Abstract
Sublytic doses of complement desensitize cells and make them resistant to lytic complement doses. This process, named complement-induced protection, requires calcium ion influx, protein kinase C activation and protein synthesis. The involvement of the extracellular signal-regulated kinase, ERK, in cell desensitization by sublytic complement was examined in erythroleukaemia K562 cells and in COS-7 cells. As shown here, ERK is activated in K562 and COS-7 cells within 10 min of sublytic immune attack and then shows a decline and a second peak of activation at 20 min. C7- and C8-deficient human sera have a small effect on ERK activity. However, a significant increase in ERK activation is observed when C7 or C8, respectively, is added back to these sera. Complement-induced ERK activation was blocked in cells treated with GF109203X or Go6976, two selective PKC inhibitors, as well as by treatment with PD098059, an inhibitor of MEK1, the ERK kinase. PD098059 treatment also sensitized K562 cells to complement-mediated lysis and prevented complement-induced protection. COS-7 cells transfected with a dominant-negative MEK plasmid were incapable of undergoing the process of complement-induced protection. In conclusion, cell desensitization by sublytic doses of the complement membrane attack complex involves a signalling cascade that includes PKC-mediated ERK activation.
Keywords: complement-resistance, MAPK, ERK, PKC, cell-desensitization
INTRODUCTION
The complement system causes cell damage by insertion of a membrane attack protein complex (MAC) comprised of the C5b, C6, C7, C8 and C9 complement proteins (C5b-9) into the plasma membrane of target cells [1]. Cells vary, to a large extent, in their sensitivity to MAC-mediated lysis. This is due to the fact that they are equipped with several basal and inducible resistance mechanisms that are expressed at different levels in different cells [reviewed in 2]. Basal resistance is provided by membrane complement regulatory proteins such as CD46, CD55 and CD59 [reviewed in 3]. Other membrane proteins, such as ecto-proteases [4] and ecto-protein kinases [5], may also contribute to protection from complement. In addition, cells have some capacity to withstand the damage caused by the C5b-9 complex and repair it [reviewed in 6–8]. Several studies have demonstrated that protein kinase C (PKC) also plays a role in cell protection from the MAC [9–13], although the substrates phosphorylated by PKC in complement attacked cells have not been identified yet.
Cells also have the ability to further amplify their basal state of complement resistance [reviewed in 2]. We have shown that pretreatment of K562 human erythroleukaemic cells with sublytic doses of MAC protects them from a subsequent lytic complement attack. This phenomenon of cellular desensitization to complement was termed complement-induced protection [14]. Influx of calcium ions and activation of PKC that are induced within 5–10 min by sublytic MAC are essential for cell desensitization to lytic MAC [13]. Protection from lytic MAC is also induced by sublytic doses of other pore-formers such as, perforin and streptolysin O (SLO) as well as by calcium ionophores [15].
Sublytic MAC was shown to trigger de novo protein synthesis in differentiated primary cells and in transformed and tumour cell lines [16–18]. MAC was also found to induce expression of c-Jun, JunD and c-Fos and enhance AP-1 DNA-binding activity in oligodendrocytes [19]. Transmission of extracellular signals from the cell surface into the nucleus may involve activation of the mitogen-activated protein kinase (MAPK) signalling cascades, via the ERK, JNK and/or p38 pathways [reviewed in 20]. Mammalian cells have several extracellular signal-regulated kinases (ERK), of them the most common ones are ERK1 (a 44-kD MAPK) and ERK2 (a 42-kD MAPK). These kinases regulate cell processes stimulated by various extracellular agents and have been implicated in control of nuclear transcriptional activity [21]. Activation of ERKs occurs as a result of phosphorylation of threonine and tyrosine residues in a -TXY- motif that is common to most MAPKs. Phosphorylation of both residues is required for full activation. The main upstream event leading to activation of ERKs is their phosphorylation by the dual specificity protein kinase, MAPK kinase or MAPK/ERK kinase (MEK) which can be activated primarily by Raf-1 [22] but also by β-Raf [23] and Mos [24].
Niculescu et al. [25] have demonstrated that sublytic MAC activates Ras, Raf-1 and ERK in a human lymphoblastic B cell line (JY25). ERK activation was found to be initiated at the level of C5b-7, and further increased by C5b-9. MAPK activation was also shown in rat glomerular epithelial cells [26] and human aortic smooth muscle cells [27] subjected to sublytic MAC.
Results presented here indicate that the following signalling pathway is induced by sublytic MAC doses in nucleated cells: activation of PKC which in turn promotes activation of MEK and then of ERK and resulting in enhanced cell resistance to complement-mediated damage.
MATERIALS AND METHODS
Reagents and antibodies
Experiments were carried out in Dulbecco’s phosphate-buffered saline (PBS), pH 7·4. The selective inhibitors GF109203X, Go6976, PD098059 and the calcium ionophores A23187 and ionomycin were purchased from Calbiochem (San Diego, CA, USA). [γ32P] ATP was from DuPont, NEN Research products (Wilmington, DE). Myelin basic protein (MBP) was purchased from Sigma (Rehovot, Israel). Chemicals employed in this study were all of analytical grade. Polyclonal rabbit anti-ERK antibodies and mouse monoclonal anti‐phospho ERK were purchased from Sigma and alkaline phosphatase-conjugated goat anti‐rabbit or anti‐mouse were from Promega (Madison, WI).
