cell cultures were stimulated every second week with phytohaemagglutinin (PHA; 2 µg/ml) (Pharmacia Biotech, Uppsala, Sweden), feeder cells (irradiated, 30 Gy, peripheral blood mononuclear cells from two blood donors, 0·5 × 106 cells/ml), and recombinant human IL-2 (rIL-2; 20 U/ml; Amersham Laboratories, Bucks, UK). The T cell clones were all CD4 +, as determined by flow cytometry, and differentially classified as Th0 or Th2 according to their cytokine profiles, determined by ELISA (see below). The T cell clones with an IL-4/IFN-γ ratio between 0·2 and 5 were defined as Th0 and those with a ratio more than 5 as Th2. The Th0 clones designated 24 : 12, 24 : 17, 29 : 19, 25 : 4 and the Th2 clones 12 : 3, 19 : 3, 24 : 6, 24 : 9, 28 : 12 were included in this study.
Activation of T cell clones
For anti-CD3 activation 24-well plates (Costar) were coated with goat-antimouse immunoglobulin G (IgG, Dakopatts, Copenhagen, Denmark, 10 µg/ml) in 0·05 m Tris buffer solution, pH 9·8, for 1 h at room temperature. The wells were washed three times with phosphate-buffered saline (PBS, pH 7·4) and thereafter incubated with anti-CD3 antibodies (dilution 1 : 5000) (OKT3 hybridoma supernatant; Ortho Diagnostic Systems, Rariton Inc., Johnson & Johnson, New Brunswick, NJ, USA) diluted in PBS with 2% heat-inactivated human AB Rh + serum for 1 h at room temperature. The antibody solution was then aspirated and approximately 1 × 106 cells were added to the wells in 1 ml of serum free medium (AIM-V, Life Technologies) supplemented with PHA (10 µg/ml) and cultured for 48 h at 37°C.
For the other experiments where cells were treated with GSH or NAC, 1 × 106 cells were added to the wells in 1 ml of AIM-V medium, in the presence of rIL-2 (20 U/ml) and different concentrations of GSH (0·5–20 mm) or NAC (0·5–20 mm) and incubated at 37°C for 16, 24 and 48 h. Culture supernatants were centrifuged at 300 g and stored at 20°C prior to analysis.
Analysis of cytokine production by ELISA
IL-4, IFN-γ and IL-5 were detected in the culture supernatants by the use of ELISA according to the manufacturer's instructions (IL-4 and IFN-γ, Mabtech AB, Stockholm, Sweden; IL-5, Becton Dickinson AB, Stockholm, Sweden). Briefly, 96-well plates (Nunc, Roskilde, Denmark) were coated with monoclonal antibodies (MoAbs) to IL-4 (IL4-I, 1 µg/ml, 100 µl/well), anti-IFN-γ MoAbs (1-D1K, 2 µg/ml, 100 µl/well) or anti-IL-5 MoAb (TRFK5, 4 µg/ml, 100 µl/well) and incubated overnight at 4°C, followed by incubation with 1% bovine serum albumine (BSA) (100 µl/well) for 1 h. Standards and samples were then added to the wells in duplicates (100 µl/well), incubated overnight at 4°C, followed by the addition of biotin-labelled anti-IL-4 (IL-4 II, 1 µg/ml), anti-IFN-γ (7-B6–1, 1 µg/ml) or anti-IL-5 (JES1–5A10, 1 µg/ml). Next, streptavidin-alkaline phosphatase (Mabtech AB, dilution 1/1000) was added (100 µl/well), followed by addition of the substrate (p-nitro-phenyl-phosphatase disodium: Sigma, St. Louis, MO, USA), diluted in 1 m diethanolamine buffer, pH 9·8. After this, an ELISA plate reader (Labsystems Multiskan RC, Helsinki, Finland) measured the absorbance at 405 nm. As standards, human recombinant (rh) IFN-γ (PreproTech Inc. London, UK), rhIL-4 (PreproTech Inc.) and rhIL-5 (Pharmingen, Becton Dickinson AB, Stockholm, Sweden) were used. The detection limit for IFN-γ, IL-4 and IL-5 was 0·03 ng/ml.
Analysis of sCD30 by ELISA
The concentration of sCD30 in the culture supernatants was determined by a sandwich enzyme linked immunosorbent assay (CD30, Ki-1 antigen, ELISA; Dako, Glostrup, Denmark), as previously described [25], where the mouse monoclonal antibodies (MoAbs) used for coating and conjugation, respectively, are noncompetitive and react with different epitopes on the CD30 molecule. Samples were analysed in duplicate. According to the manufacturer's description, for each of 4 specimens, the imprecision was calculated from the means of up to 5 duplicate determinations, for 5 separate runs. The coefficient of variation (CV) was estimated to be < 8%. By determination of the mean absorbance + 2 SD of 20 measurements of the 0 U/ml standard, the detection limit of the assay was estimated to be 1 U/ml. The culture supernatants were kept frozen at 20°C until analysed.
Flow cytometry
Cell surface expression of CD4, CD30, CD40L and CD28 was analysed by flow cytometry. Briefly, cells (5 × 105) were stained with fluoresceine isothiocyanate (FITC)-conjugated anti-CD30 MoAb (Ber-H2, Dakopatts), FITC-conjugated anti-CD4 (Leu3a), phycoerythrin (PE)-conjugated anti-CD28 (L293), PE-conjugated anti-CD154 (antihuman CD40L), FITC-and PE-IgG1 isotype matched control antibody (all from Becton Dickinson AB) for 30 min at 4°C. Cells were then washed twice with PBS, resuspended in 0·5 ml of PBS and 104 cells were analysed by flow cytometry with fluorescence excitation at 488 nm with a 15-nW argon laser (FACSCalibur, Becton Dickinson AB). Data were analysed using Cellquest software (Version 1·2.2) Becton Dickinson AB). The flow cytometer was calibrated according to the manufacturer's instructions before each acquisition. Dead cells were excluded with propidium iodide (PI) and/or by cell size. According to PI staining > 90% of the cells in the studied population were viable when analysed. Treatment with 0·5–20 mm NAC did not change the viability.