Sera and complement proteins
Normal human serum (NHS) used as a source of complement components was prepared from healthy donors. Heat inactivation (HI) of complement in NHS was performed by heating it for 30 min at 56°C. C7- or C8- deficient human sera were prepared from donors genetically deficient in complement components C7 and C8, respectively [28]. Human C7 and C8 were purified from NHS as described [29]. All sera and complement proteins were kept frozen at − 70°C until used. The concentrations of C7 (165 µg/ml) and C8 (110 µg/ml) required to restore haemolytic activity of the deficient sera were higher than the normal C7 and C8 concentrations in NHS (around 55 µg/ml). This was probably due to the fact that the purified C7 and C8 lost, upon storage, some of their activity.
Cell cultures
K562 human erythroleukaemia cells were kept in culture in RPMI-1640 supplemented with 10% (v/v) heat-inactivated fetal calf serum (HI-FCS) (Gibco Laboratories, Grand Island, NY), 2% sodium pyruvate, 1% glutamine and an antibiotic mixture of penicillin (100 µ/ml), streptomycin (0·4 mg/ml) and fungizon (0·02 mg/ml) (Biolab, Jerusalem, Israel). COS-7 monkey kidney cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) HI-FCS, glutamine, antibiotics (as above) and 2% sodium bicarbonate. Cells were incubated at 37°C under 5% CO2/air. COS-7 cells were periodically harvested with trypsin-EDTA from confluent cultures. Serum-starved cells were cultured for 18 h in medium containing 0·1% HI-FCS.
Preparation of antibodies to K562
Polyclonal antibodies directed against K562 were prepared in rabbits by subcutaneous injections of 1 × 107 intact cells in PBS. The antibody titre was determined in a cytotoxicity assay using the trypan blue exclusion method. Usually, 15-to 30-fold dilution of the rabbit antiserum (depending on the batch) yielded 10–20% cell lysis by NHS diluted 1 : 2, and this antibody dilution was employed to produce a sublytic complement attack. IgG used against COS-7 cells was purified from rabbit anti-K562 antiserum by precipitation with a 40% solution of ammonium sulphate as described [30].
Cytotoxicity assay
Lysis of K562 and COS-7 cells
Cells (0·5 × 106) were washed twice with PBS and then incubated for 30 min on ice with 50 ml of whole antiserum or purified IgG serially diluted (1:2–1:64) in PBS. Next, 50 µl of NHS was added to a final dilution of 1:2, for 60 min at 37°C. HI-NHS served as control. Percentage of mortality in presence of HI-NHS was usually 1–3%. The cells were then washed with PBS and resuspended in 0·2% (w/v) trypan blue (Fluka AG, Buchs, Switzerland) in PBS. Percent lysis was determined microscopically.
Lysis of red blood cells
Hemolytic activity of all sera (HI-NHS, NHS and C7- or C8-deficient human sera, supplemented or not with purified C7 or C8, respectively) was tested on rabbit erythrocytes as follows. Fresh, washed rabbit erythrocytes (107/50 µl) diluted in gelatin veronal buffer (GVB) (5 mm veronal, pH 7·4 containing 145 mm NaCl, 0·15 mm CaCl2, 0·5 mm MgCl2 and 0·1% (w/v) gelatin) in presence of 10 mm EGTA were incubated for 30 min at 37°C with 50 ml human serum (diluted 1:2–1:64) in duplicates. Cell lysis was stopped by adding 1 ml cold GVB. Percent haemolysis was determined spectroscopically at 412 nm after sedimentation of the intact erythrocytes.
Complement-induced protection assay
Cells were treated with sublytic antibody and complement doses. First, they were incubated for 30 min on ice with a sublytic dilution of antibodies (0·5 ml diluted antibody/5 × 106 cells), and then mixed with 0·5 ml NHS and incubated for 50 min at 37°C. Non induced control cells were treated with HI-NHS. Percent lysis resulting from the sublytic complement attack was determined by trypan blue exclusion. Next, the sublytically treated cells were washed and subjected to lytic antibody and complement doses. 5 × 105 cells were incubated for 30 min on ice with 50 µl antibody diluted 1:10–1:15 and then for 60 min at 37°C with 50 µl NHS serially diluted 1:4–1:32. The preincubation and sublytic stages were performed on cells in batch and the lytic stage on aliquoted 5 × 105 cells in triplicates. Percent protection was determined as follows:
Protection (%) = [(Lysis in HI–NHS − Lysis in NHS)/Lysis in HI–NHS] × 100
Preparation of cell extracts
Cell extracts were prepared by sonication in Hepes lysis buffer (40 mm Hepes, pH 7·5, 5 mm EGTA, 5 mm MgCl2, 1 mm benzamidine, 1 mm DTT, 1 mm sodium orthovanadate, 10 mm sodium pyrophosphate, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 2 µg/ml pepstatin A) and cleared by centrifugation at 100 000 × g for 15 min at 4°C (Beckman's Ultracentrifuge, Fullerton, CA). The supernate contained the cytosolic extracts to be examined. All subsequent steps were performed at 4°C. Protein concentration was determined with the Protein Assay dye reagent (BioRad, Richmond, CA) according to Bradford [31] and using BSA to derive the standard curve.