Immunocytochemistry
TCC were dripped onto three-well glass slides (4 × 104 cells per well) (Novakemi AB, Enskede, Sweden), air-dried, and stored at − 80°C. Before staining, the cells were fixed with acetone. For staining with peroxidase antiperoxidase (PAP), the cells were incubated in 0·3% H2O2 in PBS for 15 min at room temperature to block endogenous peroxidase, followed by incubation with normal rabbit serum (diluted 1/10) for 10 min to reduce nonspecific staining. After this the cells were incubated for 60 min with the primary antibody Ber-H2 (anti-CD30, dilution 1/80, Dakopatts), and with isotype matched antibodies as negative control (mouse IgG, dilution 1/200, Dakopatts). Biotinylated horse antimouse IgG (diluted 1/400, Vector Laboratories, Inc., Burlingame, CA, USA) was then allowed to react with the cells for 30 min, followed by avidin-biotin-peroxidase complexes (Vectastain Elite ABC Kit; Vector Laboratories, Inc.). The peroxidase reaction was developed with 3-amino-9-ethylcarbazole (Aldrich-Chemie, Steinhelm, Germany). To allow assessment in both an ordinary light microscope and a confocal laser-scanning microscope (CLSM), cells were also stained with alkaline phosphatase antialkaline phosphatase (APAAP) [33]. After incubation with the primary antibody (Ber-H2), followed by a secondary antibody (rabbit antimouse, dilution 1/40, Dakopatts), complexes of APAAP (dilution 1/20, Dakopatts) were added. These two steps were repeated twice. This was followed by application of Fast Red-solution (Dakopatts) to the cells. The cells were counterstained with Meyer's haematoxylin and mounted in Kaiser's glycerin-gelatin (Merck, Darmstadt, Germany), and were examined under a conventional light microscope (Laborux K, Leitz, Wetzlar) or in a Leica TCS SP confocal laser-scanning microscope (CLSM) equipped with argon gas laser. Excitation was stimulated with the 543 nm laser line and induced fluorescence was detected in the wavelength region between 548 and 702 nm.
Determination of IL-4 reduction by NAC
An ELISA plate was coated overnight at 4°C with 0·05 ml of rhIL-4 (Nordic BioSite, Täby, Sweden) (1000 ng/ml) diluted in carbonate buffer, pH 9·6. After blocking with PBS/0·1% Tween 20 for 1 h at 37°C, the plate was washed with TPBS (PBS/0·05% Tween 20) four times. Thereafter 0·05 ml containing indicated amounts of NAC (100 mm stock solution was prepared in PBS, see above) or dithiothreitol (DTT) (5 mm) (Saveen Biotech AB, Ideon, Malmö, Sweden), diluted in TPBS was added to each well, in triplicates, and incubated for 6 h at 37°C. After four washes with TPBS, 0·05 ml (250 µm) of Nα-(3-maleimidylpropionylbiocytin) (Molecular Probes, Eugene, OR, USA) diluted in 50 mm HEPES, pH 7·6/130 mm NaCl/0·05% Tween 20 was added to each well, in triplicate, and incubated for 30 min at 37°C. After four washes with TPBS, 0·05 ml of alkaline phosphatase-conjugated streptavidine (Mabtech) diluted 1 : 1000 in TPBS was added to each well. After incubation at room temperature for 30 min the wells were washed five times in TPBS, and 0·05 ml of 1 mg/ml p-nitrophenylphosphate (Sigma) dissolved in substrate buffer (10% diethanolamine pH 9·8 containing 0·5 mm MgCl2) was added to each well. Absorbance at 405 nm was detected with a microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA).
Statistics
Data were compared using the nonparametric Wilcoxon matched pairs test. Analysis of variance by the Friedman's anova test was used to compare the differences in CD30, CD28 and CD40L expression with or without NAC treatment. Two way repeated measures anova was used to compare CD30 mean fluorescence intensity (MFI) at different concentrations of NAC and with or without the addition of IL-4. P < 0·05 was considered statistically significant.
RESULTS
GSH and NAC decrease the production of cytokines in Th0 and Th2 clones
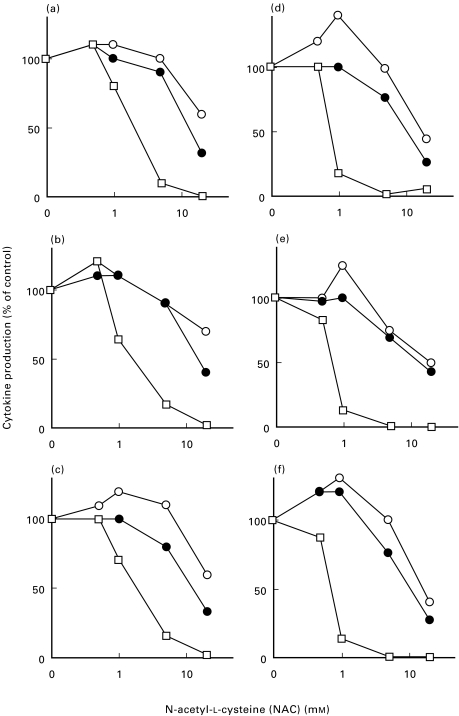
To analyse whether there was a change in the cytokine pattern in human TCC treated with thiols, we examined the production of IL-4, IL-5 and IFN-γ in Th0 clones (n = 3) and Th2 clones (n = 3) by ELISA. TCC were activated with anti-CD3 antibodies for 16 h in the presence or absence of NAC (0·5–20 mm) or GSH (0·5–20 mm). The concentrations of GSH/NAC used in this study were chosen according to a previous study where T helper clones were used [9]. At 1 mm NAC, there was a 20–36% decrease in the concentration of IL-4 in the Th0 clones and a 84–87% decrease of IL-4 in the Th2 clones (Fig. 1). This decrease was further pronounced at 20 mm NAC (Fig. 1). At 5–20 mm NAC, IL-5 levels were reduced 10–68% in the Th0 clones and 30–83% in the Th2 clones (Fig. 1). The levels of IFN-γ were not as strongly affected by 5–20 mm NAC; in the Th0 clones the decrease in IFN-γ production ranged from 0 to 40%, and in the Th2 clones from 0 to 60% (Fig. 1). Similar results were observed in three Th0 clones treated with GSH (0·5–20 mm) for 16 h. Activation of Th0 (n = 2) and Th2 (n = 4) clones with anti-CD3 antibodies for 48 h in the presence or absence of NAC (20 mm), also had only a modest effect on IFN-γ production, while a clear decrease in IL-4 and IL-5 was observed (data not shown). Thus, these data indicate that NAC, in a dose dependent manner, preferentially down-regulates IL-4, has a moderate down-regulating effect on IL-5, and an even smaller effect on IFN-γ.