Determination of MAPK activity
To measure MAPK activity, cytosolic extracts were fractionated on DEAE-Cellulose mini-columns as follows. DE-52 resin (Whatman) was prepared in buffer A (50 mm β-glycerophosphate, pH 7·3, 1·5 mm EGTA, 1 mm EDTA, 1 mm DTT and 0·1 mm sodium orthovanadate) and 0·4 ml of this resin was packed into small columns and further washed with buffer A + 0·02 m NaCl. Cell extracts (0·5 ml) were loaded on the mini columns, which were washed twice with 1 ml buffer A + 0·02 m NaCl, and the MAPK was eluted with 0·75 ml of buffer A + 0·22 m NaCl. MAPK activity was tested by measuring phosphate incorporation into myelin basic protein (MBP) as follows. Aliquots (12·5 µl) of the DE-52 eluted MAPK were mixed with 4·2 µl MBP (0·5 mg/ml), and the phosphorylation reactions were initiated by adding 8·3 µl of assay buffer containing 0·3 mm[γ32P] ATP (specific activity ∼ 2000 cpm/pmol) in 75 mm β-glycerophosphate, pH 7·3, 3·7 mm EGTA, 0·15 mm Na3VO4, 1·5 mm dithiothreitol, 30 µm calmidazolium, 6 µm PKI and 30 mm MgCl2. After 15 min at 30°C, the reaction was terminated by spotting 20 µl of the assay mixture onto P-81 phosphocellulose filter paper squares (Whatman, Clifton, NJ), which were then washed several times with 150 mm phosphoric acid, dried and counted in scintillation vials after adding Opti-fluor scintillation liquid (Packard, Meriden, CT). Counts obtained in reactions in absence of MBP (usually less than 20% of the specific phosphorylation) were subtracted from those obtained in presence of MBP. Results were calculated as pmol phosphate incorporated per minute per mg MBP. The tested fractions contained both ERK1 and ERK2 and represented > 85% of MAP kinase activity [32].
Western blotting
Proteins separated on SDS-PAGE gels were blotted as described [13]. Briefly, the nitrocellulose membrane was incubated for 1 h with rabbit anti-ERK antibodies or mouse antiphospho ERK and then for 45 min with alkaline phosphatase-conjugated goat antirabbit or antimouse antibodies. Detection was performed with NBT/BCIP.
Immunoprecipitation of MAPK
COS-7 cells were washed, lysed as described above and immunoprecipitated with anti ERK C-terminus antibody (Antibodies Unit, Weizmann Institute of Science) using protein A-Sepharose (20 µl, Sigma). ERK activity was determined by the phosphorylation of MBP as previously described [33]. Samples were analysed by SDS-PAGE in a 15% gel. Gels were dried and then subjected to autoradiography.
COS-7 cell transfection with DN-MEK
The dominant negative MAPKK (DN-MEK) was prepared as previously described [32]. This mutant was obtained by deleting amino acids 32–51 from the N-terminal region by removing the StuI 95–154 fragment [34]. The construct was introduced into the expression vector pcDNA1·1 for transient expression (Invitrogen, Carlsbad, CA) to express N-terminal 6 histidine (6His)-linked MEK construct in bacteria. DN-MEK in pcDNA1·1 was propagated in competent E. coli. strain XL1-Blue grown until OD600 = 0·7–1·0. Plasmid DNA was prepared using an adaptation of the alkaline lysis method [35] and purified using Qiagen Tip-100 columns as recommended by the manufacturer (Qiagen GmbH, Hilden, Germany). COS-7 cells grown to subconfluency (50–60%) were transfected with plasmid DNA (10 µg) using the DEAE-dextran method [36]. Freshly thawed cells (not more than six passages) were used throughout the experiments.
Statistical analysis
Statistical significance was analysed by using the two-sided unpaired student t-test.
RESULTS
ERK activation by complement
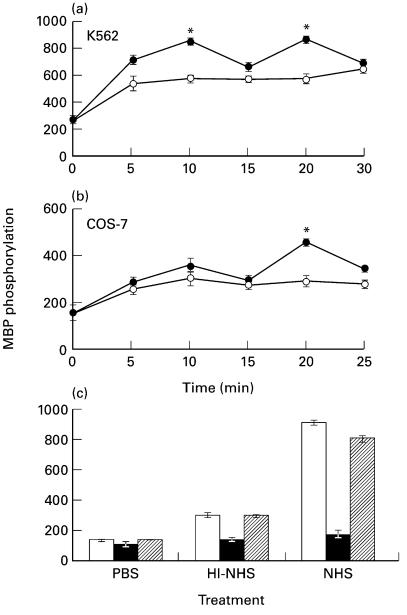
The kinetics of ERK activation by sublytic complement doses was examined in serum starved (0·1% HI-FCS, 18 h) K562 and COS-7 cells. Cells were treated with sublytic doses of antibody (anti-K562 antiserum or purified immune rabbit IgG) and human serum (NHS or HI-NHS). Following stimulation, ERK activity was determined in DE-52 fractionated cell lysates by measuring phosphorylation of MBP. In both cell lines, significant ERK activation was observed already after 5 min treatment (Fig. 1b). In K562 cells, ERK activity reached a first peak of activation at 10 min after addition of sublytic complement and a second peak of activation at 20 min of treatment (Fig. 1a). This was consistently observed in 4 independent experiments with K562 cells as well as with HL-60 cells activated by sulytic complement (data not shown). ERK activation by complement in COS-7 cells was pronounced at 20 min postactivation (Fig. 1b), but lower after 10 min. In general, the specific activity of ERK was higher in K562 cells than in COS-7 cells. Treatment with HI-NHS also produced ERK activation, although significantly less than NHS. As discussed later, this is probably attributed to serum effects [37].
Fig. 1.
Complement-induced MAPK activation in (a) K562 and (b) COS-7 cells. (a,b) K562 and COS-7 cells were serum-starved by growth in RPMI or DMEM, respectively, in presence of 0·1% HI-FCS for 18 h in a 5% CO2 incubator and then treated for 30 min on ice with antibody (diluted 1 : 25) and then with complement (HI-NHS (○) or NHS (•) diluted 1 : 2) at 37°C for 5–30 min. Cells treated with PBS served to determine basal MAPK activity (Time 0). Following incubation, cell extracts were prepared and fractionated. MAPK activity was determined in triplicates by MBP phosphorylation. The results are representative of 4 independent experiments. (c) Serum-starved K562 cells were preincubated without (control □) or with 1 µm PD098059 (▪) or 0·02% DMSO ( ) (60 min, 37°C) and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. MAPK activity was determined as above. Results are representative of 3 independent experiments. *P < 0·01, **P < 0·001.
) (60 min, 37°C) and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. MAPK activity was determined as above. Results are representative of 3 independent experiments. *P < 0·01, **P < 0·001.
The effect of PD098059, a specific inhibitor of the ERK kinase (MEK), on complement-induced ERK activation was tested. K562 cells were preincubated with PD098059 (1 µm) or the equivalent concentration of DMSO (0·02%) for 60 min and then treated with antibody and NHS or HI-NHS. As shown in Fig. 1c, PD098059 significantly inhibited complement-induced ERK activation, reducing activity to basal levels. PD098059 inhibited also the activation of ERK by antibody and HI-NHS.
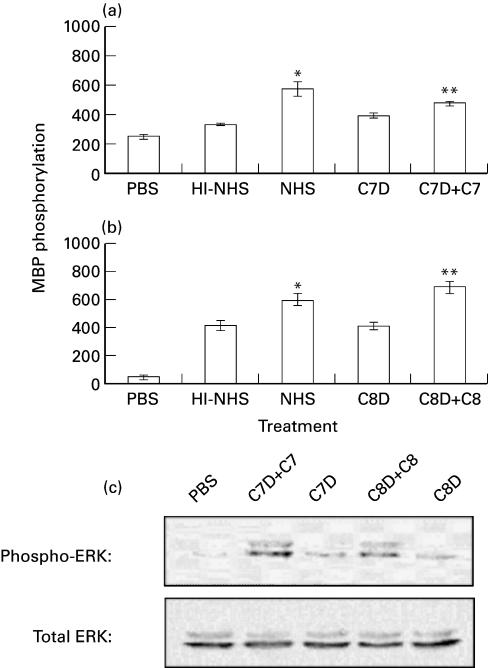
To prove that ERK activation is effected by complement and not by other serum factor(s), we used genetically C7- or C8-deficient human sera. K562 cells were treated first with a sublytic dose of antibody and then with the C7-deficient (C7D) or C8-deficient (C8D) human serum supplemented or not with purified human C7 or C8, respectively. The effect of the complement-deficient sera on ERK activity is presented in Fig. 2a. The results showed that antibody and C7D or C8D induced low ERK activation, to the same extent as HI-NHS. Reconstitution of the C7D with purified human C7 (Fig. 2a) and of the C8D with purified human C8 (Fig. 2b) potentiated the capacity of these sera to induce ERK activation to the level of ERK activation by NHS. ERK activation was also examined by Western Blotting with specific anti‐phospho ERK antibodies. Clearly sublytic complement produced in K562 cells activation of both ERK1 and ERK2 (Fig. 2c).
Fig. 2.
ERK1,2 activation by reconstituted C7-and C8-deficient serum. Serum-starved and antibody coated K562 cells were stimulated for 10 min at 37°C with complement: (a) HI-NHS, NHS, C7-deficient human serum (C7D) and C7D supplemented with C7; (b) HI-NHS, NHS, C8-deficient human serum (C8D) and C8D supplemented with C8; (c) C7D or C8D with or without C7 or C8, respectively. PBS-treated cells served as control. (a,b): MAPK activity was determined in cell extracts by MBP phosphorylation. Statistical significance was analysed by the student t-test. * NHS versus HI-NHS; P < 0·01. ** C7D + C7 versus C7D; P < 0·05. ** C8D + C8 versus C8D; P < 0·001. (c) Active and total ERK levels were examined by Western Blotting with anti‐phospho ERK and anti-ERK antibodies.
Effect of calcium ionophores, PMA and PKC inhibitors on ERK activity
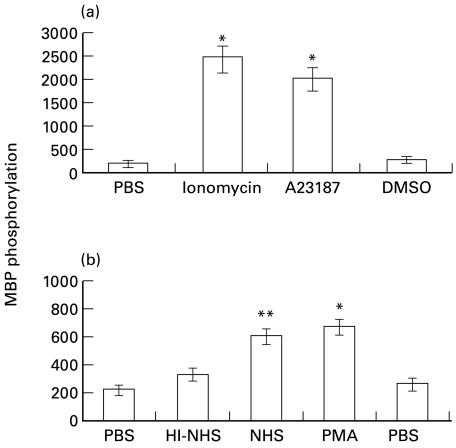
Our previous results demonstrated that calcium ionophores can reproduce some of the the effects of sublytic complement [15]. The effect of the calcium ionophores, ionomycin and A23187, on ERK activity in K562 cells was examined. As shown in Fig. 3a, both ionophores strongly activated ERK in K562 cells, as compared to control cells treated with PBS or with the solvent (DMSO) alone. The phorbol ester PMA which is known to activate PKC [38] also enhanced ERK activity in K562 cells to a similar extent as sublytic antibody and NHS (Fig. 3b).
Fig. 3.
Effect of calcium ionophores and PMA on MAPK activity. Serum-starved K562 cells were treated with (a) ionophores: 3 µm ionomycin, 25 µm A23187 or 0·01% DMSO (b) antibody and complement (HI-NHS or NHS) or PMA (10 µg/ml) or DMSO (0·01%) for 10 min at 37°C. PBS-treated cells served as control. Statistical significance of differences (ionophores or PMA versus DMSO; NHS versus HI-NHS) was analysed by the student t-test. * P < 0·001, ** P < 0·01. Results are representative of 3 independent experiments.