Fig. 1.
IL-4, IL-5 and IFN-γ production is decreased in human Th0 and Th2 clones after treatment with NAC. Three Th0 clones (29 : 19, 24 : 12, 25 : 4; a, b and c, respectively) and three Th2 clones (24 : 9, 24 : 6, 12 : 3; d, e and f) were activated with anti-CD3 antibodies for 16 h in serum-free medium (1 × 106 cells in 1 ml medium), supplemented with IL-2 (20 U/ml) and with or without NAC (0·5–20 mm). The concentrations of IL-4 (□), IL-5 (•) and IFN-γ (O) were assayed in the culture supernatants with ELISA. The results are shown as decrease in cytokine production (% of control). The concentration of IL-4, IL-5 and IFN-γ, respectively, at 16 h without addition of NAC was (7, 10 and 5 ng/ml) (16, 53 and 5 ng/ml) and (2, 5 and 2 ng/ml) in the Th0 clones and (2, 20 and 0·4 ng/ml) (28, 16 and 0·3 ng/ml) and (35, 18 and 0·2 ng/ml) in the Th2 clones.
The expression of CD30 is down-regulated after GSH and NAC treatment
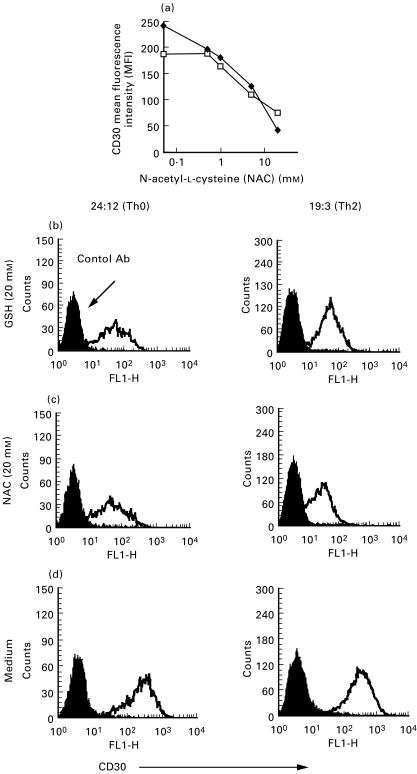
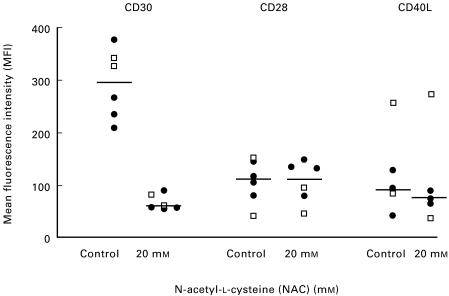
Next we wanted to determine whether there was a change in the expression of CD30 by the human TCC after GSH and NAC treatment. When Th0 clones (n = 3) were activated for 16 h with anti-CD3 antibodies in the absence or presence of different concentrations of NAC (0·5–20 mm), the expression of CD30, as analysed by flow cytometry, declined in a dose dependent manner (Fig. 2a-d). Similar results were found when the cells were treated with GSH (0·5–20 mm) (data not shown). After 16 h of stimulation with anti-CD3 antibodies, in the absence of thiols, 59–99% of the cells were CD30+ (data not shown). Thereafter we analysed both Th0 (n = 2) and Th2 (n = 4) clones for CD30 surface expression 48 h after activation with anti-CD3 antibodies in the presence of 20 mm NAC. The CD30 mean fluorescence intensity (MFI), as analysed by flow cytometry, was significantly decreased (P < 0·05, n = 6) when the cells were treated with NAC compared to untreated control cells (Fig. 3). A down-regulation of CD30 immunoreactivity after NAC treatment could also be shown by peroxidase antiperoxidase (PAP) staining or alkaline phosphatase antialkaline phosphatase (APAAP) of acetone-fixed cells (Fig. 4). To assess whether the downregulation of surface protein was a general response to thiols, we also examined the expression of CD28 and CD40 ligand (CD40L) after treating the TCC with 20 mm NAC for 48 h (Fig. 3). However, not even at this concentration of NAC did we observe any significant change in surface expression of CD28 or CD40L (Fig. 3). The decrease in CD30 expression was not due to toxic effects of these thiols since analysis of the cells with both trypan blue and propidium iodide indicated that GSH and NAC did not decrease cell viability. It has also previously been shown by several research groups that NAC is not toxic and that 0·5–20 mm NAC induces proliferation in a dose dependent manner in stimulated T cells and TCC [7–9].
Fig. 2.
Cell surface expression of CD30 in human Th0 and Th2 clones is decreased after treatment with NAC. Surface CD30 expression was investigated with flow cytometry (using the anti-CD30 MoAb Ber-H2, Dakopatts), in Th0 (□) and Th2 (•) clones after activation with anti-CD3 antibodies for 16 h in serum-free medium supplemented with IL-2 (20 U/ml) and in the presence or absence of NAC (0·5–20 mm) (a). The two clones shown (24 : 12 and 24 : 9) are representative of experiments with three Th0 clones and two Th2 clones. The histograms (b-d), show CD30 mean fluorescence intensity from one Th0 clone (left) and one Th2 clone (right), activated with anti-CD3 antibodies for 16 h in the presence of GSH (20 mm) (B), NAC (20 mm) (c) or without any treatment (d). These figures are representative of 3–5 independent experiments.
Fig. 3.
NAC significantly reduces CD30 expression on human T cell clones, but does not change the expression of CD28 and CD40L. Th0 (24 : 17; 24 : 12) (□) and Th2 (28 : 12; 24 : 9; 12 : 3; 24 : 6) (•) clones, were activated for 48 h with anti-CD3 antibodies in serum-free medium (1 × 106 cells in 1 ml medium) supplemented with IL-2 (20 U/ml) and with or without NAC (20 mm). Surface expression of CD30, CD28 and CD40L was investigated by flow cytometry. The differences in CD30 expression, with or without 20 mm NAC, were significantly larger (P < 0·05) than the differences in CD28 and CD40L expression. Each dot shows the mean value of one to three experiments per clone. The central line shows the median value in each group.
Fig. 4.
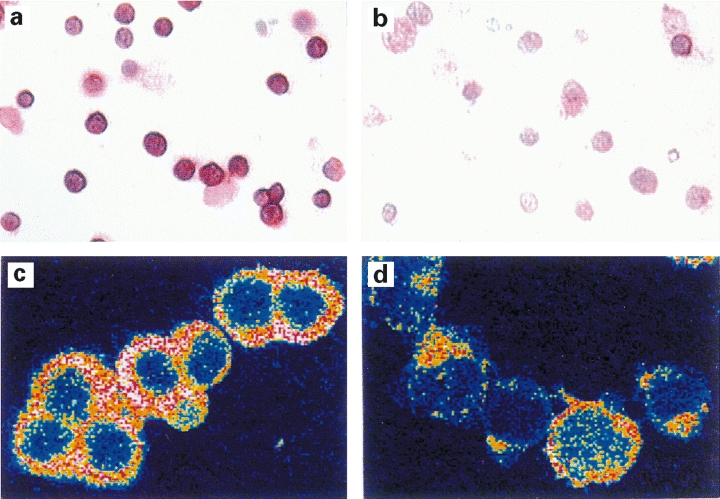
CD30 expression is down-regulated after NAC treatment as shown by immunocytochemistry. One Th0 clone (24 : 12) (A, B) and one Th2 clone (24 : 9) (C, D) was activated for 48 h with anti-CD3 antibodies in the absence (A, C) or presence (B, D) of 20 mm NAC. Immunocytochemical staining was performed using peroxidase antiperoxidase (PAP) (clone 24 : 12; A, B) or alkaline phosphatase antialkaline phosphatase (APAAP) (clone 24 : 9; C, D). There is a reduction in detected immunoreactivity after treatment with NAC, as shown by light microscopy (cf. A with B) and an optical section of the cells using confocal laser-scanning microscope (CLSM) (cf. C with D). Four different TCC (12 : 3; 24 : 6; 24 : 9; 24 : 12) stained with either PAP or APAAP showed similar results. Magnification × 370 (A, B) and × 950 (C, D).
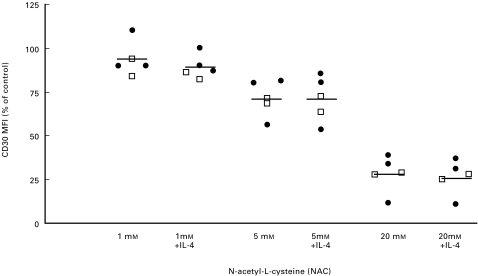
The decrease in CD30 expression after GSH and NAC treatment cannot be inhibited by the addition of exogenous IL-4
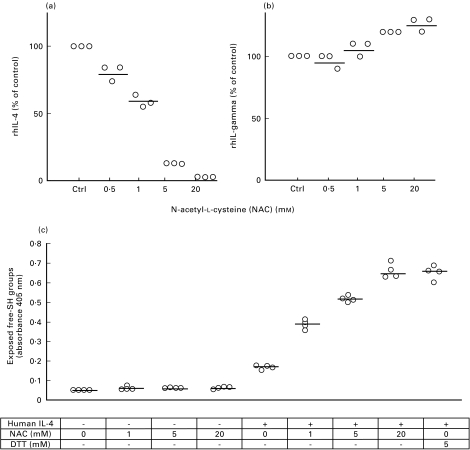
Since it has recently been reported that CD30 expression is dependent on IL-4 [21,34], we were interested in analysing whether the down-regulation of CD30 after GSH/NAC treatment was due to the decreased levels of IL-4. We therefore added recombinant human IL-4 (1000 U/ml) to the TCC in addition to 1, 5 and 20 mm of NAC. The TCC were activated with anti-CD3 and cultured for 16 h, whereafter the cells were analysed for surface CD30 expression by flow cytometry (Fig. 5). As noted previously, there was a dose dependent down-regulation of CD30 after treatment with NAC. A significant reduction was observed after treatment with 5 and 20 mm NAC (P < 0·05). The addition of rhIL-4, however, did not affect the CD30 expression significantly (Fig. 5). Similar results were found when rhIL-4 was added together with GSH (data not shown). To test whether NAC had a direct effect on the concentration of IL-4 in the medium, rhIL-4 (dissolved in AIM-V) was incubated with or without NAC (0·5–20 mm) for 6 h at 37°C and then its concentration was determined by ELISA. The levels of IL-4 decreased in a dose dependent manner with increasing amounts of NAC (Fig. 6a). However, when rhIFN-γ was incubated with 0·5–20 mm NAC under the same conditions, no change in IFN-γ was observed (Fig. 6b). This may indicate that NAC reduces the structural disulphide bonds in IL-4; this could result in a conformational change of the protein. To further analyse this, rhIL-4 was coated onto ELISA plates and incubated with or without NAC (1–20 mm) (Fig. 6c). Free SH-groups were then detected by labelling with Nα-(3-maleimidylpropionylbiocytin) (Fig. 6c). The results show a dose dependent increase in free SH-groups as measured by absorbance, which supports the hypothesis that IL-4 is sensitive to reduction by NAC.