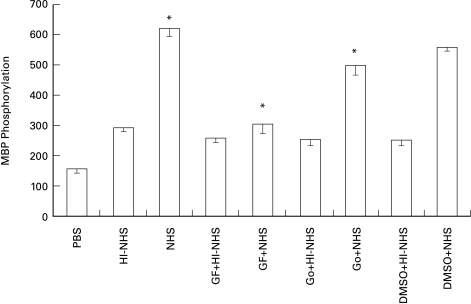
The possible involvement of PKC in complement-induced ERK activation was next studied. Pretreatment with the PKC inhibitors GF109203X and Go6976, abrogated the capacity of sublytic doses of complement to activate ERK in K562 cells (Fig. 4). GF109203X and Go6976 are very potent inhibitors of PKC and they both display a high degree of specificity to PKC over other serine/threonine kinases. GF109203X appears to inhibit all PKC isoforms with similar potency [39], whereas Go6976 has been found to discriminate between PKC isoforms in vitro, showing higher selectivity for the conventional isotypes PKCα and PKCβ [40]. Our results showed (Fig. 4) that GF109203X produced almost a complete inhibition of ERK activation by complement, while Go6976 produced only a partial inhibition. ERK activation by antibody and HI-NHS (i.e. the serum effect) was not inhibited by the PKC inhibitors.
Fig. 4.
Effect of PKC inhibitors on complement-induced MAPK activity. Serum-starved K562 cells were preincubated with or without GF109203X (0·5 µm) or Go6976 (2 nm) or DMSO (0·5%) for 60 min at 37°C and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. PBS-treated cells served as control. Statistical significance was analysed by the student t-test. * NHS versus HI-NHS, GF109203X + NHS versus NHS, Go6976 + NHS versus NHS or versus DMSO + NHS; P < 0·001. Results are representative of 4 independent experiments.
Involvement of ERK in cell resistance to complement
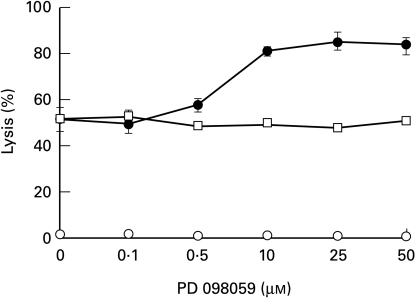
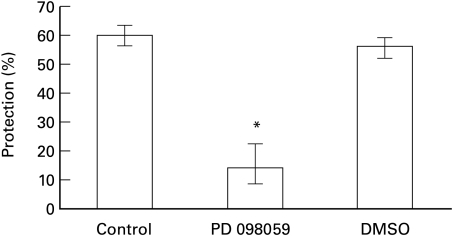
In order to examine the involvement of ERK in cell resistance to complement, the effect of PD098059 on complement-mediated lysis and complement-induced protection was examined. Inhibition of MEK by PD098059 was found to prevent the activation of ERK (ERK1 and ERK2) and the subsequent phosphorylation of ERK substrates both in vitro and in vivo [41]. Pretreatment with PD098059, but not with DMSO, enhanced the basal sensitivity of K562 cells to complement-mediated lysis (Fig. 5). In addition, pretreatment of K562 cells with PD098059 prior to treatment with sublytic doses of antibody and complement, also abolished the capacity of these cells to acquire resistance to lytic complement doses (Fig. 6).
Fig. 5.
Effect of PD098059 on complement-mediated cell lysis. K562 cells were pretreated with increasing concentrations of PD098059 or DMSO for 60 min at 37°C and then incubated with anti-K562 antibodies (30 min, 4°C) and with HI-NHS or NHS (1 : 2) for 60 min at 37°C. Percent lysis was determined in triplicates by trypan blue exclusion. Results are expressed as the mean percent lysis ± SD. Results are representative of 3 independent experiments. ○ PD098059 + HI-NHS; • PD098059 + NHS; □ DMSO+NHS.
Fig. 6.
Effect of PD098059 on complement-induced protection. K562 cells were pretreated without (control) or with: PD098059 (1 µm) or DMSO (0·02%) for 60 min at 37°C and then treated with sublytic antibody and HI-NHS or NHS. Next the cells were subjected to lytic antibody and NHS diluted 1:8 in PBS. Percent protection in induced cells (treated with NHS) was calculated relative to noninduced cells (treated with HI-NHS). Results are expressed as the mean percent protection ± SD (n = 4). The experiment is representative of 3 independent experiments. * P < 0·001.