Fig. 5.
Exogenously added IL-4 does not inhibit the downregulation of CD30 on human TCC. Th0 (29 : 19, 25 : 4) (□) and Th2 clones (24 : 9, 12 : 3, 19 : 3) (•) were activated for 16 h with anti-CD3 antibodies in serum-free medium (1 × 106 cells in 1 ml medium) supplemented with IL-2 (20 U/ml) and with 1 mm NAC, 1 mm NAC + IL-4 (1000 U/ml), 5 mm NAC, 5 mm NAC + IL-4 (1000 U/ml), 20 mm NAC and 20 mm NAC + IL-4 (1000 U/ml). The CD30 expression was analysed with flow cytometry using the anti-CD30 MoAb Ber-H2 (Dakopatts). The addition of hrIL-4 (1000 U/ml) did not change the expression of CD30 significantly. Results are shown as decrease in CD30 MFI (% of control). The central line shows the mean value. The MFI values with no addition of NAC (control) were 157 and 83 in the Th0 clones, and 276, 58 and 124 in the Th2 clones.
Fig. 6.
Characterization of NAC as a reductant of human IL-4. Recombinant human IL-4 (rhIL-4) (1000 U) or rhIFN-γ (10 ng) was dissolved in 1 ml AIM V medium, with or without NAC (0·5–20 mm). After incubation at 37°C for 6 h, the concentration of IL-4 (a) and IFN-γ (b) was measured, in duplicates, by ELISA. Results are shown as the relative change in IL-4 or IFN-γ levels (% of control). Two independent experiments per cytokine are shown.An ELISA plate coated with (+) or without (–) rhIL-4 was incubated with or without NAC (1–20 mm) and dithiothreitol (DTT) (5 mm), for 6 h at 37°C (c). After washing, free SH-groups were labelled with Nα-(3-maleimidylpropionylbiocytin). See ‘Materials and methods’ for details. Each dot represent one independent experiment, the central line shows the median value.
GSH and NAC reduce the levels of sCD30 in culture supernatants
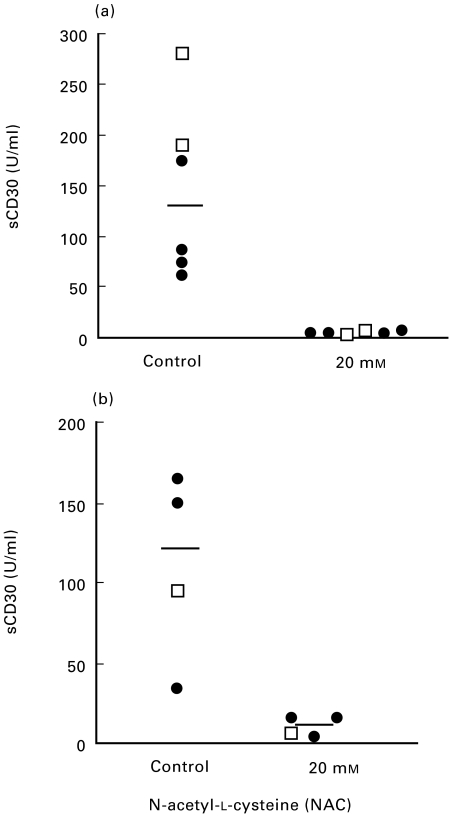
sCD30 is generated when the surface bound molecule is proteolytically cleaved and released from the cell surface [35]. To study the effect of GSH and NAC treatment on this 88-kD protein, we analysed the culture supernatants with a highly sensitive sCD30 ELISA kit. First, TCC of Th0 type were activated with anti-CD3 antibodies in the presence or absence of GSH (0·5–20 mm) or NAC (0·5–20 mm) for 16 h. The culture supernatants were collected and analysed for the presence of sCD30. The results from three different Th0 clones showed a dose dependent decrease in the concentration of sCD30 at the different concentrations of GSH and NAC (data not shown). A significant reduction (P < 0·05, n = 6) of sCD30 in the culture supernatants was determined after 48 h in Th0 (n = 2) and Th2 (n = 4) clones after treatment with NAC (20 mm) (Fig. 7a). Next we incubated culture supernatants that were no longer in contact with the TCC for 24 h with or without NAC (20 mm). The supernatants had been collected from TCC (two Th0 and three Th2 clones) that had been activated with anti-CD3 antibodies and thus contained high levels of sCD30. Interestingly, we noted an apparent reduction in the concentration of sCD30 in the supernatants that had been incubated with 20 mm NAC (Fig. 7b). These results may indicate that CD30, which contains six cysteine rich repeats, is sensitive to reduction, and that this could lead to conformational changes of the protein.
Fig. 7.
The concentration of sCD30 in culture supernatants of human TCC is decreased after treatment with GSH and NAC. The concentration of sCD30 was determined with ELISA in culture supernatants from two Th0 (29 : 19; 24 : 12) (□) and four Th2 (12 : 3; 24 : 6; 24 : 9; 28 : 12) (•) clones, after activation with anti-CD3 antibodies for 48 h in serum-free medium supplemented with IL-2 (20 U/ml) and in the presence or absence of NAC (20 mm) (a). sCD30 levels were significantly reduced in the supernatants collected from the NAC treated clones (P < 0·05, n = 6). Each dot shows the mean value from 1 to 2 experiments. Culture supernatants collected from one Th0 (□) and three Th2 (•) clones activated with anti-CD3 antibodies and thus containing high sCD30 levels (b), were incubated at 37°C for 24 h with serum-free medium as control, or with NAC (20 mm). The central line shows the median value.
DISCUSSION
The results in this study show a marked decrease in CD30 surface expression after treatment of allergen specific human T cells with GSH or NAC. They also indicate that the levels of sCD30 in culture supernatants are down regulated. In addition, we also show a dose-dependent down-regulation of IL-4, IL-5 and IFN-γ by NAC, with the most pronounced decrease of IL-4.
It has previously been shown both in vitro and in vivo that GSH and NAC appear to favour Th1 responses by down-regulating type 2 cytokines [9–11]. The biochemical properties of NAC may explain the differential effects on cytokine production. The transport of NAC into cells will increase the level of GSH, affecting intracellular redox potential and the thioredoxin and glutaredoxin systems, which regulate transcription factors by thiol redox control [36–38]. Changes in the effective thiol concentration in the endoplasmatic reticulum or at the cell surface may also destabilize cytokines or their receptors, resulting in widely different effects on specific cytokines.
When we activated TCC in the presence of NAC (0·5–20 mm), we observed a preferential down-regulation of IL-4, followed by IL-5, in the culture supernatants. The levels of IFN-γ, however, were not as dramatically affected by GSH/NAC treatment. Since NAC has a reducing capacity, we wanted to assess whether NAC had a direct effect on IL-4 in the medium. When we incubated rhIL-4 in AIM-V with different concentrations of NAC, we found a dose-dependent reduction of IL-4 levels by ELISA (Fig. 6). Since AIM-V medium should lack any thiol-inducible proteolytic activity one explanation could be that NAC chemically reduced one or more of the three structural disulphide bonds in IL-4 [39], and thereby cause a conformational change in the protein. The antibodies used in the ELISA test may be dependent on conformational epitopes and would thus not recognize this modified IL-4. To further test this hypothesis we developed a method by which free SH-groups are detected by labelling with a biotinylated maleimide. The result revealed a clear dose-dependent increase in free SH-groups in IL-4 with increasing concentrations of NAC (Fig. 6c). Our results demonstrate that the low levels of IL-4 can be due to an action of NAC on IL-4 at the protein level, and not only to the inhibition of IL-4 RNA transcription, as previously shown by Jeanin et al. [9].
In addition, a distinct down-regulation of CD30 was noted in all TCC after treatment with either GSH or NAC. We also investigated the effect of GSH and NAC on the expression of CD28 and CD40L, both very important costimulatory molecules expressed by T cells. Even at the highest concentration of NAC used (20 mm), we did not detect any significant changes in surface expression of CD28 or CD40L as analysed by flow cytometry. Our results suggest that GSH and NAC, acting via some as yet unknown mechanism, regulate the CD30 expression. This finding is interesting, since it has previously been shown that Th2 cells have a sustained expression of CD30 [40], and that increased CD30 expression appears to play a role in human disorders with elevated levels of CD4 or CD8 lymphocytes expressing Th2 cytokines [23,24]. In studies with mice, it has recently been demonstrated that the expression of CD30 requires either stimulation via CD28, or the presence of exogenous IL-4 [21,34]. We therefore added rhIL-4 to the activated TCC in addition to GSH and NAC to see if this could prevent the downregulation of CD30. However, rhIL-4 did not reverse the down-regulatory effect induced by GSH or NAC. One possibility is that GSH/NAC reacted directly with the soluble rhIL-4 in the medium, and affected its biological activity by reducing structural disulphides.
When the extracellular form of CD30 is cleaved by a zinc metalloproteinase at the cell surface, a soluble form is generated, which can be detected in culture supernatants or in patients' sera. In several human diseases associated with an abnormal Th2 response, elevated levels of sCD30 have been detected, e.g. in atopic conditions [25, 41–43] and HIV-infection [28]. It is not yet clear whether sCD30 plays a role in the pathogenesis of atopic diseases. However, it has been demonstrated that sCD30 can bind to CD30L [44], and that a bidirectional signalling can occur between the ligand and the receptor [45]. Moreover, several studies have shown a correlation between sCD30 levels and disease activity in atopic dermatitis and asthma [41–43]. Interestingly, data from this study show that in culture supernatants from activated TCC treated with NAC there is a striking difference in sCD30 levels compared to those in culture supernatants from untreated cells. This was not only due to the downregulation of the membrane-bound molecule, since we found a reduction of sCD30 also in culture supernatants containing high sCD30 levels, after incubation with GSH and NAC.
As shown here, IL-4, IL-5 and sCD30 all decreased, while IFN-γ was not as strongly affected by GSH/NAC treatment as determined by ELISA. Interestingly, it has previously been shown that the structural disulphides in IL-4 and IL-5 [39, 46] are necessary for their biological activity [47, 48]. Indeed, when we incubated rhIFN-γ with different concentrations of NAC, the levels of IFN-γ did not change. The amino acid sequence of CD30 contains six cysteine residues [49]. These are likely to be in disulphide form and might also be essential for biological activity [49]. In contrast, IFN-γ lacks structural disulphide bonds [50], which may explain why it was unaffected by GSH/NAC treatment. Further studies have to be performed to determine whether the biological activities of IL-4, IL-5, CD30 and sCD30 are all redox-regulated. Such a mechanism may be operating in vivo, since instead of high extra cellular GSH levels, dithiol-dependent disulphide reductases may be expressed on the cell surface [51].