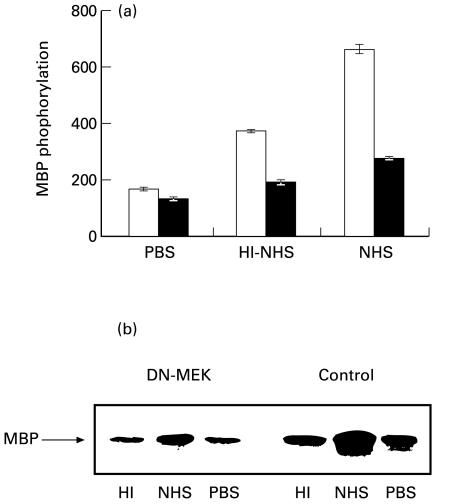
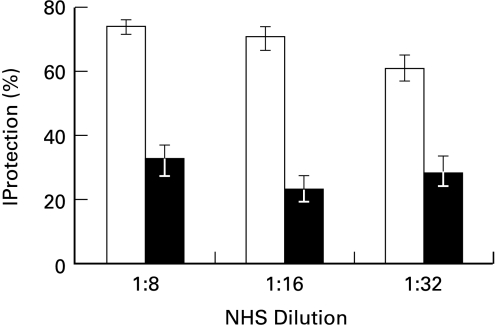
To elucidate the physiological roles of the ERK pathway, one approach has been to generate dominant negative mutants and overexpress them in cells. Being the only known activator of ERK1 and ERK2, MEK is a useful target for manipulation of the pathway. Dominant negative forms of MEK (ERKK1) have been found to inhibit the activation of ERK1 and ERK2 as well as growth factors-induced activities in several cells [42]. To further study the involvement of the MEK/ERK activities in cell response to sublytic complement, COS-7 cells were transiently transfected with a pcDNA1·1 expression vector containing the dominant negative mutant MEK (DN-MEK). As shown in Fig. 7, the presence of this DN-MEK mutant decreased complement-induced ERK activity as well as the HI-NHS-mediated activation of the enzyme. Similar results were obtained 48 h (Fig. 7a) and 72 h (data not shown) after transfection. Reduced ERK activation was also detected in transfectants by kinase immunoprecipitation from cell extracts and MBP phosphorylation (Fig. 7b). Accordingly, DN-MEK transfected COS-7 cells had a reduced capacity, relative to control nontransfected cells, to respond to sublytic complement and become protected from lytic complement (Fig. 8).
Fig. 7.
Complement-induced MAPK activity in COS-7 cells transfected with dominant-negative MEK. Serum-starved COS-7 cells 48 h after transfection with DN-MEK mutant (▪) and nontransfected cells (□ Control) were treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 20 min at 37°C. PBS-treated cells were used as control. (a) MAPK activity was analysed by MBP phosphorylation (b) MAPK activity was assayed by phosphorylation of MBP after immunoprecipitation of cell extracts with anti ERK C-terminus antibodies and protein A-Sepharose. The immunoprecipitates were subjected to SDS-PAGE in a 15% gel. PBS-treated cells were used as control. Autoradiogram of phosphorylated MBP is shown.
Fig. 8.
Effect of DN-MEK on complement-induced protection in COS-7 cells. COS-7 cells after transfection (▪) or nontransfected cells (□) were treated with a sublytic dose of antibody and HI-NHS or NHS. Next, the cells were subjected to lytic antibody and NHS serially diluted 1:8–1:32 in PBS. Percent protection in induced cells (treated with NHS) was calculated relative to noninduced cells (treated with HI-NHS). Results are expressed as the mean percent protection + SD (n = 3). The experiment is representative of 3 independent experiments.
DISCUSSION
The involvement of ERK in cell resistance to immune lysis was studied. As shown here, treatment of K562 and COS-7 cells with sublytic doses of antibody and complement activates ERK within 10 min. This is demonstrated both by Western Blotting and activity assays. After reaching a peak activity at 10 min, ERK activity then declines for 5 min and rises up again at 20 min after treatment. This observation was reproducible and was seen also with HL-60 cells treated with sublytic complement (data not shown). This might be indicative of activation of an ERK phosphatase activity that peaks at 15 min after complement treatment. ERK is activated by MEK by phosphorylation on threonine-183 and tyrosine-185 [20,43], and dephosphorylation at any of these sites will abolish its activity. Activation of such a phosphatase by complement has not been reported yet. Activation of an ERK or a MEK inhibitor is also possible. Both ERK1 and ERK2 were observed in K562 cells and following treatment with sublytic complement, both were activated. This was evident in Western Blotting analysis with anti‐active ERK antibodies (Fig. 2c).
MAPK can be activated in vitro in cells by serum treatment, due to the action of growth factors [37]. This ‘serum effect’ is seen in our experiments in control cells treated with heat-inactivated normal human serum (Figs 2–4) or with complement-deficient sera (Fig. 2). However, a significant activation of ERK, above the serum effect, is seen in cells that are incubated in complement sufficient sera, such as NHS and C7- or C8-deficient sera reconstituted with C7 or C8, respectively. Clearly, the terminal complement proteins, inserted into K562 cell membrane, trigger ERK activation. Similar activation of ERK1 was seen in human aortic smooth muscle cells [27] treated with sublytic complement. Also, sublytic complement has been shown to activate Ras, Raf-1 and ERK1 in JY25 human B lymphoblastoid cells [25]. The main upstream mechanism leading to the activation of ERK is its phosphorylation by the dual specificity protein kinase, known as the MAPK kinase or ERK kinase or MEK. The MEKs are in turn regulated by phosphorylation on serine residues by MEK kinases.
The fact that complement can activate ERK does not necessarily implicate it in complement resistance. However, as shown here, pretreatment of cells with PD098059 inhibits ERK activation by sublytic complement, sensitizes cells to complement-mediated lysis and prevents the desensitization of K562 cells by sublytic complement doses (Figs 1c, 5, 6). PD098059 selectively blocks MEK activity, thus inhibiting both phosphorylation and activation of ERK in vitro and in a variety of intact cells [41]. PD098059 has not been found to block other protein kinases even at higher concentrations. It binds to the inactive form of MEK and prevents its activation by Raf-1, MEKK1 and other upstream activators [44]. A dominant-negative MEK (DN-MEK) was also used to determine the physiological relevance of ERK to cell resistance to complement. Our results show that unlike COS-7 cells which are desensitized to lytic complement by pretreatment with sublytic complement, COS-7 cells transfected with DN-MEK can not undergo desensitization in response to sublytic complement (Fig. 8). However, DN-MEK had only a small insignificant effect on basal cell resistance to complement (results not shown). The results obtained with PD098059 and with DN-MEK prove that signalling via the ERK pathway is essential for the complement-induced protection. The fact that PD098059 but not DN-MEK significantly sensitizes cells to lysis, raises a question regarding ERK’s involvement in basal resistance mechanisms. It is possible that due to incomplete inhibition by DN-MEK, transfected cells still contain low but sufficient ERK activity to support the ERK-dependent protective mechanism. Therefore, the data suggests that ERK may also contribute to basal cell resistance to complement.