A wide range of human diseases is associated with an abnormal Th1 or Th2 response. Thus, it is important to generate and maintain the appropriate Th1/Th2 balance for an effective and nonpathological immune function. The results from this and previously published studies, suggest that NAC can favour Th1 responses by down regulating Th2 cytokines. In a recently published paper, CD30 is suggested to play an important role as a costimulatory molecule in the negative selection in the thymus [52]. The immunological function of CD30 in mature cells is, however, still unclear. Experiments in our laboratory are in progress to try to clarify the role of CD30–CD30L interaction, and the possible immunological function of sCD30. If CD30 and its soluble counterpart are shown to contribute to the pathogenesis of Th2 related diseases such as allergy, NAC could be considered as a future therapeutic agent to treat these diseases.
Acknowledgments
We would like to express our gratitude to Ms Catharina Johansson and Ms Eva Buentke for their help with establishing the T cell clones, Mr Jan Kowalski and Mr Ulf Brodin, Department of Medical Informatics and Educational Development, Medical Statistics Unit, Karolinska Institutet, for their help with statistical calculations. This study was supported by grants from Karolinska Institutet, the Swedish Association against Asthma and Allergy, the Swedish Medical Research Council (grant numbers 16X-7924 and 13X-3529), the Swedish Council for Work Life Research and the Swedish Foundation for Health Care Sciences and Allergy Research. JAC is the recipient of a fellowship from AMF-Sjukförsäkrings Jubilee Foundation for Research in National Diseases.
REFERENCES
- 1..Dröge W, Schulze-Osthoff K, Mihm S. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1992;8:1131–8. [PubMed] [Google Scholar]
- 2.Uhlig S, Wendel A. The physiological consequences of glutathione variations. Life Sci. 1992:1083–94. doi: 10.1016/0024-3205(92)90509-n. [DOI] [PubMed] [Google Scholar]
- 3.Ventresca G, Cicchetti V, Ferrari V. Acetylcysteine. In: Braga P, Allegra L, editors. Drugs in bronchial mucology. New York: Raven Press; 1989. pp. 77–102. [Google Scholar]
- 4.De Quay B, Malinverni R, Lauterburg B. Glutathione depletion in HIV-infected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992;6:815–9. [PubMed] [Google Scholar]
- 5.Roederer M, Ela S, Staal F, Herzenberg L. N-acetylcysteine: a new approach to anti-HIV therapy. AIDS Res Hum Retroviruses. 1992;8:209–17. doi: 10.1089/aid.1992.8.209. [DOI] [PubMed] [Google Scholar]
- 6.Martinez E, Domingo P. N-acetylcysteine as a chemoprotectant in cancer chemotherapy. Lancet. 1991;338:249. doi: 10.1016/0140-6736(91)90383-z. [DOI] [PubMed] [Google Scholar]
- 7.Eylar E, Rivero-Quinones C, Molina C, et al. Acetylcysteine enhances T cell functions and T cell growth in culture. Int Immunol. 1993;5:97–101. doi: 10.1093/intimm/5.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Liang C-M, Lee N, Cattell D, Liang S-M. Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem. 1989;264:13519–23. [PubMed] [Google Scholar]
- 9.Jeannin P, Delneste Y, Lecoanet-Henchoz S, et al. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. J Exp Med. 1995;182:1785–92. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eylar E, Báez I, Vázquez A, Yamamura Y. N-acetylcysteine (NAC) enhances interleukin-2 but suppresses interleukin-4 secretion from normal and HIV+ CD4+ T-cells. Cell Mol Biol. 1995;41:S35–40. [PubMed] [Google Scholar]
- 11.Peterson J, Herzenberg L, Vasquez K, Waltenbaugh C. Gluathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–6. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagihara Y, Basaki Y, Kajiwara K, Ikizawa K. A thiol antioxidant regulates IgE isotype switching by inhibiting activation of nuclear factor-kB. J Allergy Clin Immunol. 1997;100:33–8. doi: 10.1016/s0091-6749(97)70002-2. [DOI] [PubMed] [Google Scholar]
- 13.Verhasselt V, Van den Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-Acetyl-l-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-κB inhibition. J Immunol. 1999;162:2569–74. [PubMed] [Google Scholar]
- 14.Andreesen R, Osterholtz J, Löhr G, Bross K. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984;63:1299–302. [PubMed] [Google Scholar]
- 15.Ellis T, Simms P, Slivnick D, Jäck H-M, Fisher R. CD30 is a signal transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993;151:2380–9. [PubMed] [Google Scholar]
- 16.Schwarting R, Gerdes J, Durkop H, Falini B, Pileri S, Stein H. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood. 1989;74:1678–89. [PubMed] [Google Scholar]
- 17.Stein H, Mason D, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 18.Smith C, Gruss H, Davis T, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 19.Grüss HJ, Hermann F. CD30 ligand, a member of the TNF ligand superfamily, with growth and activation control for CD30+ lymphoid and lymphoma cells. Leukemia Lymphoma. 1996;20:397–409. doi: 10.3109/10428199609052421. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson Å, Lundberg M, Avila-Cariño J. Crosslinking of CD30 on activated human Th clones enhances their cytokine production and down regulates the CD30 expression. Scand J Immunol. 52:595–601. doi: 10.1046/j.1365-3083.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 21.Gilfillan MC, Noel PJ, Podack ER, Reiner SL, Thompson CB. Expression of the costimulatory receptor CD30 is regulated by both CD28 and cytokines. J Immunol. 1998;160:2180–7. [PubMed] [Google Scholar]
- 22.Del Prete G, De Carli M, D'Elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Elios M, Romagnani P, Scaletti C, et al. In vivo CD30 expression in human diseases with predominant activation of Th2-like T cells. J Leukoc Biol. 1997;61:539–44. [PubMed] [Google Scholar]
- 24.Manetti R, Annunziato F, Biagiotti R, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines: Evidence for large numbers of CD8+CD30+ T cell clones in human immuno deficiency virus infection. J Exp Med. 1994;180:2407–11. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bengtsson Å, Holm L, Bäck O, Fransson J, Scheynius A. Elevated serum levels of soluble CD30 in patients with atopic dermatitis (AD) Clin Exp Immunol. 1997;109:533–7. doi: 10.1046/j.1365-2249.1997.4731373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dummer W, Brocker EB, Bastian BC. Elevated serum levels of soluble CD30 are associated with atopic dermatitis, but not with respiratory atopic disorders and allergic contact dermatitis. Br J Dermatol. 1997;137:185–7. doi: 10.1046/j.1365-2133.1997.18031887.x. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S, Annunziato F, Manetti R, et al. Role for CD30 in HIV expression. Immunol Lett. 1996;51:83–8. doi: 10.1016/0165-2478(96)02559-x. [DOI] [PubMed] [Google Scholar]
- 28.Pizzolo G, Vinante F, Morosato L, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–5. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Mavalia C, Scaletti C, Romagnani P, et al. Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Calgaris-Cappio F, Bertero MT, Converso M, et al. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol. 1995;13:339–43. [PubMed] [Google Scholar]
- 31.Chilosi M, Facchetti F, Notarangelo L, et al. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–34. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 32.Tengvall Linder M, Johansson C, Zargari A, et al. Detection of Pityrosporum orbiculare reactive T cells from skin and blood in atopic dermatitis and characterization of their cytokine profiles. Clin Exp Allergy. 1996;26:1286–97. doi: 10.1046/j.1365-2222.1996.d01-281.x. [DOI] [PubMed] [Google Scholar]
- 33.Murdock A, Jenkinson EJ, Johnson GD, Owen JJT. Alkaline phosphatase-Fast Red, a new fluorescent label. Application in double labelling for cell surface antigen and cell cycle analysis. J Immunol Methods. 1990;132:45–9. doi: 10.1016/0022-1759(90)90396-d. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura Y, Lee RK, Nam SY, et al. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J Immunol. 1997;158:2090–8. [PubMed] [Google Scholar]
- 35.Josimovic-Alasevic O, Durkop H, Schwarting R, Backé E, Stein H, Diamantstein T. KI-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I.Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989;19:157–62. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- 36.Holmgren A. Redox regulation and mechanisms of mammalian thioredoxin and glutaredoxin systems. In: Yodoi J, Packer L, editors. Redox regulation of cell signalling and its clinical applications. New York: Marcel Dekker, Inc.; 1999. pp. 279–98. [Google Scholar]
- 37.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 38.Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 39.Walter MR, Cook WJ, Zhao BG, et al. Crystal structure of recombinant human interleukin-4. J Biol Chem. 1992;267:20371–6. doi: 10.2210/pdb2int/pdb. [DOI] [PubMed] [Google Scholar]
- 40.Bengtsson Å, Johansson C, Tengvall Linder M, Halldén G, van der Ploeg I, Scheynius A. Not only Th2 cells but also Th1 and Th0 cells express CD30 after activation. J Leukoc Biol. 1995;58:683–9. doi: 10.1002/jlb.58.6.683. [DOI] [PubMed] [Google Scholar]
- 41.Frezzolini A, Paradisi M, Ruffelli M, Cadoni S, Depita O. Soluble CD30 in pediatric patients with atopic dermatitis. Allergy. 1997;52:106–9. doi: 10.1111/j.1398-9995.1997.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 42.Caproni M, Bianchi B, D'Elios M, De Carli M, Amedi A, Fabbri P. In vivo relevance of CD30 in atopic dermatitis. Allergy. 1997;52:1063–70. doi: 10.1111/j.1398-9995.1997.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 43.Leonard C, Tormey V, Faul J, Burke C, Poulter L. Allergen- induced CD30 expression on T cells of atopic asthmatics. Clin Exp Allergy. 1997;27:780–6. doi: 10.1046/j.1365-2222.1997.700840.x. [DOI] [PubMed] [Google Scholar]
- 44.Younes A, Consoli U, Snell V, et al. CD30 ligand in lymphoma patients with CD30+ tumors. J Clin Oncol. 1997;15:3355–62. doi: 10.1200/JCO.1997.15.11.3355. [DOI] [PubMed] [Google Scholar]
- 45.Wiley S, Goodwin R, Smith C. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–9. [PubMed] [Google Scholar]
- 46.Milburn MV, Hassel AM, Lambert MH, et al. A novel dimer configuration revealed by the crystal structure at 2.4 Å resolution of human interleukin-5. Nature. 1993;363:172–6. doi: 10.1038/363172a0. [DOI] [PubMed] [Google Scholar]
- 47.Windsor WT, Syto R, Le HV, Trotta P. Analysis of the conformation and stability of Escherichia coli derived recombinant human interleukin 4 by circular dichroism. Biochemistry. 1991;30:1259–64. doi: 10.1021/bi00219a014. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie ANJ, Ely B, Sanderson CJ. Mutated interleukin-5 monomers are biologically inactive. Mol Immunol. 1991;28:155–8. doi: 10.1016/0161-5890(91)90099-6. [DOI] [PubMed] [Google Scholar]
- 49.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–7. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 50.Ealick ES, Cook WJ, Vijay-Kumar S, et al. Three-dimensional structure of recombinant human interferon-γ. Science. 1991;25:698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- 51.Jiang XM, Fitzgerald M, Grant CM, Hogg PJ. Redox control of exofacial protein thiols/disulfides by protein disulfide isomerase. J Biol Chem. 1999;274:2416–23. doi: 10.1074/jbc.274.4.2416. [DOI] [PubMed] [Google Scholar]
- 52.Chiarle R, Podda A, Prolla G, Podack E, Thorbecke G, Inghirami G. CD30 overexpression enhances negative selection in the thymus and mediates programmed cell death via a Bcl-2-sensitive pathway. J Immunol. 1999;163:194–205. [PubMed] [Google Scholar]