Studies on cell growth and differentiation indicated that among their many substrates, the ERKs phosphorylate nuclear transcription factors such as c-Jun [45] and Elk-1 [46] and other nuclear proteins, demonstrating their role as important regulators of nuclear transcriptional activity [21]. The ERKs are known also to phosphorylate numerous cytoplasmic proteins and affect their activity [20]. Both phosphorylation and transcriptional activities of ERK are fast and may be completed before the lytic effect of the MAC is irreparable. The downstream ERK-activated proteins that promote complement resistance are still not known. One possibility is that one or more of the transcription factors activated by ERK up-regulate synthesis of ‘protective’ proteins. Indeed, complement resistance and complement-induced protection depend on de novo protein synthesis [14]. Such proteins may confer on cells some resistance against complement lysis by blocking the intracellular death process or by promoting damage repair, similar to the protective effects of Bcl-2 family members from apoptotic-type cell death [47]. Alternatively, ERK may directly or indirectly promote removal of the MAC channels by vesiculation and/or endocytosis [48, 49]. MAC channel elimination was shown to be inhibited by PKC inhibitors [48] and perhaps is also driven by ERK. Yet, another possibility is that ERK amplifies somehow the protective capacity of the membrane complement regulatory proteins CD46, CD55 and/or CD59. However, the fact that cells triggered by sublytic MAC doses (and thus have an elevated ERK level) bind as much C3 and C9 as control cells [14] and express the same amount of membrane regulators [50 and Jurianz et al., personal communication], opposes the latter possibility.
Sublytic complement is known to produce calcium ion influx and activation of PKC in various target cells [9–12, 26]. We have recently shown that calcium-mediated PKC activation is an essential early event in the process of desensitization induced in K562 cells subjected to sublytic complement attack [13]. Results presented here (Fig. 4), demonstrate that the activation of PKC by complement is an upstream event in the activation of ERK. PKC inhibitors prevented ERK activation by sublytic complement. Two PKC inhibitors had no effect on the ‘serum-induced ERK activation’ observed in cells treated with HI-NHS (Fig. 4), excluding PKC involvement in ‘serum’ activation. GF109203X reduced complement-induced ERK activation to the level of ‘serum’ effect, whereas Go6976 inhibited it only partially. Since GF109203X is a general PKC inhibitor [39] and Go6976 is selective to the conventional type PKC [40], it is concluded that both conventional and nonconventional PKC types are activating ERK1 in complement-induced K562 cells. It is not clear which of the two conventional PKC types, PKCα and PKCβ2, that are activated in K562 cells by sublytic complement [13], promotes ERK activation.
Acknowledgments
This work was supported in part by research grants from The Israel Science Foundation and the Israel Cancer Association and by the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel’s Ministry of Science (MOS). The excellent technical assistance of Ms. Tamar Hanoch is acknowledged.
REFERENCES
- 1.Muller-Eberhard HJ. The membrane attack complex of complement. Ann Rev Immunol. 1986;4:503–428. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- 2.Jurianz K, Ziegler S, Garcia-Schuler H, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–39. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 3.Morgan BP, Meri S. Membrane Proteins That Protect Against Complement Lysis. Springer SeminImmunopathol. 1994;15:369–96. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 4.Ollert MW, Frade R, Fiandino A, et al. C3- cleaving membrane proteinase: a new complement regulatory protein of human melanoma cells. J Immunol. 1990;144:3862–7. [PubMed] [Google Scholar]
- 5.Paas Y, Bohana-Kashtan O, Fishelson Z. Phosphorylation of the complement component, C9, by an ecto-protein kinase of human leukemic cells. Immunopharmacology. 1999;42:175–85. doi: 10.1016/s0162-3109(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 6.Ohanian SH, Shlager SI. Humoral immune killing of nucleated cells: Mechanisms of complement- mediated attack and target cell defense. CRC Crit Rev Immunol. 1981;1:165–209. [PubMed] [Google Scholar]
- 7.Shin ML, Carney DF. Cytotoxic action and other metabolic consequences of terminal complement proteins. Prog Allergy. 1988;40:44–81. [PubMed] [Google Scholar]
- 8.Morgan BP. Complement membrane attack on nucleated cells: Resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–1. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishelson Z, Kopf E, Reiter Y. Phosphorylation, phosphatidyl inositol turnover and resistance to complement damage. FASEB J. 1988;2:A872. [Google Scholar]
- 10.Fishelson Z, Kopf E, Paas Y, Roos L, Reiter Y. Protein phosphorylation as a mechanism of resistance against complement damage. Prog Immunol. 1989;7:205–8. [Google Scholar]
- 11.Carney DF, Lang TJ, Shin ML. Multiple signals are generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol. 1990;145:623–9. [PubMed] [Google Scholar]
- 12.Cybulsky AV, Bonventre JV, Quigg RJ, Liebenthal W, Salant DJ. Cytosolic calcium and protein kinase C reduce complement- mediated epithelial injury. Kidney Int. 1990;38:803–11. doi: 10.1038/ki.1990.274. [DOI] [PubMed] [Google Scholar]
- 13.Kraus S, Fishelson Z. Induction of protection from complement in tumor cells depends on calcium influx and activation of protein kinase C. Eur J Immunol. 2000;30:1272–80. doi: 10.1002/(SICI)1521-4141(200005)30:5<1272::AID-IMMU1272>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Reiter Y, Ciobotariu A, Fishelson Z. Sublytic complement attack protects tumor cells from lytic doses of antibody and complement. Eur J Immunol. 1992;22:1207–13. doi: 10.1002/eji.1830220515. [DOI] [PubMed] [Google Scholar]
- 15.Reiter Y, Ciobotariu A, Jones J, Morgan BP, Fishelson Z. Complement membrane attack complex, perforin, and bacterial exotoxins induce in K562 cells calcium- dependent cross- protection from lysis. J Immunol. 1995;155:2203–10. [PubMed] [Google Scholar]
- 16.Reiter Y, Fishelson Z. Complement membrane attack complexes induce in human leukemic cells rapid expression of large proteins (L-CIP) Mol Immunol. 1992;29:771–81. doi: 10.1016/0161-5890(92)90187-3. [DOI] [PubMed] [Google Scholar]
- 17.Halperin JA, Taratuska A, Nicholson-Weller A. Terminal complement complex C5b-9 stimulates mitogenesis in 3T3 cells. J Clin Invest. 1993;91:1974–8. doi: 10.1172/JCI116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet derived growth factor from endothelial cells. J Exp Med. 1994;179:985–92. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes: S phase induction is dependent on c-jun activation. J Immunol. 1996;156:4892–900. [PubMed] [Google Scholar]
- 20.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 21.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opinion Cell Biol. 1996;8:205–15. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 22.Kyriakis JM, App H, Zhang XF, et al. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–21. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 23.Traverse S, Cohen P. Identification of a latent MAP kinase kinase in PC12 cells as B-raf. FEBS Lett. 1994;350:13–8. doi: 10.1016/0014-5793(94)00723-3. [DOI] [PubMed] [Google Scholar]
- 24.Posada J, Yew N, Ahn NG, Vande-Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–53. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niculescu F, Rus H, Van Biesen T, Shin ML. Activation of ras mitogen-activated protein kinase pathway by terminal complement complexes is G protein dependent. J Immunnol. 1997;158:4405–12. [PubMed] [Google Scholar]
- 26.Cybulsky AV, Papillon J, McTavish AJ. Complement activates phospholipases and protein kinases in glomerular epithelial cells. Kidney Int. 1998;54:360–72. doi: 10.1046/j.1523-1755.1998.00013.x. [DOI] [PubMed] [Google Scholar]
- 27.Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger M, Nave Z, Levy Y, Slater PE, Fishelson Z. Prevalence of hereditary properdin, C7 and C8 deficiences in patients with meningococcal infections. Clin Exp Immunol. 1990;81:423–7. doi: 10.1111/j.1365-2249.1990.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammer CH, Wirtz GH, Renfer L, Gersham HD, Tack BF L. arge scale isolation of functionally active components of the human complement system. J Biol Chem. 1981;256:3995–4006. [PubMed] [Google Scholar]
- 30.Hardow E, Lane D. Storing and purifying antibodies. In: Hardow E, Lane D, editors. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor; 1988. pp. 285–318. [Google Scholar]
- 31.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Seger R, Seger D, Reska AA, et al. Over expression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH3T3 cells. J Biol Chem. 1994;269:25699–709. [PubMed] [Google Scholar]
- 33.Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–7. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansour SJ, Matten WT, Hermann AS, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–9. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 35.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res. 1979;7:1513–23. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keown WA, Campbell CR, Kucherlapati RS. Methods for introducing DNA into mammalian cells. Meth Enzymol. 1990;185:527–37. doi: 10.1016/0076-6879(90)85043-n. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa Y, Nishizuka Y. Direct activation of calcium-activated, phospholipid dependent protein kinase by tumor promoting phorbol esters. J Biol Chem. 1982;257:7847–51. [PubMed] [Google Scholar]
- 39.Toullec D, Pianetti P, Coste H, et al. The bisindolylmaleimide GF109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–81. [PubMed] [Google Scholar]
- 40.Martiny-Baron G, Mischak H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go6976. J Biol Chem. 1993;268:9194–7. [PubMed] [Google Scholar]
- 41.Dudley DT, Pang L, Decker ST, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–9. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinases is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 43.Tobe K, Kadowaki T, Tamemoto H, et al. Insulin and 12-O-tetradecanoyl phorbol-13-acetate activation of two immunologically distinct myelin basic protein/microtubule-associated protein 2 (MBP/MAP2) kinases via de novo phosphorylation of threonine and tyrosine residues. J Biol Chem. 1991;266:24793–803. [PubMed] [Google Scholar]
- 44.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;17:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 45.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–4. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 46.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Coob MH, Shaw PE. ERK phosphorylation potentiates Elk-1 mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–62. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 48.Carney DF, Hammer CH, Shin ML. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: Influence of extracellular Ca2+ and association with cellular Ca2+ J Immunol. 1986;137:263–70. [PubMed] [Google Scholar]
- 49.Morgan BP, Dankert JR, Esser AF. Recovery of human neutrophils from complement attack: Removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987;138:246–53. [PubMed] [Google Scholar]
- 50.Marchbank KJ, Morgan BP, Van Den Berg CW. Regulation of CD59 expression on K562 cells: effects of phorbol myristate acetate, cross-linking antibody and non-lethal complement attack. Immunology. 1995;85:146–52. [PMC free article] [PubMed] [Google Scholar]